Abstract
Hyperketonemia (HYK), defined by blood beta-hydroxybutyrate (BHB) ≥ 1.2 mmol/L, is described as a significant risk factor for cows developing postpartum (pp) diseases and impaired reproductive performance. The goal of the present study was to observe metabolic challenges in transition cows and to identify systemic markers reflecting HYK associated with lessened reproductivity. Fifty-four Simmental cows were monitored, revealing approximately 30% prevalence of HYK at the early pp period on 7, 14, or 28 days in milk (DIM). We assessed the dry matter intake, rumination time (RT), serum liver activity index, non-esterified fatty acids (NEFAs), acute phase proteins, and uterine and oviductal health. Elevated NEFA and reduced RT 14 days antepartum were a good predictor for HYK at 7 DIM. Hyperketonemia at 14 DIM resulted in higher milk yield compared with controls. We could neither detect differences in uterine health nor in reproductive key performance parameters between hyperketonemic and control cows, whereby the proportion of polymorphonuclear neutrophils in oviductal epithelia was significantly lower in hyperketonemic cows 14 DIM. We conclude that elevated concentrations of BHB in HYK 7, 14, or 28 DIM indicated energy supply to support physiological metabolic adaptations and lactation and that, in the absence of excessive inflammation during the transition period, HYK was not a risk factor for impaired fertility.
1. Introduction
The transition period of dairy cows from gestation to the onset of lactation is characterized by adaptive physiological changes that support postpartum health, fertility, and productivity [1]. In the early postpartum (pp) period, most dairy cows undergo a negative energy balance (NEB) reflected by elevated serum concentrations of non-esterified fatty acids (NEFAs) and beta-hydroxybutyrate (BHB) [2]. Hyperketonemia, also referred to as subclinical ketosis (SCK), most frequently reflected by increased concentration of circulating BHB without clinical signs of disease, may present a significant risk factor for developing clinical ketosis, displaced abomasum, and reproductive sequelae [3,4,5]. The definition of SCK and HYK is based on BHB concentrations exceeding 1.0 to 1.4 mmol/L, depending on the study model [3,5,6]. Many studies reported that elevated concentrations of BHB or NEFA in the first two weeks pp were associated with increased risk for metritis or displaced abomasum [7,8], purulent vaginal discharge (PVD) or endometritis [9,10], not having a detected estrus [11], reduced pregnancy per artificial insemination (AI) [12,13] and culling [14].
Excessive NEB in the early pp period, reflected by elevated concentrations of NEFA and BHB, increases the susceptibility to periparturient diseases, such as ketosis and fatty liver [15]. Optimal liver function in dairy cows is crucial to cope with pp body fat mobilization under NEB in the transition period and to support lactation and animal health. Proxies of negative acute phase proteins (APPs), such as albumin (ALB), cholesterol (CHOL), and vitamin A (VIT A), are hepatic protein products used as metabolic markers that reflect the adaptations of cows. Reduced production of negative APPs and elevation of positive APPs, e.g., haptoglobin (HP), serum amyloid A, and ceruloplasmin, are associated with fatty liver and with a variety of metabolic and infectious sequelae [16,17,18]. Bertoni and Treviso [19] used these metabolic markers to estimate liver activity and to evaluate the inflammatory status of transition cows. Overall, transient effects on metabolism were enhanced in antepartum (ap) overfed cows, leading to reduced VIT A levels pp [20]. Less VIT A, in combination with lower CHOL concentration, results in less lipoprotein synthesis and sequelae of liver lipidosis [21]. In addition, oversupply during a far-off period resulted in higher liver lipid accumulation, lower liver activity index (LAI), and larger spike HP concentration pp, while VIT A was lowered by a restricted close-up diet. Consequently, high adiposity in the dry period tends to increase the incidence of SCK [22].
Excessive fat mobilization is associated with immunosuppression, indicated by a decrease in polymorphonuclear neutrophil (PMN) activity [23,24], which increases the cows’ susceptibility to uterine infections [25]. Thus, immunosuppression has a negative impact on the endometrial environment, resulting in metritis, clinical endometritis (CE), and lower reproductive performance [26,27]. In this context, endometritis was associated with salpingitis and led to poorer fertility in cows [28].
There is an ongoing discussion in the literature about whether the immense metabolic changes in transition cows impose a risk of disease or are adaptive to support health and productivity [17]. The positive role of ketone bodies as an energy source for dairy cow health and productivity is gaining in importance [29]. A recent review concluded that normal metabolic adjustments, reflected by elevated NEFA and BHB, are associated with immune activation, while these markers in combination with other unfavorable variables (e.g., elevated concentration of HP, hypocalcemia, or pathogens) lead to dysregulated immune function and contribute to subsequent negative outcomes [30]. Only a few studies evaluated systemic markers of metabolic and inflammatory disorders comprehensively [12].
We designed an observational cohort study using a real-life set-up within a 75-cow herd to assess whether HYK pp is a physiological adaptation or reflects maladaptation to the metabolic challenges in transition dairy cows. We hypothesized that HYK in the first 2 weeks pp is adaptive, whereas HYK occurring after the transition period, 28 DIM, imposes a risk for reduced fertility. We evaluated systemic markers, animal health behavior, and level of production to identify metabolic maladaptation and to predict reproductive outcome.
2. Materials and Methods
This study was approved by the institutional ethics committee and the national authority according to §8 of the Law for Animal Experiments, Tierversuchsgesetz-TVG (BMWFW-68.205/0162-WF/V/3b/2017). The study was conducted at the research and teaching farm Kremesberg (renamed VetFarm), University of Veterinary Medicine Vienna, Austria, between June 2018 and December 2019. The year-round-calving dairy herd is in Pottenstein, Lower Austria (47°57′12.654″ N 16°6′56.023″ E), and comprises 75 milking Simmental cows, housed in a free-stall barn with straw-bedded cubicles. Cows were milked twice a day, in the morning at 4:30 a.m. and in the evening at 4:30 p.m., in a 2 × 6 tandem milking parlor. The annual average milk production per cow was 9247 and 8555 kg in 2018 and 2019, respectively. Sixty-seven cows in 1st to 4th parity were selected alternatively for an individual feeding trial starting from approximately 42 days before expected calving until 42 DIM. During the trial, cows were kept in a loose-housing system with deep straw bedding and single feeding troughs (Hokofarm, Emmeloord, The Netherlands) equipped with scales to measure the daily feed intake. Cows had access to the troughs via a collar transponder (Compident Tiris, Schauer, Prambachkirchen, Austria). We excluded cows with systemic clinical signs after calving (downer syndrome, clinical ketosis, or fever, such as in acute puerperal metritis or clinical mastitis), dystocia, or Cesarean section from the study, with the intention to only describe the effects of HYK/SCK on liver metabolism and reproduction. Hyperketonemic cows eligible for this study did not receive any treatment, e.g., oral propylene glycol.
A partial mixed ration was fed with an automatic feeding system (Trioliet, Oldenzaal, The Netherlands) once a day for dry cows and lactating cows, respectively. The two rations were either fed balanced according to the recommendations of the Bavarian Institute for Agriculture [31] for Simmental cows through the entire study period (group BAL) or fed ad libitum ap and restricted to 75% of energy requirements pp (group LIB/REST). The two feeding regimes were part of another trial; the feeding details can be found in the supplement (Table S1). In general, lactating cows in BAL that yielded > 25 kg/d milk received concentrate via an individual feeding station. If the energy intake exceeded the experimental targets, we restricted the feed supply to match the aimed 100% of nutrient requirements in BAL or 75% in REST, respectively. In the context of the presented study, we used the feeding regimes to increase the number of HYK cows. Additionally, daily dry matter intake (DMI) was estimated by determining the dry matter content of the feed components with a drying incubator (BD 53, Binder GmbH, Tüttlingen, Germany) once a week. We used backfat thickness (BFT) measurement with ultrasound [32] to evaluate the body condition of cows at the beginning of the trial (d 42 ap), at calving (d 0), and at 28 DIM. Rumination time was measured with an ear-tag sensor system (SmartBow GmbH, Weibern, Austria) as described in detail by Reiter et al. [33].
We took capillary blood samples on d 14 ap and 7, 14, and 28 DIM from the vulva skin to measure BHB concentrations with an on-site test (Freestyle Precision, Abbott, IL, USA), described in detail by Kanz et al. [34]. Diagnosis of HYK was based on blood BHB ≥ 1.2 mmol/L pp [3,11,35], while BHB < 1.2 mmol/L defined CON. We performed BHB measurements at 7, 14, and 28 DIM to categorize them into HYK7, HYK14, HYK28, and CON, respectively. In addition, we collected blood samples from the coccygeal vein with VACUETTE tubes (Greiner bio-one, Kremsmünster, Austria) on d 14 ap, 7, 14, and 28 DIM. The tubes were centrifuged at 1800× g for 5 min at room temperature, serum was pipetted into 2 mL Eppendorf tubes and stored until further analysis at –20° C. Then we submitted aliquots of serum to the Central Laboratory of the University of Veterinary Medicine, Vienna, Austria, for evaluation of NEFA concentration at d 14 ap. Serum metabolites of HP at 7 DIM, ALB, total protein (TP), and CHOL (all APPs, representing liver function), and VIT A (marker for oxidative stress) at d 14 ap, 14, and 28 DIM were analyzed in a commercial laboratory (Laboklin GmbH & Co. KG, Bad Kissingen, Germany) by photometric determination (Cobas 8000, Roche Holding GmbH, Ludwigsburg, Germany) and reverse phase HPLC (Waters Chromatography Europe B.V., Etten-Leur, The Netherlands). We calculated globulin as the difference between TP and ALB and used the LAI to determine liver function as described elsewhere [19]. In brief, the mean and standard deviation of serum ALB, CHOL, and VIT A concentrations were calculated to determine LAI on d 14 ap and 14 and 28 DIM, reflecting the liver function of individual cows compared with the average of all cows.
As one objective of the study was to assess the association between NEB and reproduction, we examined the uterus and the oviducts. Thus, we performed vaginoscopy on 7, 14, and 28 DIM. Vaginal mucus was scored according to Williams et al. [36] to diagnose CE (score 0 = no CE; score 1 = mild, mucus containing flecks of pus; score 2 = moderate, <50% mucopurulent; score 3 = severe CE, ≥50% purulent or sanguineous). In addition, we took cytobrush samples from the endometrium [37] to identify subclinical endometritis (SE), defined by a proportion of ≥5% PMN from 300 cells in total [38,39]. Cytological evaluation of the smears was described in detail elsewhere [40].
To assess the effects of NEB on the oviduct, as a second crucial organ of reproduction, we collected bilateral oviductal samples on 28 DIM by transvaginal endoscopy. For this, we used a bi-tubular endoscopic system (50 cm length, Ø11 mm, Karl Storz, Vienna, Austria) as described in detail by Besenfelder et al. [41]. We obtained oviductal epithelia samples with a mini-cytobrush, stored them immediately at −80 °C, and analyzed them later, as described by Neubrand et al. [42].
Reproductive performance and milk yield (average daily milk yield, total milk yield at 305 DIM of the current lactation, milk fat content, and milk protein content) of groups HYK and CON were analyzed based on milk record data, which are provided monthly by the regional Dairy Herd Improvement Service (Landeskontrollverband NÖ, Zwettl, Austria). Reproductive performance parameters comprised calving-to-first service interval, submission rate, days open, pregnancy at 1st service, overall proportion of pregnant cows, 150-day in-calf rate, and conception risk (pregnancies by number of all services).
Statistical Analyses
Beta-hydroxybutyrate values from cows 7, 14, or 28 DIM were defined as independent and identically distributed data, resulting in groups HYK7, HYK14, and HYK28 or CON, respectively. Independence of HYK between the time points was evaluated with the Chi2 test and Cohen’s kappa statistics.
We calculated mean values and SD or, where appropriate, median and 25/75 percentiles for daily DMI, RT, and for serologic and reproductive parameters in groups HYK and CON. Moreover, we assessed the proportion of PMN in endometrial (defining SE) and oviductal epithelia by cytologic evaluation. We analyzed the data with SPSS software version 29 (IBM SPSS Statistics, New York, NY, USA). Normally distributed data was compared between groups with a t-test, while non-normally distributed data was analyzed by the Mann–Whitney U test. To predict effects on HYK in fresh cows at 7, 14, or 28 DIM, predictor variables, e.g., RT, metabolic and inflammatory variables, of dry-off and early peripartum cows were analyzed by a binary logistic regression. The dependent variable was BHB, resulting in a binary outcome of HYK or CON. We used only significantly different means between HYK and CON as covariates in the multiple regression. Prevalence of CE and SE was compared with a Chi2 test between HYK and CON. Reproductive data of cows in the concurrent lactation were compared between groups: the hazard of 150-day in-calf rate was determined with Kaplan–Meier survival analyses including categorical status variable pregnancy and factor BHB (HYK or CON) at the reference 7, 14, and 28 DIM. We compared the proportion of culled cows between HYK and CON with the Chi2 test. Significance was set at p ≤ 0.05. A trend was indicated by p > 0.05 and p ≤ 0.1.
3. Results
A total of 67 cows were assigned to the study at drying-off, with 54 cows being monitored from approximately 42 days before parturition until 42 DIM and, thus, eligible for the final analysis. The remaining 13 cows were excluded during the first DIM due to the exclusion criteria: acute metritis (n = 8; 14.8%), clinical ketosis (n = 3; 5.6%), and, during the dry-off period, because of rejection of the individual feeding system (n = 2; 3.7%). The feed composition with nutritional content of energy and digestible protein is documented in the supplements (Table S1). Concentrations of metabolic biomarkers are summarized in Table S2. Distribution of cows in HYK7 and HYK14 was different and indicated no agreement (p = 0.035; κ = −0.46), revealing that occurrence of HYK was independent between the testing days.
3.1. Monitoring of Beta-Hydroxybutyrate at Day 7 pp (HYK7)
Cows in HYK14 (n = 16; prevalence 29.6%) had a mean BHB concentration of 1.5 mmol/L and showed a median BHB of 1.2 at 7 DIM and 1.4 mmol/L at 28 DIM, respectively. Antepartum NEFA concentrations were lower (p ≤ 0.05), and RT showed a tendency to be greater at 7 and 28 DIM in HYK14 compared with CON (Table 1). Hyperketonemia at 14 DIM was not predicted by any factors (e.g., NEFA ap, RT) in the regression model. Relating to liver activity, HYK14 had lower serum TP at 28 DIM (OR 0.83; p = 0.04) and partly lower LAI relative to CON (Table S2, Figure 1). Furthermore, HYK14 yielded more milk at the first test day (39.1 kg vs. 31.9 kg; p = 0.03) and in the current lactation (10,220 kg; OR 1.001; p = 0.03) and had less milk fat compared with CON (Table 2).

Table 1.
Mean or median (SD or 25/75 percentile) serum concentration of non-esterified fatty acids (NEFAs), beta-hydroxybutyrate (BHB), and haptoglobin (HP), dry matter intake (DMI), rumination time (RT), and back fat thickness (BFT) at days 14 antepartum (ap), 0 (calving), 7, and 28 postpartum (pp) of hyperketonemic (HYK, n = 16) and control (CON, n = 38) cows at day 7 pp.
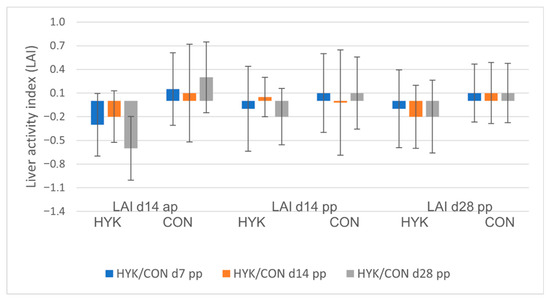
Figure 1.
Mean/standard error of liver activity index (LAI) at day (d) 14 antepartum (ap) (LAI d14 ap), d 14 postpartum (pp) (LAI d14 pp), and d28 pp (LAI d28 pp). Comparison between cows identified with hyperketonemia (HYK: left bars) or controls (CON: right bars) at sampling days: d7 (blue bars), d14 (orange bars), and d28 (gray bars).

Table 2.
Proportion of clinical endometritis (CE), subclinical endometritis (SE), and median (25/75 percentile) inflammatory cells [PMN %] in the endometrium and oviduct at sampling days 7, 14, and 28 postpartum (pp) of hyperketonemic (HYK7, 14, 28) and control (CON) cows. Key performance indicators of fertility and production data (median and 25/75 percentile of milk yield and milk components) in hyperketonemic (HYK7, 14, 28) and control cows (CON).
3.2. Monitoring of Beta-Hydroxybutyrate at Day 14 pp (HYK14)
Cows in HYK14 (n = 16; prevalence 29.6%) had a mean BHB concentration of 1.5 mmol/L and showed a median BHB of 1.2 at 7 DIM and 1.4 mmol/L at 28 DIM, respectively. Antepartum NEFA concentrations were lower (p ≤ 0.05), and RT showed a tendency to be greater at 7 and 28 DIM in HYK14 compared with CON (Table 3). Hyperketonemia at 14 DIM was not predicted by any factors (e.g., NEFA ap, RT) in the regression model. Relating to liver activity, HYK14 had lower serum TP at 28 DIM (OR 0.83; p = 0.04) and partly lower LAI relative to CON (Table S2, Figure 1). Furthermore, HYK14 yielded more milk at the first test day (39.1 kg vs. 31.9 kg; p = 0.03) and in the current lactation (10,220 kg; OR 1.001; p = 0.03) and had less milk fat compared with CON (Table 2).

Table 3.
Mean or median (SD or 25/75 percentile) serum concentration of non-esterified fatty acids (NEFAs), beta-hydroxybutyrate (BHB), and haptoglobin (HP), dry matter intake (DMI), rumination time (RT), and back fat thickness (BFT) at days 14 antepartum (ap), 0 (calving), 7, and 28 postpartum (pp) of hyperketonemic (HYK, n = 16) and control (CON, n = 38) cows at day 14 pp.
3.3. Monitoring of Beta-Hydroxybutyrate at Day 28 pp (HYK28)
Cows identified with HYK at the end of the peripartum period on 28 DIM (HYK28; n = 17; prevalence 31.5%) had a greater median BHB of 1.9 mmol/L relative to CON (p ≤ 0.05, median BHB 0.7, n = 37). Excessive NEB was also evident at 7 and 14 DIM, reflected by elevated BHB (p ≤ 0.05) and NEFA d 14 ap (p = 0.09) compared with CON. Cows in HYK28 had higher RT 14d ap (p ≤ 0.05) and lower BFT (p = 0.06) in the far dry-off period compared with CON (Table 4). Although average milk yield at 28 DIM and at the end of the current lactation were not different, HYK28 had lower milk fat % (p ≤ 0.05, Table 2). In the regression model, the odds of lower milk fat % for HYK28 were 1.17 times greater compared with CON (p = 0.05). Neither the variables of liver function nor of inflammation (i.e., HP) showed any significant difference between the groups (Table 4). The LAI in HYK28, however, remained negative over the sampling period, while it was consistently positive in CON (Figure 1, Table S2).

Table 4.
Mean or median (SD or 25/75 percentile) serum concentration of non-esterified fatty acids (NEFAs), beta-hydroxybutyrate (BHB), and haptoglobin (HP), dry matter intake (DMI), rumination time (RT), and back fat thickness (BFT) at days 14 antepartum (ap), 0 (calving), 7, and 28 postpartum (pp) of hyperketonemic (HYK, n = 17) and control (CON, n = 37) cows at day 28 pp.
3.4. Reproductive Health and Performance of HYK7, HYK14, and HYK28
To evaluate the potential impact of HYK on reproduction, we investigated the uterus and the oviduct by vaginoscopy and endoscopy, respectively, and performed cytological assessments at 7, 14, and 28 DIM. Cows in HYK7 were associated with a higher proportion of CE at 7 DIM compared with CON (61.5 vs. 44.7%); however, the prevalence of CE was not different between HYK and CON at 14 and 28 DIM, respectively. We found a similar pattern in HYK14, whereas HYK28 showed a lower proportion compared with CON (5.9 vs. 24.3% in HYK28 vs. CON; p = 0.1). Hyperketonemia had only a minor effect on the presence of SE, showing a greater number of SE at 28 DIM relative to CON (40.0 vs. 28.6% in HYK28 vs. CON) (Table 2). Overall, neither the prevalence of CE nor of SE was different between CON and HYK at all sampling days (7, 14, and 28 DIM; p > 0.05). The proportion of PMN in the oviduct was overall numerically lower in HYK at all sampling days, particularly lower in HYK14 (p ≤ 0.05), and showed a trend to lower numbers at 28 DIM (p = 0.1) compared with CON (Table 2). Reproductive performance parameters showed numerical differences between CON and HYK7, HYK14, and HYK28, respectively (Table 2). Overall, no significant differences in reproduction and risk for culling were found between CON and HYK7 as well as HYK28. Cows in HYK14 showed a longer calving-to-first service interval (78 vs. 62 days, HYK14 vs. CON, p ≤ 0.05), and a lower proportion were serviced after the end of the voluntary waiting period (submission rate within 40–60 days of 21.4 vs. 44.8%, HYK 14 vs. CON). On the contrary, HYK14 had a greater 150-day in-calf rate (46.7 vs. 13.8%, p ≤ 0.05) and a greater overall proportion of pregnancy relative to CON. According to Kaplan–Meier analysis (150-day survival time), HYK14 showed a trend towards fewer median days open compared with CON (111.4 vs. 126.6; p = 0.1), while we found no differences between CON and HYK7 or HYK28, respectively. Moreover, the risk for culling was lower in HYK14 compared to CON (12.5 vs. 35.1%; p = 0.1). Hyperketonemia at 28 DIM, closer to the starting point of breeding, had no effects on reproduction, as submission rate, overall proportion of cows pregnant, and conception risk were greater compared to CON. Moreover, HYK28 had a lower culling risk relative to CON (Table 2).
4. Discussion
The goal of this study was to observe metabolic changes in early pp hyperketonemic cows and to identify systemic metabolic and inflammatory biomarkers and then to compare the production and reproductive outcome of the cohort with controls. In many field trials, concentrations of BHB or NEFA in dairy cows were measured within the first 14 DIM, and hyperketonemia has been associated with peripartum diseases and impaired reproduction [8,10,12]. In this context, criticism was expressed that studies often did not evaluate systemic markers of metabolic and inflammatory disorders comprehensively [12]. In the last decade, elevated concentrations of ketone bodies have been identified as markers of physiological immune activation. During metabolic maladaptation, however, a dysregulated immune function led to unfavorable metabolic and reproductive outcomes [17,30]. We conducted the present study to achieve a model herd in a real-life medium-scaled dairy farm with approximately 30% HYK prevalence, which is close to the reported 28.9% in early lactation [35].
Elevated concentrations of NEFA before parturition indicate NEB [2] and were associated with metabolic and reproductive tract diseases, e.g., clinical ketosis and metritis [43,44]. As we excluded clinical diseases from our analyses, we cannot draw any conclusion regarding this relationship. Ospina et al. [45] reported a critical NEFA concentration threshold of >0.29 mmol/L ap, which was associated with diseases. In our results, median NEFA concentrations of HYK14 and HYK28 reached 0.2 mmol/L, and the previously mentioned threshold of 0.29 mmol/L was only exceeded in the third quartile of the values at all sampling days. The regression model, however, indicated NEFA 14 d ap as a strong predictor for HYK7. We interpret this finding with caution, as the factor NEFA was attenuated in the multiple regression by other covariates, e.g., RT and body condition. The high odds for HYK at 7 DIM (OR 1455) are likely due to the low range of NEFA values, combined with a limited number of cases, and are hardly comparable with the reported relative risk of 1.8 by Ospina et al. [45]. Furthermore, BHB ap was not a suitable marker for HYK pp in our study, which we explain by the fact that acetoacetate and BHB are in equilibrium ap, while the ketone body production pp shifts further towards BHB production [46]. Transition cows in excessive NEB are at risk for metabolic disorders, which were associated with pp BHB cut-offs of ≥1.0 mmol/L in a large-scale study [45]. Other authors considered cut-off values from 1.0 to 1.4 mmol/L to determine SCK [5,6]. We used the hand-held meter validated by Kanz et al. [34], where the cut-off ≥ 1.2 mmol/L fitted best to perform BHB measurements accurately. Most studies evaluated BHB in Holstein dairy herds [6,34]; thus, recommended BHB cut-offs may not be appropriate for generally lower-yielding Simmental cows. In our study, Simmental dairy cows yielded 9200 to 10,200 kg in the current lactation, which was comparable to the reported 9200 kg average milk yield of Holstein cows by Kanz et al. [34]. Lower RT 7 d ap was a significant predictor for HYK at 7 DIM in our study. This observation is of interest because ap RT showed high heterogeneity and was not an adequate predictor of SCK in a meta-analysis [47]. Evaluation of RT before calving, however, is beneficial because lower RT up to five days ap was associated with the occurrence of diseases pp [48].
During NEB, cows mobilize NEFA from fat reserves to adapt to the energetic requirements of lactogenesis, which is associated with changes in body condition and lower LAI [22]. The authors found poorer liver function, indicated by lower LAI, in ap over- and restricted-fed cows compared with balanced-fed (BAL: 100%) ones. In addition, obesity in the dry period tended to increase SCK incidence. These findings are partially consistent with our results because LAI d 14 ap and 28 DIM were consistently negative and lower in HYK7, HYK14, and HYK28 compared to CON, indicating poorer liver function in hyperketonemic cows. Occurrence of HYK and metabolic changes, however, were independent of body condition and its changes during dry-off and the transition period in our study. We found changes in biomarkers of liver activity indicated by significantly lower and a trend to lower TP in HYK14 and HYK28, respectively. Total protein is the sum of ALB (a negative APP) and globulin (a proxy of inflammation), which are negatively associated with fatty liver, and the former is significantly associated with impaired liver function [49]. Our outcomes need closer scrutiny because decreased TP in HYK was only relative to CON, and concentrations of ALB were consistently within physiological range (30–40 g/L, reference from Laboklin GmbH) in both groups. Other negative APPs, like CHOL and VIT A, are reduced in metabolically challenged cows, indicating risk for liver lipidosis [21] and indicating the importance of plane nutrition in transition cows [22]. In our study, we could not demonstrate significant alterations in liver biomarkers even though the feeding strategy resulted in approximately 30% prevalence of SCK. We assume that elevated concentrations of NEFA and BHB in transition cows of our study reflect the physiological metabolic adaptation, which is well summarized in the review by Horst et al. (2021) [17]. This assumption is supported by the low median HP concentrations at 7 DIM in our study. Moreover, HP exceeded the cut-off > 0.35 g/L only in the third quartile of values in both CON and HYK. Furthermore, elevated serum NEFA concentrations ap and elevated HP 7 DIM were significant risk factors for endometritis [50]. The interpretation of HP concentration in the week after calving, however, is under debate, because HP in this period showed poor sensitivity in determining diseases [51]. On the contrary, HP concentration > 1.6 g/L was an early indicator for metritis [52]. We measured HP at 7 DIM because Janovick et al. [22] found a peak concentration at this time point in restricted-fed cows corresponding to our feeding regime of LIB/REST.
The association between NEB, lipomobilization, and immunosuppression [24,25] and an increased susceptibility to uterine infections has often been described [25,53]. In the present study, we found no higher prevalence of CE and SE, as well as no poorer reproductive outcome in HYK in early lactation. It can be speculated that this was due to the small number of cases or because HYK was not promotive for uterine diseases. Anyway, the effects of BHB in ketonic cows on PMN functions are not consistent [54,55]. Activation of the immune system utilizes glucose stores and induces hypophagia, reflected by an extensive increase in NEFA and ketones [17]. To evaluate this association, we monitored DMI and evaluated concentrations of HP, but we did not perform measurements of glucose or glycogen. Migration of PMN into the uterus is necessary for bacterial clearance in cows after calving, but there is evidence that an excessive pro-inflammatory state early pp is a key factor for developing endometritis later [56,57]. This condition of exaggerated inflammation in fresh cows is linked with greater serum HP concentrations and has been reported in cows with SE and in cows with PVD [58]. This is in coincidence with the results of other authors reporting that higher HP and lower negative APPs in metabolically challenged cows are associated with excessive inflammation and fatty liver [16,18].
We were also interested in the inflammatory status of the oviductal epithelium, as there is evidence that inflammatory cells present in the oviduct may be sequelae of uterine infections [28]. Based on our results, this association was not reproducible, as the abundance of PMN in the oviducts was minimal and even zero in HYK14 and HYK28. The latter observation might be due to an impaired immune function in HYK because PMN seem to be involved in physiological conditions, as shown in the oviduct of naïve heifers [42]. Our conclusion, however, warrants closer scrutiny with a larger number of cases.
In the present study, we followed up the cows until the end of the current lactation to record reproductive performance and milk production. Several authors have shown that a compromised immune system in cows has a negative impact on reproductive performance [26,27,59]. The interpretation of fertility parameters, however, is difficult because reproductive performance is dependent on several factors, e.g., management (estrous detection, husbandry), nutrition, or diseases. In our study, reproductive performance parameters did not show any significant differences between HYK and CON except for HYK14, which showed a greater pregnancy rate at 1st service (p ≤ 0.05). This result seems contradictory, as nonketotic cows were 1.6 times more likely to conceive than diseased counterparts [60], whereas others found no association between hyperketonemia in the second week of lactation and poorer reproductive outcome [61]. In our study, however, HYK14 showed longer calving-to-first service intervals (p ≤ 0.05) and considerably lower submission rates than CON, which may reflect the sequelae of excessive lipomobilization and management decisions to inseminate hyperketonemic cows later in lactation. In addition, HYK14 yielded significantly more milk at the first milk record day as well as in the current lactation compared with CON. During NEB, cows use NEFA and ketones as an energy source, which can be utilized by the mammary gland to enhance milk yield [11,62] but were also associated with lower milk fat and a higher risk for removal from the herd [3]. Likewise, with our study, we observed significant milk fat depression in HYK14 and HYK28 compared with CON. We assume that elevated BHB concentrations negatively impacted de novo synthesis of milk fat, because lipolysis of fat depots reduces short-chain fatty acids (C4-14) necessary for milk fat production in the udder [63,64]. Milk yield often influences culling decisions on dairy farms, where high production is positively associated with survival, while underperforming cows are more likely to be removed from the herd [65]. This may explain the result of the present study, in which hyperketonemic cows with higher milk yield were less likely to be removed from the herd, but also underlines the role of ketone bodies as an important energy source, recently reviewed in detail [29]. Nevertheless, the main limitation of this study is the limited sample size, as the results only reflect the metabolic changes in transition cows in a single medium-sized herd.
5. Conclusions
In our study, markers of inflammation and oxidative stress were not elevated, which provides evidence that transition cows with HYK experienced physiological immune activation postpartum and adapted well to the metabolic challenges. Hyperketonemia 14 DIM was associated with higher milk yield, which underlines the role of NEFA and ketone bodies as energy sources to support the lactation of cows in NEB. Moreover, we found no clear effect of HYK at 28 DIM on uterine and oviductal health and on reproductive performance in the cohort. The results reflect the metabolic changes in transition cows with medium milk yield in a limited sample size, which is not representative of high-yielding dairy herds. Monitoring of HYK is part of good herd health management; however, in addition, optimal calving management must be implemented to avoid dystocia, hypophagia, and consequently enhanced inflammation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dairy7010002/s1, Table S1: Feed composition, average dry matter and energy content (mega-joule net-energy-lactation, MJ NEL), digestible protein (nXP, gram), and dry-matter content (DM, %), analyzed by a nutritional laboratory (Futtermittellabor Rosenau, Wieselburg, Austria) at the beginning of the study, after a new silo (grass, corn) was opened and at the end of the trial. Nutritional requirements for balanced nutrition (BAL) according to recommendations by the Bavarian Institute for Agriculture [30]; Table S2: Mean (SD) concentration of metabolic and oxidative serum biomarkers at days 14 antepartum (ap), 14, and 28 postpartum of hyperketonemic (HYK) and control (CON) cows at day 7, 14, and 28 postpartum (status).
Author Contributions
Conceptualization, H.P. and M.D.; methodology, H.P., M.D., U.B. and V.H.; software, H.P. and A.T.; validation, formal analysis, H.P. and A.T.; investigation, H.P., M.M., F.F., M.S., U.B. and V.H.; resources, H.P., M.M., F.F. and M.S.; data curation, A.T.; writing—original draft preparation, H.P.; writing—review and editing, H.P., M.D., U.B. and V.H.; visualization, H.P., M.D. and U.B.; supervision, M.D. and U.B.; project administration, H.P.; funding acquisition, H.P. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding. The funding and the publication were supported by the University of Veterinary Medicine Vienna, Austria (2025/219).
Institutional Review Board Statement
This study was approved by the institutional ethics committee and the national authority according to §8 of Law for Animal Experiments, Tierversuchsgesetz-TVG (BMWFW-68.205/0162-WF/V/3b/2017; approval date 12 September 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the farm management and the staff for the positive cooperation.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial insemination |
| ALB | Albumin |
| ap | antepartum |
| APP | Acute phase protein |
| BAL | Balanced |
| BFT | Backfat thickness |
| BHB | Beta-hydroxybutyrate |
| CE | Clinical endometritis |
| CHOL | Cholesterol |
| CON | Controls |
| d | Day |
| DIM | Days in milk |
| DMI | Dry matter intake |
| HP | Haptoglobin |
| HYK | Hyperketonemia |
| LAI | Liver activity index |
| LIB | Ad libitum |
| n | Number |
| NEB | Negative energy balance |
| NEFAs | Non-esterified fatty acids |
| PMNs | Polymorphonuclear neutrophils |
| pp | postpartum |
| PVD | Purulent vaginal discharge |
| REST | Restrictive |
| RT | Rumination time |
| SCK | Subclinical ketosis |
| SD | Standard deviation |
| SE | Subclinical endometritis |
| TP | Total protein |
| VIT A | Vitamin A |
References
- Drackley, J.K. ADSA Foundation Scholar Award: Biology of Dairy Cows during the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 15. [Google Scholar] [CrossRef] [PubMed]
- Van der Kolk, J.H.; Gross, J.J.; Gerber, V.; Bruckmaier, R.M. Disturbed bovine mitochondrial lipid metabolism: A review. Vet. Q. 2017, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.D.; Barbano, D.M.; McArt, J.A.A. The relationship of excessive energy deficit with milk somatic cell score and clinical mastitis. J. Dairy Sci. 2020, 104, 715–727. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 10. [Google Scholar] [CrossRef] [PubMed]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. Food Anim. 2004, 20, 24. [Google Scholar] [CrossRef]
- Duffield, T.F.D.; Sandals, K.E.; Leslie, K.; Lissemore, B.W.; McBride, J.H.; Lumsden, P.D.; Bagg, R. Efficacy of Monensin for the Prevention of Subclinical Ketosis in Lactating Dairy Cows. J. Dairy Sci. 1998, 81, 2866–2873. [Google Scholar] [CrossRef]
- Kerwin, A.L.; Burhans, W.S.; Mann, S.; Nydam, D.V.; Wall, S.K.; Schoenberg, K.M.; Perfield, K.L.; Overton, T.R. Transition Cow Nutrition and Management Strategies of Dairy Herds in the Northeastern United States: Part II—Associations of Metabolic- and Inflammation-Related Analytes with Health, Milk Yield, and Reproduction. J. Dairy Sci. 2022, 105, 21. [Google Scholar] [CrossRef]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of Subclinical Ketosis and Relationships with Postpartum Diseases in European Dairy Cows. J. Dairy Sci. 2013, 96, 14. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk Factors for Postpartum Uterine Diseases in Dairy Cows. J. Dairy Sci. 2010, 93, 8. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Lima, F.S.; Greco, L.F.; Bisinotto, R.S.; Monteiro, A.P.A.; Favoreto, M.; Ayres, H.; Marsola, R.S.; Martinez, N.; Thatcher, W.W.; et al. Prevalence of Periparturient Diseases and Effects on Fertility of Seasonally Calving Grazing Dairy Cows Supplemented with Concentrates. J. Dairy Sci. 2013, 96, 16. [Google Scholar] [CrossRef] [PubMed]
- Bretzinger, L.F.; Tippenhauer, C.M.; Plenio, J.-L.; Heuwieser, W.; Borchard, S. Effect of transition cow health and estrous expression detected by an automated activity monitoring system within 60 days in milk on reproductive performance of lactating Holstein cows. J. Dairy Sci. 2023, 106, 14. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Morrison, E.I.; Ribeiro, E.S.; Renaud, D.L.; Couto Serrenho, R.; LeBlanc, S.J. Postpartum health is associated with detection of estrus by activity monitors and reproductive performance in dairy cows. J. Dairy Sci. 2023, 106, 23. [Google Scholar] [CrossRef]
- Walsh, R.B.; Walton, J.S.; Kelton, D.F.; Leblanc, S.J.; Leslie, K.E.; Duffield, T.F. The Effect of Subclinical Ketosis in Early Lactation on Reproductive Performance of Postpartum Dairy Cows. J. Dairy Sci. 2007, 90, 9. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Denis-Robichaud, J. A Dairy Herd-Level Study of Postpartum Diseases and Their Association with Reproductive Performance and Culling. J. Dairy Sci. 2017, 11, 3068–3078. [Google Scholar] [CrossRef]
- Lacasse, P.; Vanacker, N.; Ollier, S.; Ster, C. Innovative dairy cow management to improve resistance to metabolic and infectious diseases during the transition period. Res. Vet. Sci. 2018, 116, 7. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 11. [Google Scholar] [CrossRef]
- Horst, E.A.; Kvidera, S.K.; Baumgard, L.H. Invited review: The influence of immune activation on transition cow health and performance—A critical evaluation of traditional dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef]
- Katoh, N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002, 64, 15. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E. Use of the Liver Activity Index and Other Metabolic Variables in the Assessment of Metabolic Health in Dairy Herds. Vet. Clin. Food Anim. 2013, 29, 19. [Google Scholar] [CrossRef]
- Graugnard, D.E.; Bionaz, M.; Trevisi, E.; Moyes, K.M.; Salak-Johnson, J.L.; Wallace, R.L.; Drackley, J.K.; Bertoni, G.; Loor, J.J. Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J. Dairy Sci. 2012, 95, 1749–1758. [Google Scholar] [CrossRef]
- Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Amadori, M.; Bionaz, M.; Trevisi, E. The role of altered immune function during the dry period in promoting the development of subclinical ketosis in early lactation. J. Dairy Sci. 2019, 102, 18. [Google Scholar] [CrossRef] [PubMed]
- Janovick, N.A.; Trevisi, E.; Bertoni, G.; Dann, H.M.; Drackley, J.K. Prepartum plane of energy intake affects serum biomarkers for inflammation and liver function during the periparturient period. J. Dairy Sci. 2022, 106, 168–186. [Google Scholar] [CrossRef]
- Nonnecke, B.J.; Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr. Effects of the Mammary Gland on Functional Capacities of Blood Mononuclear Leukocyte Populations from Periparturient Cows. J. Dairy Sci. 2003, 86, 10. [Google Scholar] [CrossRef]
- Scalia, D.; Lacetera, N.; Bernabucci, U.; Demeyere, K.; Duchateau, L.; Burvenich, C. In Vitro Effects of Nonesterified Fatty Acids on Bovine Neutrophils Oxidative Burst and Viability. J. Dairy Sci. 2006, 89, 8. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 11. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef]
- Hammon, D.S.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 2006, 113, 9. [Google Scholar] [CrossRef]
- Owhor, L.E.; Reese, S.; Kölle, S. Salpingitis Impairs Bovine Tubal Function and Sperm-Oviduct Interaction. Sci. Rep. 2019, 9, 15. [Google Scholar] [CrossRef]
- Rico, J.E.; Barrientos-Blanco, M.A. Invited review: Ketone biology—The shifting paradigm of ketones and ketosis in the dairy cow. J. Dairy Sci. 2024, 107, 22. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; LeBlanc, S.J. Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows. Animals 2025, 15, 633. [Google Scholar] [CrossRef] [PubMed]
- Spiekers, H. Gruber Tabelle zur Fütterung der Milchkühe, Zuchtrinder, Schafe, Ziegen. In Gruber Tabelle zur Fütterung der Milchkühe, Zuchtrinder, Schafe, Ziegen: Praktische Richtwerte für eine Milchkuhration, 42nd ed.; Bayerische Landesanstalt für Landwirtschaft: Munich, Germany, 2017; p. 101. [Google Scholar]
- Pothmann, H.; Tichy, A.; Drillich, M. Der Verlauf der Rückenfettdicke von Österreichischen Fleckviehkühen—Erstellung einer Referenzkurve (Back fat thickness throughout lactation for Austrian Simmental cows—A reference curve). Wien. Tierarztl. Monat—Vet. Med. Austria 2014, 101, 8. [Google Scholar]
- Reiter, S.; Sattlecker, G.; Lidauer, L.; Kickinger, F.; Öhlschuster, M.; Auer, W.; Schweinzer, V.; Klein-Jöbstl, D.; Drillich, M.; Iwersen, M. Evaluation of an ear-tag-based accelerometer for monitoring rumination in dairy cows. J. Dairy Sci. 2018, 101, 13. [Google Scholar] [CrossRef] [PubMed]
- Kanz, P.; Drillich, M.; Klein-Jöbstl, D.; Mair, B.; Borchardt, S.; Meyer, L.; Schwendenwein, I.; Iwersen, M. Suitability of capillary blood obtained by a minimally invasive lancet technique to detect subclinical ketosis in dairy cows by using 3 different electronic hand-held devices. J. Dairy Sci. 2015, 98, 11. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 11. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.W.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 15. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 2004, 62, 9–23. [Google Scholar] [CrossRef]
- Madoz, L.V.; Giuliodori, M.J.; Jaureguiberry, M.; Plöntzke, J.; Drillich, M.; de la Sota, R.L. The relationship between endometrial cytology during estrous cycle and cutoff points for the diagnosis of subclinical endometritis in grazing dairy cows. J. Dairy Sci. 2013, 96, 4333–4339. [Google Scholar] [CrossRef]
- Melcher, Y.; Prunner, I.; Drillich, M. Degree of variation and reproducibility of different methods for the diagnosis of subclinical endometritis. Theriogenology 2014, 82, 57–63. [Google Scholar] [CrossRef]
- Pothmann, H.; Müller, J.; Pothmann, I.; Tichy, A.; Drillich, M. Reproducibility of endometrial cytology using cytobrush technique and agreement for the diagnosis of subclinical endometritis between five predefined endometrial sites. Reprod. Domest. Anim. 2018, 54, 350–357. [Google Scholar] [CrossRef]
- Besenfelder, U.; Havlicek, V.; Kuzmany, A.; Brem, G. Endoscopic approaches to manage in vitro and in vivo embryo development: Use of the bovine oviduct. Theriogenology 2010, 73, 9. [Google Scholar] [CrossRef] [PubMed]
- Neubrand, L.; Pothmann, H.; Besenfelder, U.; Havlicek, V.; Gabler, C.; Dolezal, M.; Aurich, C.; Drillich, M.; Wagener, K. In vivo dynamics of pro-inflammatory factors, mucins, and polymorph nuclear neutrophils in the bovine oviduct during the follicular and luteal phase. Sci. Rep. 2023, 13, 14. [Google Scholar] [CrossRef]
- Nicola, I.; Chupin, H.; Roy, J.-P.; Buczinski, S.; Fauteux, V.; Picard-Hagen, N.; Cue, R.; Dubuc, J. Association between prepartum nonesterified fatty acid serum concentrations and postpartum diseases in dairy cows. J. Dairy Sci. 2022, 105, 9. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and β-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J. Dairy Sci. 2010, 93, 7. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Evaluation of nonesterified fatty acids and β-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 9. [Google Scholar] [CrossRef]
- Mills, S.E.; Beitz, D.C.; Young, J.W. Characterization of Metabolic Changes During a Protocol for Inducing Lactation Ketosis in Dairy Cows. J. Dairy Sci. 1986, 69, 10. [Google Scholar] [CrossRef] [PubMed]
- Cocco, R.; Andrighetto Canozzi, M.E.; Fischer, V. Rumination time as an early predictor of metritis and subclinical ketosis in dairy cows at the beginning of lactation: Systematic review-meta-analysis. Prev. Vet. Med. 2021, 189, 11. [Google Scholar] [CrossRef]
- Gusterer, E.; Kanz, P.; Krieger, S.; Schweinzer, V.; Süss, D.; Lidauer, L.; Kickinger, F.; Öhlschuster, M.; Auer, W.; Drillich, M.; et al. Sensor technology to support herd health monitoring: Using rumination duration and activity measures as unspecific variables for the early detection of dairy cows with health deviations. Theriogenology 2020, 157, 9. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited Review: Pathology, Etiology, Prevention, and Treatment of Fatty Liver in Dairy Cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Reproductive tract inflammatory disease in postpartum dairy cows. Animal 2014, 8, 10. [Google Scholar] [CrossRef]
- Humblet, M.F.; Guyot, H.; Boudry, B.; Mbayahi, F.; Hanzen, C.; Rollin, F.; Godeau, J.M. Relationship between haptoglobin, serum amyloid A, and clinical status in a survey of dairy herds during a 6-month period. Vet. Clin. Pathol. 2006, 35, 188–193. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Short communication: Haptoglobin as an early indicator of metritis. J. Dairy Sci. 2009, 92, 5. [Google Scholar] [CrossRef]
- Kehrli, M.E.; Nonnecke, B.J., Jr.; Roth, J.A. Alterations in bovine neutrophil function during the periparturient period. Am. J. Vet. Res. 1989, 50, 8. [Google Scholar] [CrossRef]
- Ster, C.; Loiselle, M.-C.; Lacasse, P. Effect of postcalving serum nonesterified fatty acids concentration on the functionality of bovine immune cells. J. Dairy Sci. 2012, 95, 10. [Google Scholar] [CrossRef]
- Suriyasathaporn, W.; Heuer, C.; Noordhuizen-Stassen, E.N.; Schukken, Y.H. Hyperketonemia and the impairment of udder defense: A review. Vet. Res. 2000, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Lilly, S.T.; Santos, N.R.; Gilbert, R.O.; Goetze, L.; Bryant, C.E.; White, J.O.; Cronin, J.; Sheldon, I.M. Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod. Biol. Endocrinol. 2009, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Price, S.B.; Cronin, J.; Gilbert, R.; Gadsby, J.E. Mechanisms of Infertility Associated with Clinical and Subclinical Endometritis in High Producing Dairy Cattle. Reprod. Domest. Anim. 2009, 44, 10. [Google Scholar] [CrossRef]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of immune function in the bovine uterus peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef]
- Bonnett, B.N.; Martin, S.W.; Meek, A.H. Associations of clinical findings, bacteriological and histological results of endometrial biopsy with reproductive performance of postpartum dairy cows. Prev. Vet. Med. 1993, 15, 16. [Google Scholar] [CrossRef]
- Gillund, P.; Reksen, O.; Groehn, Y.T.; Karlberg, K. Body Condition Related to Ketosis and Reproductive Performance in Norwegian Dairy Cows. J. Dairy Sci. 2001, 84, 7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Z.; Shepley, E.; Endres, M.I.; Cramer, G.; Caixeta, L.S. Assessment of milk yield and composition, early reproductive performance, and herd removal in multiparous dairy cattle based on the week of diagnosis of hyperketonemia in early lactation. J. Dairy Sci. 2022, 105, 11. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Collier, R.J.; Bauman, D.E. A 100-Year Review: Regulation of nutrient partitioning to support lactation. J. Dairy Sci. 2017, 100, 14. [Google Scholar] [CrossRef] [PubMed]
- Churakov, M.; Karlsson, J.; Edvardsson Rasmussen, A.; Holtenius, K. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animals 2021, 15, 100253. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, C.; Klopp, R.N.; Moraes, L.E.; Harvatine, K.J. Meta-analysis of the relationship between milk trans-10 C18:1, milk fatty acids <16 C, and milk fat production. J. Dairy Sci. 2020, 103, 12. [Google Scholar] [CrossRef]
- Kulkarni, P.; Mourits, M.; Nielen, M.; Van den Broek, J.; Steeneveld, W. Survival analysis of dairy cows in the Netherlands under altering agricultural policy. Prev. Vet. Med. 2021, 193, 8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.