Comparative Analysis of Intraoral Scanner Accuracy in a Six-Implant Complete-Arch Model: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
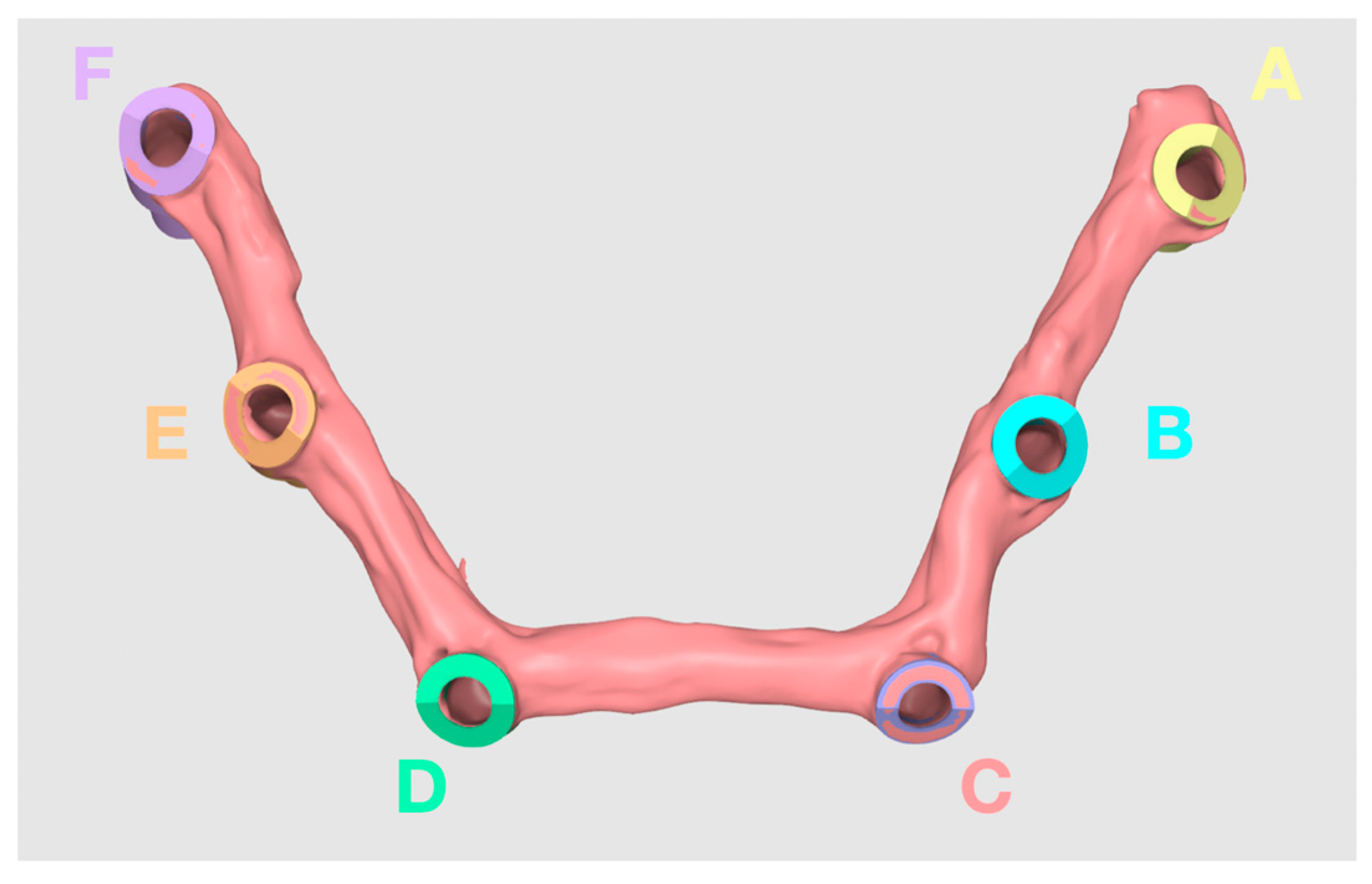
2.1. Fabrication of Master Model
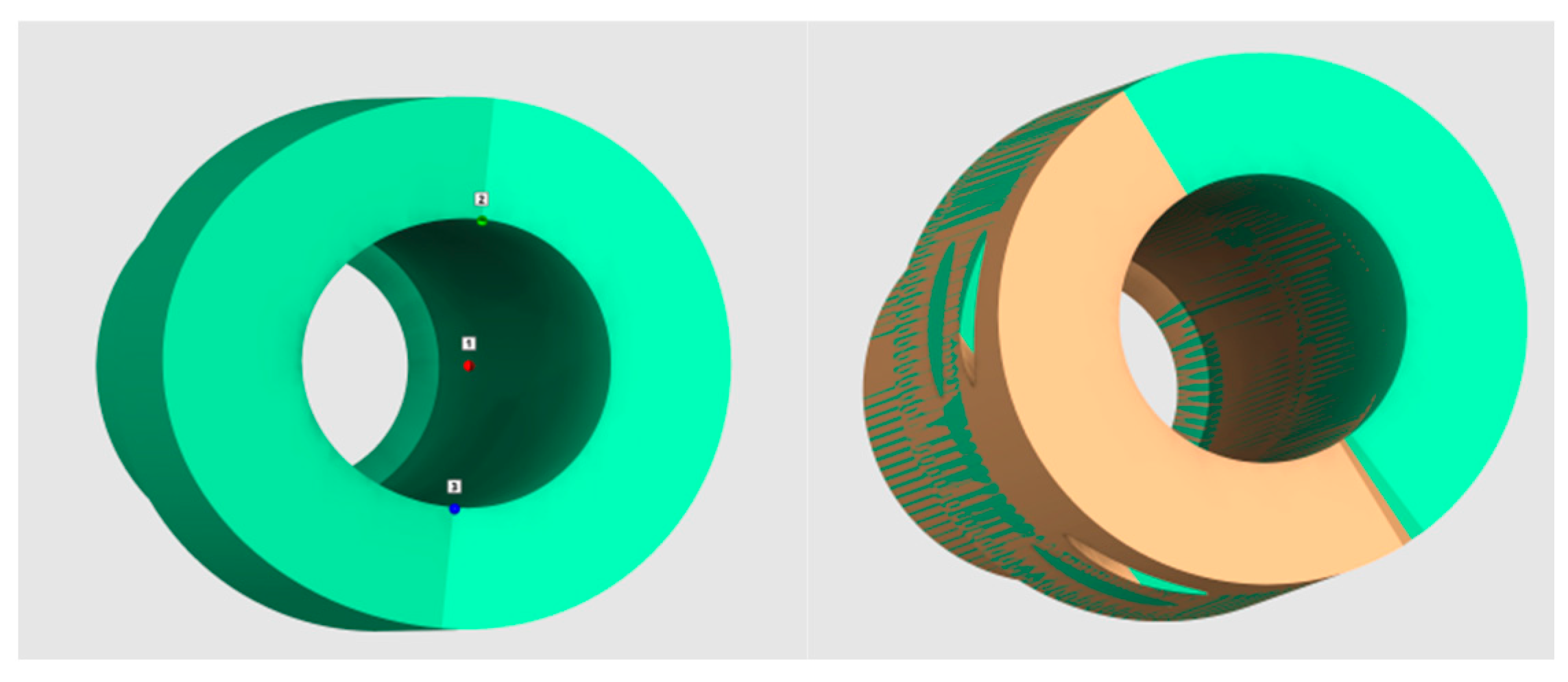
2.2. Measurements of Distances between the Abutments
2.3. Intraoral Scanners
- -
- Group 1: Medit i700 wired (Medit, Seoul, Republic of Korea)
- -
- Group 2: Medit i700 wireless (Medit, Seoul, Republic of Korea)
- -
- Group 3: CS3800 wireless (Carestream Health, Rochester, NY, USA)
- -
- Group 4: Trios4 wireless (3Shape, Copenhagen, Denmark)
2.4. Statistical Analysis
3. Results
3.1. Short Distances
3.2. Medium Distances
3.3. Long Distances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erozan, Ç.; Ozan, O.E. Evaluation of the Precision of Different Intraoral Scanner-Computer Aided Design (CAD) Software Combination in Digital Dentistry. Med. Sci. Monit. 2020, 26, e918529. [Google Scholar] [PubMed]
- Amin, S.; Weber, H.P.; Finkelman, M.; El Rafie, K.; Kudara, Y.; Papaspyridakos, P. Digital vs. conventional full-arch implant impressions: A comparative study. Clin. Oral Implant. Res. 2017, 28, 1360–1367. [Google Scholar] [CrossRef]
- Capparè, P.; Sannino, G.; Minoli, M.; Montemezzi, P.; Ferrini, F. Conventional versus digital impressions for full arch screw-retained maxillary rehabilitations: A randomized clinical trial. Int. J. Environ. Res. Publish Health 2019, 16, 829. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, F.; Gherlone, E.F.; Keeling, A.; Gastaldi, G.; Ferrari, M. Full-arch intraoral scanning: Comparison of two different strategies and their accuracy outcomes. J. Osseointegr. 2018, 10, 65–74. [Google Scholar]
- Davidowitz, G.; Kotick, P.G. The use of CAD/CAM in dentistry. Dent. Clin. N. Am. 2011, 55, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Janeva, N.M.; Kovacevska, G.; Elencevski, S.; Panchevska, S.; Mijoska, A.; Lazarevska, B. Advantages of CAD/CAM versus Conventional Complete Dentures. Open Access Maced. J. Med. Sci. 2018, 6, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Duret, F. The pratical dental CAD/CAM in 1993. J. Can Dent. Assoc. 1993, 59, 445–446, 448–453. [Google Scholar] [PubMed]
- Punj, A.; Bompolaki, D.; Garaicoa, J. Dental Impression Materials and Techniques. Dent. Clin. N. Am. 2017, 61, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Ting-Shu, S.; Jian, S.J. Intraoral Digital Impression Technique: A Review. J. Prosthodont. 2015, 24, 313–321. [Google Scholar] [CrossRef]
- Dec, L.J.H. Digital Workflow for Establishing the Posterior Palatal Seal on a Digital Complete Denture. J. Prosthodont. 2021, 30, 817–821. [Google Scholar] [CrossRef]
- Suese, K. Progress in digital dentistry: The practical use of intraoral scanners. Dent. Mater. J. 2020, 39, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Medit i700 Wireless: Scanning Made Easy with Simile Touch. Dental Economics Editors. 2022. Available online: https://www.biospace.com/article/releases/medit-i700-wireless-scanning-made-easy-with-a-simple-touch/?s=71 (accessed on 7 March 2024).
- Cheng, J.; Zhang, H.; Liu, H.; Li, J.; Wang, H.L.; Tao, X. Accuracy of maxillary full-arch digital impressions of tooth and implant models made by two intraoral scanners. Clin. Exp. Dent. Res. 2024, 10, e857. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, H.; Liu, H.; Li, J.; Wang, H.L.; Tao, X. Accuracy of edentulous full-arch implant impression: An in vitro comparison between conventional impression, intraoral scan with and without splinting, and photogrammetry. Clin. Oral Implant. Res. 2024; Ahead of print. [Google Scholar] [CrossRef]
- Kihara, H.; Hatakeyama, W.; Komine, F.; Takafuji, K.; Takahashi, T.; Yokota, J.; Oriso, K.; Kondo, H.J. Accuracy and practicality of intraoral scanner in dentistry: A literature review. J. Prosthodont. Res. 2020, 64, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, R.G.; Persson, A.S. Scanning accuracy and precision in 4 intraoral scanners: An in vitro comparison based on 3-dimensional analysis. J. Prosthet. Dent. 2014, 112, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.Y.; Jang, Y.; Kim, W.C.; Kim, H.Y.; Lee, D.H.; Kim, J.H. Comparing the accuracy (trueness and precision) of models of fixed dental prostheses fabricated by digital and conventional workflows. J. Prosthodont. Res. 2019, 63, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ender, A.; Mehl, A. In-vitro evaluation of the accuracy of conventional and digital methods of obtaining full-arch dental impressions. Quintessence Int. 2015, 46, 9–17. [Google Scholar]
- Carbajal, M.J.B.; Wakabayashi, K.; Nakamura, T.; Yatani, H. Influence of abutment tooth geometry on the accuracy of conventional and digital methods of obtaining dental impressions. J. Prosthet. Dent. 2017, 118, 392–399. [Google Scholar] [CrossRef] [PubMed]
- ISO 5725-1; Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 1: General Principles and Definitions. International Standards Organization (ISO): Geneva, Switzerland, 1994.
- Mangano, F.G.; Admakin, O.; Bonacina, M.; Lerner, H.; Rutkunas, V.; Mangano, C. Trueness of 12 intraoral scanners in the full-arch implant impression: A comparative in vitro study. BMC Oral Health 2020, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.-Y.; Benic, G.I.; Park, J.-M. Trueness of digital intraoral impression in reproducing multiple implant position. PLoS ONE 2019, 19, e0222070. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Gallucci, G.O.; Chen, C.J.; Hanssen, S.; Naert, I.; Vandenberghe, B. Digital versus conventional implant impressions for edentulous patients: Accuracy outcomes. Clin. Oral Implant. Res. 2016, 27, 465–472. [Google Scholar] [CrossRef]
- Flügge, T.V.; Att, W.; Metzger, M.C.; Nelson, K. Precision of Dental Implant Digitization Using Intraoral Scanners. Int. J. Prosthodont. 2016, 29, 277–283. [Google Scholar] [CrossRef]
- Giménez, B.; Özcan, M.; Martínez-Rus, F.; Pradíes, G. Accuracy of a digital impression system based on parallel confocal laser technology for implants with consideration of operator experience and implant angulation and depth. Int. J. Oral Maxillofac. Implant. 2014, 29, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Rutkunas, V.; Geciauskaite, A.; Jegelevicius, D.; Vaitiekunas, M. Accuracy of digital implant impressions with intraoral scanners. A systematic review. Eur. J. Oral Implantol. 2017, 1, 101–120. [Google Scholar]
- Lee, D.J.; Saponaro, P.C. Management of Edentulous Patients. Dent. Clin. N. Am. 2019, 63, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Hull, P.S.; Worthington, H.V.; Clerehugh, V.; Tsirba, R.; Davies, R.M.; Clarkson, J.E. The reason for tooth extraction in adults and their validation. J. Dent. 1997, 25, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Istat. Il Ricorso Alle Cure Odontoiatriche e la Salute Dei Denti in Italia. 2013. Available online: https://www.istat.it/it/archivio/164054 (accessed on 7 March 2024).
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Agliardi, E. Tilted Implants—Riabilitazione Implanto-Protesica del Paziente Atrofico. s.l.; Quintessence Publishing: Batavia, IL, USA, 2018. [Google Scholar]
- Aiuto, R.; Barbieri, C.; Garcovich, D.; Dioguardi, M.; Redaelli, M.; De Micheli, L. Rehabilitation of Edentulous Jaws with Full-Arch Fixed Implant-Supported Prostheses: An Approach with Short and Ultrashort Implants and Metal-Free Materials. Case Rep. Dent. 2020, 2020, 8890833. [Google Scholar] [CrossRef]
- Crespi, R.; Vinci, R.; Capparé, P.; Romanos, G.E.; Gherlone, E. A clinical study of edentulous patients rehabilitated according to the “all on four” immediate function protocol. Int. J. Oral Maxillofac. Implant. 2012, 27, 428–434. [Google Scholar]
- Cattoni, F.; Chirico, L.; Merlone, A.; Manacorda, M.; Vinci, R.; Gherlone, E.F. Digital Smile Designed Computer-Aided Surgery versus Traditional Workflow in “All on Four” Rehabilitations: A Randomized Clinical Trial with 4-Years Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 3449. [Google Scholar] [CrossRef] [PubMed]
- Gómez-de Diego, R.; Mang-de la Rosa Mdel, R.; Romero-Pérez, M.J.; Cutando-Soriano, A.; López-Valverde-Centeno, A. Indications and contraindications of dental implants in medically compromised patients: Update. Med. Oral Patol. Oral Cir. Bucal. 2014, 19, e483–e489. [Google Scholar] [CrossRef] [PubMed]
- Kullar, A.S.; Miller, C.S. Are There Contraindications for Placing Dental Implants? Dent. Clin. N. Am. 2019, 63, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, I. A Proposal of New Classification for Dental Implant Complications. J. Contemp. Dent. Pract. 2018, 19, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Freymiller, E.G.; Leizerovitz, M. Management of complications of dental implant surgery. Alpha Omegan 2014, 107, 56. [Google Scholar] [PubMed]
- Ozcelik, O.; Haytac, M.C.; Akkaya, M. Iatrogenic trauma to oral tissues. J. Periodontol. 2005, 70, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Custodio, A.L. Mandibular fractures associated with endosteal implants. Oral Maxillofac. Surg. 2009, 13, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, K.; Ryncarz, W.; Yüksel, O.; Goncalves, P.; Baek, K.W.; Cok, S.; Dard, M. Image analysis of immediate full-arch prosthetic rehabilitations guided by a digital workflow: Assessment of the discrepancy between planning and execution. Int. J. Implant. Dent. 2019, 5, 26. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, R.; Crespi, G.; Cosola, S.; Covani, U. A Retrospective Digital Analysis of Contour Changing after Tooth Extraction with or without Using Less Traumatic Surgical Procedures. J. Clin. Med. 2022, 11, 922. [Google Scholar] [CrossRef]
- Kapos, T.; Evans, C. CAD/CAM technology for implant abutments, crowns, and superstructures. Int. J. Oral Maxillofac. Implant. 2014, 29, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Ellakany, P.; Al-Harbi, F.; El Tantawi, M.; Mohsen, C. Evaluation of the accuracy of digital and 3D-printed casts compared with conventional stone casts. J. Prosthet. Dent. 2022, 127, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Kim, D.; Kim, J.; Kim, W. Accuracy of dental replica models using photopolymer materials in additive manufacturing: In vitro three-dimensional evaluation. J. Prosthodont. 2019, 28, e557–e562. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, R.M.; Yilmaz, B.; McGlumphy, E.A.; Seidt, J.; Johnston, W.M. Accuracyof different digital scanning techniques and scan bodies for complete-arch implant- supported prostheses. J. Prosthet. Dent. 2020, 123, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Imbrurgia, M.; Kois, J.; Marino, E.; Lerner, H.; Mangano, F.G. Continuous scan strategy (CSS)–A novel technique to improve the accourcie of intraoral digital impressions. Eur. J. Prosthodont. Restor. Dent. 2020, 28, 128–141. [Google Scholar]
- Abdel-Azim, T.; Zandinejad, A.; Elathamna, E.; Lin, W.; Morton, D. The influence of digital fabrication options on the accuracy of dental implant-based single units and complete-arch frameworks. Int. J. Oral Maxillofac. Implant. 2014, 29, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Baghani, M.T.; Shayegh, S.S.; Johnston, W.M.; Shidfar, S.; Hakimaneh, S.M.R. In vitro evaluation of the accuracy and precision of intraoral and extraoral complete-arch scans. J. Prosthet. Dent. 2021, 126, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Setti, P.; Pera, F.; Pera, P.; Pesce, P. Accuracy of multi-unit implant impression: Traditional techniques versus a digital procedure. Clin. Oral Investig. 2018, 22, 1253–1262. [Google Scholar] [CrossRef]
- Pesce, P.; Pera, F.; Setti, P.; Menini, M. Precision and Accuracy of a Digital Impression Scanner in Full-Arch Implant Rehabilitation. Int. J. Prosthodont. 2018, 31, 171–175. [Google Scholar] [CrossRef]
- García-Gil, I.; Cortés-Bretón-Brinkmann, J.; Jiménez-García, J.; Peláez-Rico, J.; Suárez-García, M.J. Precision and practical usefulness of intraoral scanners in implant dentistry: A systematic literature review. J. Clin. Exp. Dent. 2020, 12, e784–e793. [Google Scholar] [CrossRef]
- Chiu, A.; Chen, Y.W.; Hayashi, J.; Sadr, A. Accuracy of CAD/CAM Digital Impressions with Different Intraoral Scanner Parameters. Sensors 2020, 20, 1157. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The use of PEEK in digital prosthodontics: A narrative review. BMC Oral Health 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, P.; Ann, R. Australas Coll Dent Surg. CAD/CAM Dent. 1996, 13, 99–107. [Google Scholar]
- Preston, J.D.; Duret, F. CAD/CAM in dentistry. Oral Health 1997, 87, 17–20, 23–24, 26–27. [Google Scholar] [PubMed]
- Revell, G.; Simon, B.; Mennito, A.; Evans, Z.P.; Renne, W.; Ludlow, M.; Vág, J. Evaluation of complete-arch implant scanning with 5 different intraoral scanners in terms of trueness and operator experience. J. Prosthet. Dent. 2022, 128, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Meneghello, R.; Graiff, L.; Savio, G.; Vigolo, P.; Monaco, C.; Stellini, E. Full arch digital scanning systems performances for implant-supported fixed dental prostheses: A comparative study of 8 intraoral scanners. J. Prosthodont. Res. 2019, 63, 396–403. [Google Scholar] [CrossRef]
- Natsubori, R.; Fukazawa, S.; Chiba, T.; Tanabe, N.; Kihara, H.; Kondo, H. In vitro comparative analysis of scanning accuracy of intraoral and laboratory scanners in measuring the distance between multiple implants. Int. J. Implant. Dent. 2022, 8, 18. [Google Scholar] [CrossRef]
- Zarone, F.; Ruggiero, G.; Ferrari, M.; Mangano, F.; Joda, T.; Sorrentino, R. Comparison of different intraoral scanning techniques on the completely edentulous maxilla: An in vitro 3-dimensional comparative analysis. J. Prosthet. Dent. 2020, 124, 762.e1–762.e8. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, E.; Wee, A.G.; Carr, A.B.; Urban, I.; Margaritis, V. Accuracy of complete-arch implant impression made with occlusal registration material. J. Prosthet. Dent. 2020, 123, 143–148. [Google Scholar] [CrossRef]
- Ender, A.; Attin, T.; Mehl, A. In vivo precision of conventional and digital methods of obtaining complete-arch dental impressions. J. Prosthet. Dent. 2016, 115, 313–320. [Google Scholar] [CrossRef]
- Mehl, A.; Reich, S.; Beuer, F.; Güth, J.F. Accuracy, trueness, and precision—A guideline for the evaluation of these basic values in digital dentistry. Int. J. Comput. Dent. 2021, 24, 341–352. [Google Scholar] [PubMed]
- Nedelcu, R.; Olsson, P.; Thulin, M.; Nyström, I.; Thor, A. In Vivo Trueness and Precision of Full-Arch Implant Scans Using Intraoral Scanners with Three Different Acquisition Protocols. J. Dent. 2023, 128, 104308. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Graiff, L.; Savio, G.; Granata, S.; Basilicata, M.; Bollero, P.; Meneghello, R. Investigation of the Accuracy of Four Intraoral Scanners in Mandibular Full-Arch Digital Implant Impression: A Comparative In Vitro Study. Int. J. Environ. Res. Public Health 2022, 19, 4719. [Google Scholar] [CrossRef]

| Short Distances (<15 mm) (n = 3) | Medium Distances (15–40 mm) (n = 7) | Long Distances (>40 mm) (n = 5) | ||||
|---|---|---|---|---|---|---|
| Mean | Min–Max | Mean | Min–Max | Mean | Min–Max | |
| Alicona | 14.507 | 13.392–15.730 | 28.085 | 16.579–35.981 | 44.796 | 43.144–48.035 |
| Short (μm) | Medium (μm) | Long (μm) | |
|---|---|---|---|
| Group 1 | 36 | 38 | 83 |
| Group 2 | 45 | 56 | 46 |
| Group 3 | 30 | 49 | 61 |
| Group 4 | 42 | 58 | 85 |
| Short (μm) | Medium (μm) | Long (μm) | |
|---|---|---|---|
| Group 1 | 128 | 160 | 333 |
| Group 2 | 118 | 120 | 322 |
| Group 3 | 126 | 110 | 324 |
| Group 4 | 1.22 | 170 | 319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrini, F.; Mazzoleni, F.; Barbini, M.; Coppo, C.; Di Domenico, G.L.; Gherlone, E.F. Comparative Analysis of Intraoral Scanner Accuracy in a Six-Implant Complete-Arch Model: An In Vitro Study. Prosthesis 2024, 6, 401-412. https://doi.org/10.3390/prosthesis6020030
Ferrini F, Mazzoleni F, Barbini M, Coppo C, Di Domenico GL, Gherlone EF. Comparative Analysis of Intraoral Scanner Accuracy in a Six-Implant Complete-Arch Model: An In Vitro Study. Prosthesis. 2024; 6(2):401-412. https://doi.org/10.3390/prosthesis6020030
Chicago/Turabian StyleFerrini, Francesco, Federica Mazzoleni, Matteo Barbini, Carlotta Coppo, Giovanna Laura Di Domenico, and Enrico Felice Gherlone. 2024. "Comparative Analysis of Intraoral Scanner Accuracy in a Six-Implant Complete-Arch Model: An In Vitro Study" Prosthesis 6, no. 2: 401-412. https://doi.org/10.3390/prosthesis6020030
APA StyleFerrini, F., Mazzoleni, F., Barbini, M., Coppo, C., Di Domenico, G. L., & Gherlone, E. F. (2024). Comparative Analysis of Intraoral Scanner Accuracy in a Six-Implant Complete-Arch Model: An In Vitro Study. Prosthesis, 6(2), 401-412. https://doi.org/10.3390/prosthesis6020030





