Biofabrication Approaches for Peri-Implantitis Tissue Regeneration: A Focus on Bioprinting Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Analysis
3. Results
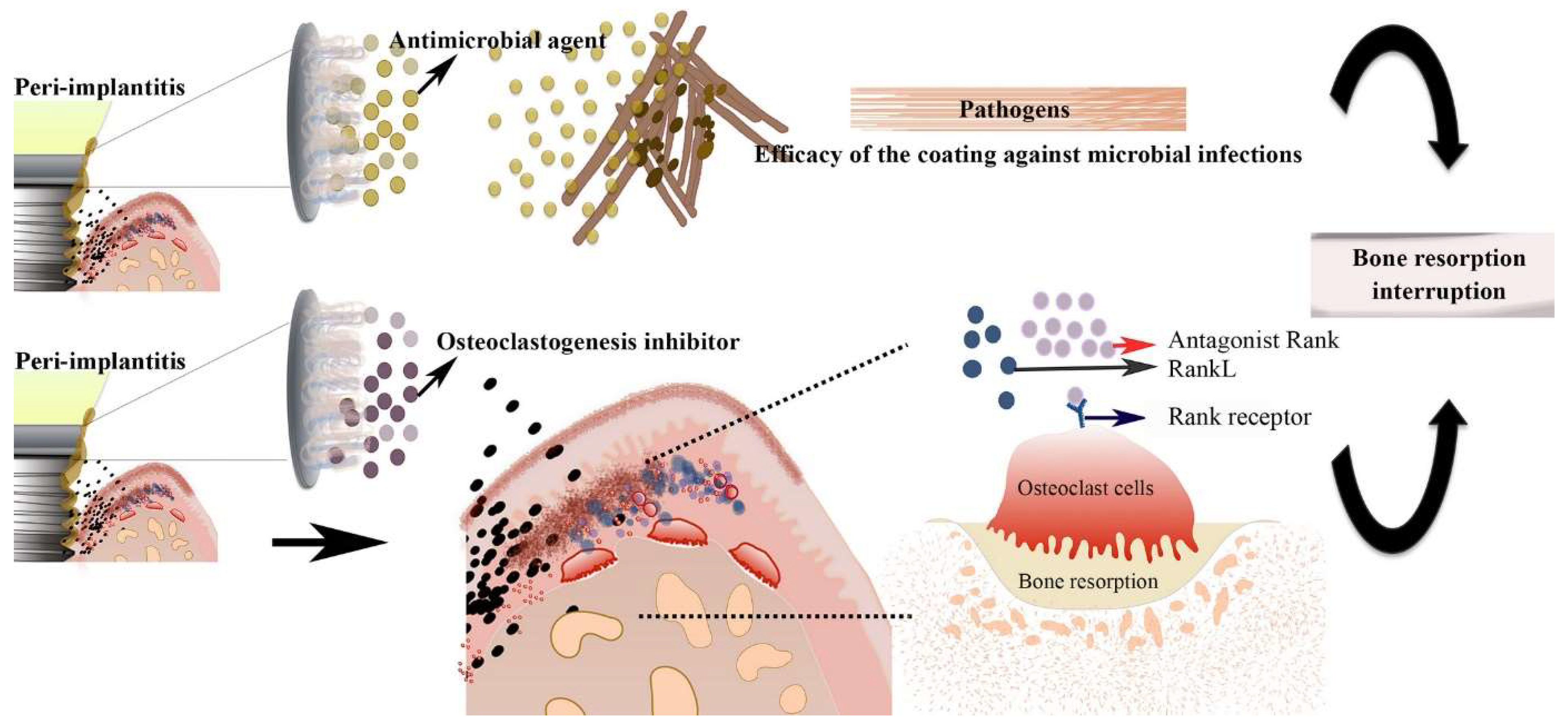
3.1. Implant Coating
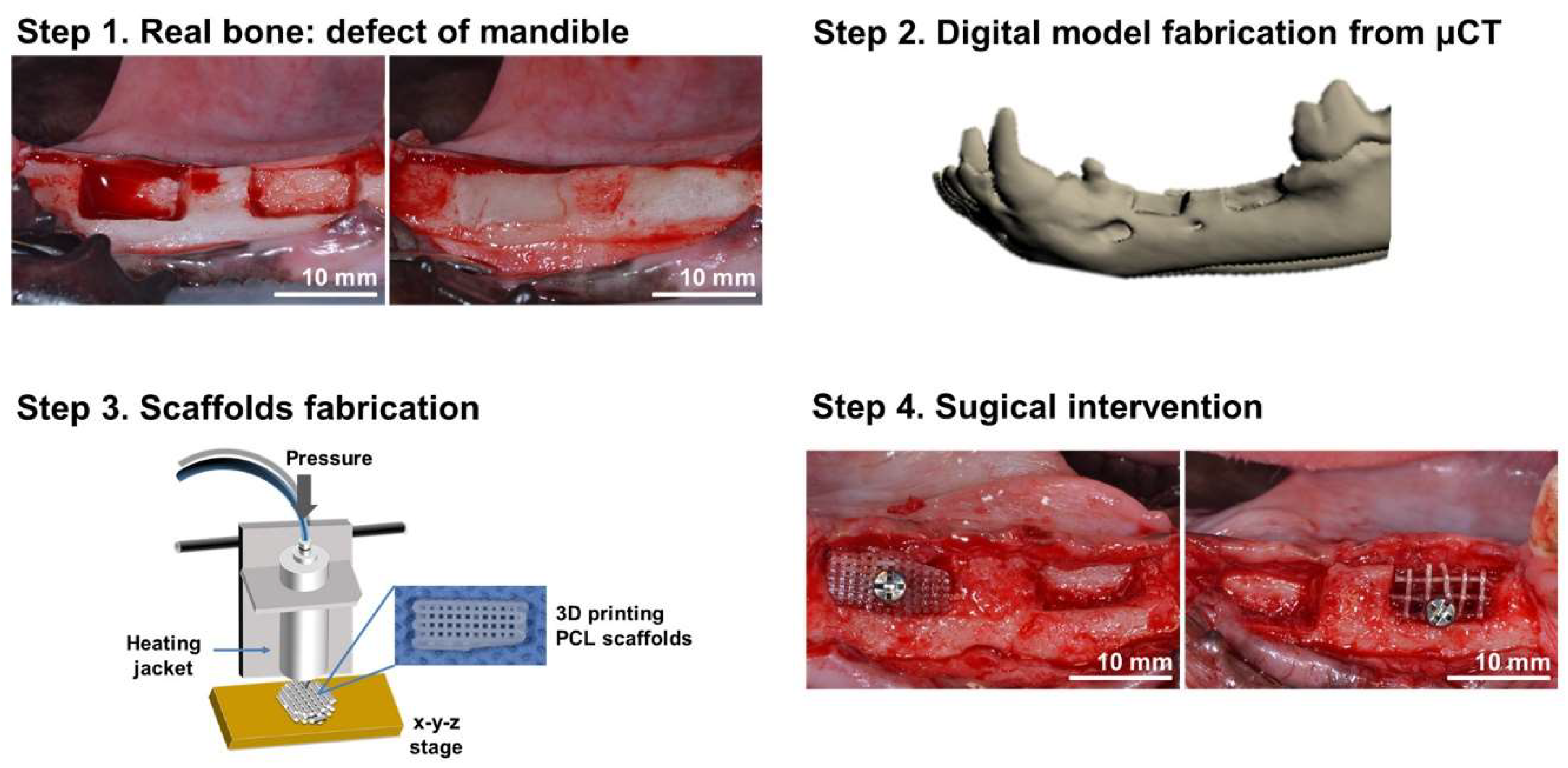
3.2. Bioprinted Scaffolds
3.3. Cell-Seeded Constructs
3.4. Growth Factors and Bioactive Molecules
3.5. Vascularization
3.6. Immunomodulation
4. Discussion
5. Conclusions and Future Perspectives
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef]
- Sigaux, N.; Pourchet, L.; Breton, P.; Brosset, S.; Louvrier, A.; Marquette, C.A. 3D Bioprinting: Principles, fantasies and prospects. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 128–132. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Jang, J.; Yi, H.G.; Cho, D.W. 3D printed tissue models: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Yu, J.; Park, S.A.; Kim, W.D.; Ha, T.; Xin, Y.-Z.; Lee, J.; Lee, D. Current Advances in 3D Bioprinting Technology and Its Applications for Tissue Engineering. Polymers 2020, 12, 2958. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Arpanaei, A.; Andresen, T.L.; Dolatshahi-Pirouz, A. 3D biomaterial microarrays for regenerative medicine: Current state-of-the-art, emerging directions and future trends. Adv. Mater. 2016, 28, 771–781. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Fahmy, M.D.; Razavi, M.; Stein, B.E.; Nowman, A.; Masri, R.M.; Tayebi, L. Dental applications of natural-origin polymers in hard and soft tissue engineering. J. Prosthodont. 2016, 25, 510–517. [Google Scholar] [CrossRef]
- Robertson, K.; Shahbazian, T.; MacLeod, S. Treatment of peri-implantitis and the failing implant. Dent. Clin. 2015, 59, 329–343. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental implant complications. Semin. Ultrasound CT MRI 2015, 36, 427–433. [Google Scholar] [CrossRef]
- Schwarz, M.S. Mechanical complications of dental implants. Clin. Oral Implant. Res. 2000, 11, 156–158. [Google Scholar] [CrossRef]
- Mistry, S.; Pal, K.; Shete, O.; Sawant, D. Hydrogels in Dentistry–Applications and Advances. J. Prosthodont. Dent. Mater. 2022, 3, 13–25. [Google Scholar]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment Note. Clin. Oral Implant. Res. 2000, 11, 146–155. [Google Scholar] [CrossRef]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Schwartz-Arad, D.; Samet, N.; Samet, N.; Mamlider, A. Smoking and complications of endosseous dental implants. J. Periodontol. 2002, 73, 153–157. [Google Scholar] [CrossRef]
- McDermott, N.E.; Chuang, S.K.; Woo, V.V.; Dodson, T.B. Complications of dental implants: Identification, frequency, and associated risk factors. Int. J. Oral Maxillofac. Implant. 2003, 18, 846. [Google Scholar] [CrossRef]
- Kasat, V.; Ladda, R. Smoking and dental implants. J. Int. Soc. Prev. Community Dent. 2012, 2, 38. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Manfredini, D.; Poggio, C.E.; Lobbezoo, F. Is bruxism a risk factor for dental implants? A systematic review of the literature. Clin. Implant Dent. Relat. Res. 2014, 16, 460–469. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef]
- Sahm, N.; Becker, J.; Santel, T.; Schwarz, F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study. J. Clin. Periodontol. 2011, 38, 872–878. [Google Scholar] [CrossRef]
- Del Amo, F.S.L.; Yu, S.H.; Wang, H.L. Non-surgical therapy for peri-implant diseases: A systematic review. J. Oral Maxillofac. Res. 2016, 7, e13. [Google Scholar]
- Choe, R.; Balhaddad, A.A.; Fisher, J.P.; Melo, M.A.S.; Huang, H.C. Photodynamic therapy for biomodulation and disinfection in implant dentistry: Is it feasible and effective? Photochem. Photobiol. 2021, 97, 916–929. [Google Scholar] [CrossRef]
- Schou, S.; Berglundh, T.; Lang, N.P. Surgical treatment of peri-implantitis. Int. J. Oral Maxillofac. Implant. 2004, 19, 140. [Google Scholar]
- Claffey, N.; Clarke, E.; Polyzois, I.; Renvert, S. Surgical treatment of peri-implantitis. J. Clin. Periodontol. 2008, 35, 316–332. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef]
- Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.M.; Panda, S.; Marchetti, E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials 2021, 14, 3319. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef]
- Boot, W.; Gawlitta, D.; Nikkels, P.G.J.; Pouran, B.; van Rijen, M.H.P.; Dhert, W.J.A.; Vogely, C.H. Hyaluronic Acid-Based Hydrogel Coating Does Not Affect Bone Apposition at the Implant Surface in a Rabbit Model. Clin. Orthop. Relat. Res. 2017, 475, 1911–1919. [Google Scholar] [CrossRef]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef]
- de Avila, E.D.; van Oirschot, B.A.; van den Beucken, J.J. Biomaterial-based possibilities for managing peri-implantitis. J. Periodontal Res. 2020, 55, 165–173. [Google Scholar] [CrossRef]
- Tao, B.; Zhao, W.; Lin, C.; Yuan, Z.; He, Y.; Lu, L.; Chen, M.; Ding, Y.; Yang, Y.; Xia, Z.; et al. Surface modification of titanium implants by ZIF-8@ Levo/LBL coating for inhibition of bacterial-associated infection and enhancement of in vivo osseointegration. Chem. Eng. J. 2020, 390, 124621. [Google Scholar] [CrossRef]
- Govindharajulu, J.P.; Chen, X.; Li, Y.; Rodriguez-Cabello, J.C.; Battacharya, M.; Aparicio, C. Chitosan-Recombinamer Layer-by-Layer Coatings for Multifunctional Implants. Int. J. Mol. Sci. 2017, 18, 369. [Google Scholar] [CrossRef]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An antimicrobial dental light curable bioadhesive hydrogel for treatment of peri-implant diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Q.; Wang, Y. Preparation and characterization of antibacterial and anti-inflammatory hyaluronic acid-chitosan-dexamethasone hydrogels for peri-implantitis repair. J. Biomater. Appl. 2022, 36, 1141–1150. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Ansari, S.; Zadeh, H.H.; Moshaverinia, M.; Chee, D.; Marques, M.M.; Shi, S.; Moshaverinia, A. Gingival mesenchymal stem cell (GMSC) Delivery system based on RGD-coupled alginate hydrogel with antimicrobial properties: A novel treatment modality for peri-implantitis. J. Prosthodont. 2016, 25, 105–115. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Peng, S.; Lin, Y.; Ye, Z. Hydrogels in dental medicine. Adv. Ther. 2023, 7, 2300128. [Google Scholar] [CrossRef]
- Ayala-Ham, A.; López-Gutierrez, J.; Bermúdez, M.; Aguilar-Medina, M.; Sarmiento-Sánchez, J.I.; López-Camarillo, C.; Sanchez-Schmitz, G.; Ramos-Payan, R. Hydrogel-based scaffolds in oral tissue engineering. Front. Mater. 2021, 8, 708945. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. [Google Scholar]
- Kim, S.; Hwangbo, H.; Chae, S.; Lee, H. Biopolymers and Their Application in Bioprinting Processes for Dental Tissue Engineering. Pharmaceutics 2023, 15, 2118. [Google Scholar] [CrossRef] [PubMed]
- Sordi, M.B.; Cruz, A.; Fredel, M.C.; Magini, R.; Sharpe, P.T. Three-dimensional bioactive hydrogel-based scaffolds for bone regeneration in implant dentistry. Mater. Sci. Eng. 2021, 124, 112055. [Google Scholar] [CrossRef] [PubMed]
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163. [Google Scholar] [CrossRef] [PubMed]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for tissue engineering: Addressing key design needs toward clinical translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Atila, D.; Kumaravel, V. Advances in Antimicrobial Hydrogels for Dental Tissue Engineering: Regenerative Strategies for Endodontics and Periodontics. Biomater. Sci. 2023, 11, 6711–6747. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Bahrami, N.; Mohamadnia, A.; Mousavi, S.; Gholami, M.; Ai, J.; Moayeri, R.S. Scaffolds in dental tissue engineering: A review. Arch. Neurosci. 2020, 7, e97014. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. 2019, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Zhang, M.; Ma, Y.; Yang, Y.; Han, X.; Wang, X. Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomater. Sci. 2020, 8, 3138–3146. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N.J.M.S. Temperature-and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Tian, J.; Gu, P.; Cao, H.; Fan, X.; Zhang, W. In situ bone regeneration enabled by a biodegradable hybrid double-network hydrogel. Biomater. Sci. 2019, 7, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-triggered, self-assembled, and bioprintable hybrid hydrogel scaffold for mesenchymal stem cell based bone tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan–aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf. B Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef] [PubMed]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.; Abdeltawab, N.F.; Awad, G.A. Chitosan-calcium phosphate composite scaffolds for control of post-operative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr. Polym. 2020, 244, 116482. [Google Scholar] [CrossRef]
- Moreira, C.D.; Carvalho, S.M.; Florentino, R.M.; França, A.; Okano, B.S.; Rezende, C.M.; Mansur, H.S.; Pereira, M.M. Injectable chitosan/gelatin/bioactive glass nanocomposite hydrogels for potential bone regeneration: In vitro and in vivo analyses. Int. J. Biol. Macromol. 2019, 132, 811–821. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Tan, Y.; Richards, D.J.; Trusk, T.C.; Visconti, R.P.; Yost, M.J.; Kindy, M.S.; Drake, C.J.; Argraves, W.S.; Markwald, R.R.; Mei, Y. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication 2014, 6, 024111. [Google Scholar] [CrossRef]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Kinsella, J.M.; Tran, S.D. The Applications of 3D Printing for Craniofacial Tissue Engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef]
- Proksch, S.; Galler, K.M. Scaffold materials and dental stem cells in dental tissue regeneration. Curr. Oral Health Rep. 2018, 5, 304–316. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Trovato, L.; Naro, F.; D’Aiuto, F.; Moreno, F. Promoting tissue repair by micrograft stem cells delivery. Stem Cells Int. 2020, 2020, 2195318. [Google Scholar] [CrossRef] [PubMed]
- Ercal, P.; Pekozer, G.G.; Kose, G.T. Dental stem cells in bone tissue engineering: Current overview and challenges. In Cell Biology and Translational Medicine, Volume 3: Stem Cells, Bio-Materials and Tissue Engineering; Springer: Cham, Switzerland, 2018; pp. 113–127. [Google Scholar]
- Almansoori, A.A.; Kwon, O.J.; Nam, J.H.; Seo, Y.K.; Song, H.R.; Lee, J.H. Mesenchymal stem cells and platelet-rich plasma-impregnated polycaprolactone-β tricalcium phosphate bio-scaffold enhanced bone regeneration around dental implants. Int. J. Implant Dent. 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst. Eng. 2011, 34, 505–513. [Google Scholar] [CrossRef]
- Arefin, A.M.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D printing review: Materials, process, and design strategies for medical applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.-J.; Kim, K.-S.; Lee, S.J.; Lee, J.-T.; Kim, S.-Y.; Chang, N.-H.; Park, S.-Y. In Vivo Evaluation of 3D-Printed Polycaprolactone Scaffold Implantation Combined with β-TCP Powder for Alveolar Bone Augmentation in a Beagle Defect Model. Materials 2018, 11, 238. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, Κ.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, Μ. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Zheng, R.C.; Park, Y.K.; Cho, J.J.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H. Bone regeneration at dental implant sites with suspended stem cells. J. Dent. Res. 2014, 93, 1005–1013. [Google Scholar] [CrossRef]
- Fan, J.; Lee, C.S.; Kim, S.; Zhang, X.; Pi-Anfruns, J.; Guo, M.; Chen, C.; Rahnama, M.; Li, J.; Wu, B.M.; et al. Trb3 controls mesenchymal stem cell lineage fate and enhances bone regeneration by scaffold-mediated local gene delivery. Biomaterials 2021, 264, 120445. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- Cheng, Z.A.; Alba-Perez, A.; Gonzalez-Garcia, C.; Donnelly, H.; Llopis-Hernandez, V.; Jayawarna, V.; Childs, P.; Shields, D.W.; Cantini, M.; Ruiz-Cantu, L.; et al. Nanoscale coatings for ultralow dose BMP-2-driven regeneration of critical-sized bone defects. Adv. Sci. 2019, 6, 1800361. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Pitacco, P.; van Dommelen, L.H.; Nulty, J.; Browe, D.C.; Shin, J.Y.; Alsberg, E.; Kelly, D.J. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci. Adv. 2020, 6, eabb5093. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, M.J. Tissue engineering in periodontics and implantology using rhBMP-2. Ann. R. Australas. Coll. Dent. Surg. 2000, 15, 144–149. [Google Scholar] [PubMed]
- Huang, B.; Yuan, Y.; Liu, C. Biomaterial-guided immobilization and osteoactivity of bone morphogenetic protein-2. Appl. Mater. Today 2020, 19, 100599. [Google Scholar] [CrossRef]
- Sanchez-Casanova, S.; Martin-Saavedra, F.M.; Escudero-Duch, C.; Uceda, M.I.F.; Prieto, M.; Arruebo, M.; Acebo, P.; Fabiilli, M.L.; Franceschi, R.T.; Vilaboa, N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials 2020, 241, 119909. [Google Scholar] [CrossRef] [PubMed]
- On, S.W.; Park, S.Y.; Yi, S.M.; Park, I.Y.; Byun, S.H.; Yang, B.E. Current Status of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Maxillofacial Surgery: Should It Be Continued? Bioengineering 2023, 10, 1005. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Vimalraj, S.; Saravanan, S. Tooth-derived stem cells integrated biomaterials for bone and dental tissue engineering. Cell Tissue Res. 2023, 394, 245–255. [Google Scholar] [CrossRef]
- Pandya, M.; Saxon, M.; Bozanich, J.; Tillberg, C.; Luan, X.; Diekwisch, T.G. The glycoprotein/cytokine erythropoietin promotes rapid alveolar ridge regeneration in vivo by promoting new bone extracellular matrix deposition in conjunction with coupled angiogenesis/osteogenesis. Int. J. Mol. Sci. 2021, 22, 2788. [Google Scholar] [CrossRef] [PubMed]
- Malek-Khatabi, A.; Javar, H.A.; Dashtimoghadam, E.; Ansari, S.; Hasani-Sadrabadi, M.M.; Moshaverinia, A. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomater. 2020, 108, 326–336. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, E.; Valat, A.; Picart, C.; Cavalcanti-Adam, E.A. Tuning cellular responses to BMP-2 with material surfaces. Cytokine Growth Factor Rev. 2016, 27, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.P.; Toksoy, Z.; Davis, B.A.; Geibel, J.P. 3D Bioprinting of Vascularized Tissues for in vitro and in vivo Applications. Front. Bioeng. Biotechnol. 2021, 9, 664188. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Xiang, Z.; Rommens, P.M.; Ritz, U. 3D Bioprinting for Vascularized Tissue-Engineered Bone Fabrication. Materials 2020, 13, 2278. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Nasrollahi Boroujeni, N.; Jahangiri, S.; Rabiee, N.; Rabiee, M.; Makvandi, P.; Akhavan, O.; Varma, R.S. Prevascularized micro-/nano-sized spheroid/bead aggregates for vascular tissue engineering. Nano-Micro Lett. 2021, 13, 182. [Google Scholar] [CrossRef]
- Simunovic, F.; Finkenzeller, G. Vascularization Strategies in Bone Tissue Engineering. Cells 2021, 10, 1749. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, J.K.Y.; Teoh, S.-H. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J. Tissue Eng. Regen. Med. 2012, 9, 85–105. [Google Scholar] [CrossRef]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.C.; Kießling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef] [PubMed]
- Nulty, J.; Freeman, F.E.; Browe, D.C.; Burdis, R.; Ahern, D.P.; Pitacco, P.; Bin Lee, Y.; Alsberg, E.; Kelly, D.J. 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater. 2021, 126, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Pan, C.-C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Röderer, G.; Günther, K.-P.; Brenner, R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell. Biochem. 2002, 87, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Elalouf, A. Immune response against the biomaterials used in 3D bioprinting of organs. Transpl. Immunol. 2021, 69, 101446. [Google Scholar] [CrossRef]
- Chung, L.; Maestas Jr, D.R.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Lotti, F.; Ranieri, F.; Vadalà, G.; Zollo, L.; Di Pino, G. Invasive intraneural interfaces: Foreign body reaction issues. Front. Neurosci. 2017, 11, 497. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Clark, N.M.; Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 2018, 182, 202–215. [Google Scholar] [CrossRef]
- Yu, T.; Tutwiler, V.J.; Spiller, K. The role of macrophages in the foreign body response to implanted biomaterials. In Biomaterials in Regenerative Medicine and the Immune System; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–34. [Google Scholar]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Schricker, S.R.; Paine, M.L.; Moradian-Oldak, J.; Khademhosseini, A.; Snead, M.L.; et al. Regulation of the Stem Cell–Host Immune System Interplay Using Hydrogel Coencapsulation System with an Anti-Inflammatory Drug. Adv. Funct. Mater. 2015, 25, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Grunert, S.; Woelber, J.P.; Nelson, K.; Semper-Hogg, W. Vitamin D deficiency in early implant failure: Two case reports. Int. J. Implant Dent. 2016, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.; McCauley, L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Zernial, O.; Stürmer, E.; Fiedler, I.; Schäfer, S.; Gosau, M.; Gaudin, R.; Stolzer, C.; Reinelt, A.; et al. New and innovative biomaterials, techniques and therapy concepts: Biologization in maxillofacial surgery, oral surgery and dentistry is in full swing. PRF, PRGF, PRP, blood plasma-stabilized augmentations, supplementation of micronutrients and vitamins–what opportunities do such “biological” approaches actually offer? We introduce them here. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2022, 11, Doc05. [Google Scholar]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Shahabipour, F.; Ashammakhi, N.; Oskuee, R.K.; Bonakdar, S.; Hoffman, T.; Shokrgozar, M.A.; Khademhosseini, A. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl. Res. 2020, 216, 57–76. [Google Scholar] [CrossRef]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral Biol. 2021, 128, 105174. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef]

| Key Benefit/Topic | Area of Application/Significance | References |
|---|---|---|
| Implant coating | Antibiotics | Boot et al., 2017 [31] De Avila et al., 2020 [33] Tao et al., 2020 [34] |
| Antibiotics and rhBMP-2 | Min et al., 2014 [32] | |
| Chitosan | Govindharajulu et al., 2017 [35] | |
| Chitosan and dexamethason | Zhou et al., 2022 [37] | |
| Silver lactate hydrogel | Diniz et al., 2016 [38] | |
| Hydrogel precursor | Sani et al., 2019 [36] | |
| Bioprinted Scaffolds | Hydrogels | Chen et al., 2023 [39] Ayala-Ham et al., 2021 [40] Yang et al., 2020 [41] Kim et al., 2023 [42] Sordi et al., 2021 [43] Mistry et al., 2019 [14] Dorishetty et al., 2020 [44] Advincula et al., 2021 [45] Xu et al., 2022 [46] Atila et al., 2023 [47] Farzin et al., 2020 [48] |
| PLA/collagen | Martin et al., 2019 [49] | |
| Collagen/monocycline | Wan et al., 2020 [50] | |
| Composite hydrogel/moxifloxacin | Radwan et al., 2020 [57] | |
| Chitosan | Tang et al., 2020 [51] Bagheri et al., 2019 [56] | |
| Chitosan/calcium phosphate | Zhao et al., 2019 [55] | |
| Chitosan/gelatin | Bakopoulou et al., 2019 [70] | |
| Thermo-responsive composite hydrogel | Moreira et al., 2019 [58] | |
| Poly(ε) caprolactone (PCL) scaffold | Park et al., 2011 [67] Arefin et al., 2021 [68] | |
| PCL-TCP (β-tricalcium phosphate) scaffold | Almansoori et al., 2021 [66] Park et al., 2018 [69] | |
| Injectable hydrogels | Li et al., 2020 [52] Lavanya et al., 2020 [53] Zhang et al., 2019 [54] | |
| Scaffold-free cell aggregates | Norrote et al., 2009 [59] Tan et al., 2014 [60] Tao et al., 2019 [61] | |
| Cell-Seeded Constructs | Mesenchymal stem cells (MSCs) | Proksch et al., 2018 [62] Zakrzewski et al., 2019 [63] Trovato et al., 2020 [64] Ercal et al., 2018 [65] |
| Mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) | Almansoori et al., 2021 [66] | |
| Dental pulp stem cells (DPSCs) | Bakopoulou et al., 2019 [70] | |
| Human exfoliated deciduous teeth (SHED)/bone marrow mesenchymal stem cells (hBMSCs) | Nakajima et al., 2018 [71] | |
| Bone marrow-derived mesenchymal stem cells (BMMSCs) | Zheng et al., 2014 [72] | |
| Mesenchymal stem cells (MSCs)/tribbles homolog 3 (Trb3) | Fan et al., 2021 [73] | |
| Growth Factors and Bioactive Molecules | Vascular endothelial growth factor (VEGF) | Pandya et al., 2021 [83] |
| Bone morphogenetic proteins (BMPs) | Gugliandolo et al., 2021 [74] Cheng et al., 2019 [75] Freeman et al., 2020 [76] Danesh-Meyer et al., 2000 [77] Huang et al., 2020 [78] Sanchez-Casanova et al., 2020 [79] On et al., 2023 [80] Malek-Khatabi et al., 2020 [84] | |
| Bone morphogenetic proteins (BMPs)—side effects | James et al., 2016 [85] Halloran et al., 2020 [86] Migliorini et al., 2016 [87] | |
| Vascularization | Create vascular networks within the scaffold | Chen et al., 2021 [88] Xing et al., 2020 [89] Rahimnejad et al., 2021 [90] Simunovic et al., 2021 [91] Liu et al., 2012 [92] Tomasina et al., 2019 [93] Schöneberg et al., 2018 [94] Nulty et al., 2021 [95] Kang et al., 2016 [96] Kuss et al., 2017 [97] Adana et al., 2019 [98] |
| Bone morphogenetic proteins (BMPs) and vascular endothelial growth factor (VEGF) | Fiedler et al., 2002 [99] Ferrara et al., 2003 [100] | |
| Immunomodulation | Foreign body response (FBR) | Elalouf et al., 2021 [101] Chung et al., 2017 [102] Anderson et al., 2008 [103] Mariani et al., 2019 [104] Sridharan et al., 2015 [105] Yu et al., 2015 [108] Franz et al., 2011 [109] |
| Modifications of the interface surface | Hotchkiss et al., 2018 [107] Lotti et al., 2017 [106] | |
| Anti-inflammatory drug | Moshaverinia et al., 2015 [110] | |
| Vitamins deficiency (C, D) | Fretwurst et al., 2016 [111] Bashutski et al., 2011 [112] Smeets et al., 2022 [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shopova, D.; Mihaylova, A.; Yaneva, A.; Bakova, D.; Dimova-Gabrovska, M. Biofabrication Approaches for Peri-Implantitis Tissue Regeneration: A Focus on Bioprinting Methods. Prosthesis 2024, 6, 372-392. https://doi.org/10.3390/prosthesis6020028
Shopova D, Mihaylova A, Yaneva A, Bakova D, Dimova-Gabrovska M. Biofabrication Approaches for Peri-Implantitis Tissue Regeneration: A Focus on Bioprinting Methods. Prosthesis. 2024; 6(2):372-392. https://doi.org/10.3390/prosthesis6020028
Chicago/Turabian StyleShopova, Dobromira, Anna Mihaylova, Antoniya Yaneva, Desislava Bakova, and Mariana Dimova-Gabrovska. 2024. "Biofabrication Approaches for Peri-Implantitis Tissue Regeneration: A Focus on Bioprinting Methods" Prosthesis 6, no. 2: 372-392. https://doi.org/10.3390/prosthesis6020028
APA StyleShopova, D., Mihaylova, A., Yaneva, A., Bakova, D., & Dimova-Gabrovska, M. (2024). Biofabrication Approaches for Peri-Implantitis Tissue Regeneration: A Focus on Bioprinting Methods. Prosthesis, 6(2), 372-392. https://doi.org/10.3390/prosthesis6020028








