1. Introduction
Dairy products constitute a fundamental component of the human diet [
1] and consumer interest in products derived from the milk of non-bovine species is steadily increasing, driven by their characteristic nutritional, functional, and sensory attributes [
2,
3]. This trend is particularly evident in the growing popularity of cheeses made from buffalo’s and goat’s milk, which are gaining recognition in both traditional and specialized markets.
Compared to cow’s milk, buffalo’s milk is characterized by a higher content of fat and key fatty acids, such as trans-vaccenic acid and conjugated linoleic acid (CLA), as well as a large amount of protein [
4]. In particular, it contains whey proteins such as lactoferrin and immunoglobulins, along with bioactive compounds exhibiting antioxidant and anti-inflammatory properties, including δ-valerobetaine and acetyl-L-carnitine, as reviewed by Liao et al. [
5]. Conversely, goat’s milk, widely recognized for its digestibility, contains greater amounts of short-chain fatty acids, smaller fat globules, and a distinctive profile of protein-derived peptides and prebiotic oligosaccharides [
6,
7].
Many soluble milk proteins undergo glycosylation, a post-translational modification in which carbohydrate chains are covalently attached to specific amino acid residues. The terminal portions of these glycans frequently contain sialic acids (SIAs), a family of nine-carbon acidic monosaccharides that typically occupy the terminal positions of glycan chains of glycoproteins and glycolipids, where they are linked through α-O- or N-glycosidic bonds [
8,
9,
10]. Due to their exposed position and structural variability, sialic acids play crucial biological roles, including the modulation of immune responses, mediation of microbial interactions, and support of neural development [
10].
In mammals, the two predominant forms of SIA are N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). Neu5Ac, the only form synthesized by humans, is particularly concentrated in the brain and has been linked to neurodevelopment and cognitive performance [
8,
11]. It has also been recognized by the European Food Safety Authority (EFSA) for its nutraceutical potential [
12].
Milk represents a major dietary source of sialic acids. In particular, human milk provides a high amount of sialylated compounds essential for early brain development and immune maturation in infants. Their well-recognized benefits have led to the supplementation of infant formula with Neu5Ac [
13,
14]. Conversely, Neu5Gc is synthesized from Neu5Ac by the enzyme CMP-Neu5Ac hydroxylase (CMAH), which is functional in most mammals but inactive in humans due to a genetic deletion. Consequently, Neu5Gc is not produced endogenously in humans and can only be acquired through the consumption of animal-derived foods. Its incorporation into human tissues has been linked to chronic inflammation and associated pathologies [
8,
15,
16].
Bovine, buffalo, and goat milk also contain substantial amounts of SIA; however, unlike human milk, they include Neu5Gc, which is exogenous and poorly tolerated in humans. A study by Kawanishi et al. [
17] reported that a high dietary intake of Neu5Ac can inhibit the intestinal absorption of Neu5Gc, thereby mitigating its potential adverse effects. Given their contrasting physiological roles, the Neu5Ac/Neu5Gc ratio represents an important nutritional indicator.
The different composition of milk markedly affects the nutritional characteristics of derived dairy products, in particular concerning the protein fraction, which is extensively sialylated [
18]. Milk proteins are classified into caseins (α-, β-, and κ-caseins and their variants), whey proteins (including lactoferrin, β-lactoglobulin, α-lactalbumin, and immunoglobulins), and milk fat globule membrane proteins [
19,
20]. While caseins represent the main structural proteins in cheese, whey proteins and other soluble components can be partially released into the whey. In addition, the glycosylated fragment of κ-casein (glycomacropeptide) is cleaved and released into the whey during rennet coagulation, resulting in a higher concentration of sialic acids in the soluble fraction [
21].
Despite the well-established biological relevance of sialic acids, current knowledge regarding their distribution in dairy products remains limited, particularly in those derived from non-bovine species. To address this gap, the present study aimed to quantify the concentrations of Neu5Ac and Neu5Gc in dairy products made from buffalo, cow, and goat milk. A selection of representative cheeses was analysed, including mozzarella, stracchino, caciotta, robiola, and ricotta. These dairy products differ notably in their inclusion of whey proteins, which are known to be highly sialylated and may therefore contribute to differences in the overall sialic acid composition. By investigating these variations, this study seeks to elucidate interspecies differences in sialic acid profiles and provide novel insights into the sialylation patterns of dairy products from different animal species.
2. Materials and Methods
2.1. Dairy Product Collection and Characterization
Dairy products made from cow (C), buffalo (B), and goat (G) milk were obtained from local dairies in the Italian regions of Lazio and Campania: three different producers for cow and buffalo products and two for goat products, as shown in
Figure 1.
The selected products included mozzarella (Mz), stracchino (St), a semi-ripened cheese commonly referred to as caciotta (Ca), robiola (Ro), and ricotta (Ri). A total of 40 dairy products were collected from eight dairies (three processing cow’s milk, three buffalo’s milk, and two goat’s milk), each producing all five types of cheese.
All cheeses analysed were produced using traditional Italian cheesemaking processes, with minor variations between production units, as expected due to differences in individual cheesemaking practices adopted by the different dairies. The main technological steps that distinguish different cheese varieties include coagulation, curd cutting, heating, draining, pressing, salting, and, in many cases, ripening. Among these, the method and timing of curd cutting are particularly critical, as they significantly influence whey expulsion and, consequently, affect the moisture content, texture, and yield of the final product [
22]. The cheesemaking procedures for each product were not described in full detail, as individual dairies may apply specific proprietary practices that cannot be disclosed. The description provided below offers a general classification of the five cheese types and highlights the main processing mechanisms that may have influenced their sialic acid composition, reflecting the overall variability of dairy products. To complement this information, the main technological features are reported in a
Supplementary Table (Table S1).
Buffalo mozzarella, unlike the other cheeses, undergoes additional acidification and stretching steps in hot water following curd formation. The resulting stretched curd, or
pasta filata, is then cut and shaped either manually or mechanically [
23]. Mozzarella made from goat milk is uncommon; in this study, it was specifically produced by the dairy upon request for experimental purposes. In cheeses such as stracchino and robiola, the curd is typically cut into large pieces, approximately the size of walnuts, whereas in semi-ripened cheeses, such as caciotta, the curd is broken down into smaller pieces resembling hazelnuts. Ricotta, unlike cheese, is not produced directly from milk but rather from whey, a by-product of the cheesemaking process. The mixture is then acidified and heated to 85–90 °C, leading to the denaturation and coagulation of whey proteins [
24].
All cheeses were divided into two equal portions: one was used from the Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “M. Aleandri” laboratory (IZS) for proximate chemical analysis, while the other one was analysed at the CREA-ZA laboratory for sialic acid determination. Upon arrival at the laboratories, samples were portioned, vacuum-packed, and stored at –20 °C until analysis.
2.2. Mini-Cheesemaking Trial in the Laboratory
Cheesemaking, ripening, and packaging processes involve numerous variables that are often specific to individual dairies. To minimize the influence of these confounding factors, a standardized cheesemaking trial was conducted under controlled laboratory conditions. This approach aimed to reduce process-related variability and better highlight species-specific differences in sialic acid content. In addition, the experiment allowed to quantify the distribution of sialic acid between the curd and the whey fraction. The trial was conducted at the laboratory using bulk milk from the three species under investigation (cow, buffalo, and goat), obtained from the experimental farm of CREA-ZA.
The cheese was produced using 1000 g of milk, following the artisanal processing method of a fresh cheese (
Figure 2). The same cheesemaking process was applied for each type of milk. Before processing, raw milk was pasteurized by heating at 65 °C for 20 min under gentle magnetic agitation, and then it was rapidly cooled to 40 °C. This mild heat treatment ensured microbial safety while preserving the structural integrity of sialic acids. These compounds are known to remain stable under moderate temperature conditions (60–70 °C for approximately 2 h) and within a pH range from 2 to 10, as reported by Zhu et al. [
25].
For each cheesemaking, a starter culture was added to the milk. Then, the milk was coagulated using liquid rennet 130 IMCU (1 mL/L, Caglificio Clerici, Como, Italy). The curd was cut into 1 cm grains (approximately the size of hazelnuts). The curd was separated from the whey and transferred into a mold (10 cm diameter). At this stage, samples of whey were collected and stored at –20 °C for further analysis.
To promote syneresis, the cheeses were placed in a warm room (28 ± 1 °C and relative humidity 90%) and, once a pH of 5.4–5.5 was reached, the cheeses were transferred to a cold room (4 °C). When the internal temperature of the cheeses reached approximately 15 °C, dry salting was carried out using 2.5% (w/w) salt. The cheeses were then stored at 4 °C for two days before being frozen at −20 °C for further analysis.
All milk, whey, and fresh cheese samples were prepared in two technical replicates, and each sample was transported under refrigerated conditions to the IZS laboratory for proximate chemical analysis. For each type of milk and process, 1000 g of milk were used as the starting material, and after cheesemaking, the weights of the resulting cheese and whey obtained were recorded to calculate the yield and evaluate the content of sialic acids compared to the quantities of cheese and whey produced.
2.3. Chemical Analysis
2.3.1. Proximate Composition of Milk, Whey and Dairy Products
Approximately 60 mL of cow, buffalo, and goat milk and whey samples (without preservatives) were collected. The samples were transported under refrigerated conditions (4 °C) and analysed within 36 h of collection at the quality milk laboratory of the IZS. The chemical composition of milk and whey (total fat, total protein, casein, lactose, and dry matter content) was determined using FTIR analysis with a MilkoScan™ 7 RM (Foss Analytical A/S, Hillerød, Denmark), which was calibrated using appropriate cow, buffalo, and goat milk standards, obtained from the Milk Standard Laboratory of the “Associazione Italiana Allevatori” (Maccarese, Italy); whilst for whey a specific whey standard was used (Muva Reference Material), which was obtained from Muva GmbH (Kempten, Germany).
For each dairy product sample, a cross-sectional slice approximately 5 cm thick was taken from the centre of the product. The slice was then finely ground and homogenized to achieve a uniform particle size between 1 and 2 mm. The homogenized sample was transferred into a Petri dish (10 cm in internal diameter) and subjected to near-infrared (NIR) analysis. NIR spectra were collected by averaging 16 sub-spectra acquired at different positions during automatic rotation of the Petri cup. For each sample, three technical replicate readings were performed and used for chemometric processing.
Dairy product samples were analysed for dry matter, total fat, and total protein using the NIR spectrum, by FoodScan™ Dairy Analyser (FOSS, Hillerød, Denmark). The FoodScan is pre-calibrated with unique Artificial Neural Network (ANN) calibrations that have received national approvals for the measurement of key quality parameters following the standard procedures, based on the commercial calibration model provided by the manufacturer. Data were reported as g/100 g of sample.
2.3.2. Sialic Acid Analysis
Sialic acids Neu5Ac and Neu5Gc were quantified as reported in Crisà et al. [
26] with some modifications. For dairy products, 1 g of product was homogenized with 2.5 mL of 50 mM sulfuric acid, whilst for milk and whey, 2 mL of sample was mixed with 2 mL of 50 mM sulfuric acid in a 1:1 (
v/
v) ratio. Samples were hydrolysed at 80 °C for 1.5 h; during this time, samples were shaken every 15 min. After cooling in an ice bath, the samples were centrifuged at 5000 rpm for 10 min at 4 °C. An aliquot of supernatant was centrifuged again at 14,000 rpm for 5 min and used for 1,2-diamino-4,5-methylenedioxybenzene (DMB) derivatization. Derivatization was performed using a 1:1 (
v/
v) hydrolysed sample to DMB ratio, followed by incubation in a heating block at 60 °C for 2.5 h.
After derivatization, the samples were injected into the HPLC Alliance 2695, Waters (Waters Corporation, Framingham, MA, USA), equipped with an ACME C18 column (250 × 4.6 mm 5 µm) (CPS Analitica, Milano, Italy). The retention times and peak identities of Neu5Ac and Neu5Gc in the samples were determined by comparison with those of pure Neu5Ac and Neu5Gc standards (Merck, Darmstadt, Germany). Quantification was based on external calibration curves obtained from standard solutions of Neu5Ac and Neu5Gc, and concentrations in samples were expressed as µg/g of product.
2.4. Statistical Analysis
Data were analysed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Two separate experiments were conducted and analysed accordingly.
For the first experiment, involving commercial dairy products collected from eight different local cheese dairies, the experimental design included two fixed factors: animal species (C, B, G) and dairy product (Mz, St, Ca, Ro, Ri). Each product was sampled in two technical replicates. Given the lack of standardization in cheesemaking conditions across facilities, cheese dairy and technical replicates were modelled as random effects to account for unmeasured processing variability.
Response variables following a normal distribution (e.g., Neu5Ac) were analysed using a linear mixed-effects model fitted with PROC MIXED. The model included species, product, and their interaction as fixed effects. Random terms were specified for cheese dairy and technical replicates nested within dairy. Least-squares means were compared using Tukey’s adjustment for multiple comparisons, and statistical significance was set at p < 0.05.
For the second experiment, a mini-cheesemaking trial was carried out under controlled laboratory conditions. In this case, the cheese dairy effect was excluded, as all processing was performed in a single facility. The experimental design included two fixed factors: species (cow, buffalo, goat) and sample type (milk, whey, and fresh cheese). Replication was again included as a random effect to reflect experimental repeatability.
In both experiments, Neu5Gc concentrations and the Neu5Ac/Neu5Gc ratio exhibited non-normal distributions, particularly in goat-derived samples. Normality was assessed using the Shapiro–Wilk test and visual inspection of residual plots. Conventional transformations (logarithmic, square root) failed to normalize the data or homogenize variance. Therefore, a generalized linear mixed model (GLMM) was applied using PROC GLIMMIX, with the method = quad option to accommodate the non-normal residual distribution. In the GLIMMIX model, species, dairy product (or sample type), and their interaction were specified as fixed effects, while cheese dairy (when applicable) and technical replicates were included as random effects. Least-squares means were compared using Tukey adjustment. This approach allowed estimation of fixed effects while accounting for random sources of variation across processing batches and technical replicates. In both experiments, results were presented as least-squares means ± standard deviation. For pairwise comparisons, statistically significant differences (p < 0.05) were indicated by different superscript letters in the same column, based on Tukey-adjusted p-values.
3. Results
3.1. Dairy Products
As expected, the different types of dairy products significantly influenced the proximate chemical composition, as illustrated in
Figure 3, with a generally consistent trend across species. Among individual products, Ca showed the highest dry matter content in all species, with values ranging from 49.75 ± 3.62 g/100 g in G to 56.22 ± 3.44 g/100 g in B. In contrast, Ri consistently had the lowest dry matter (e.g., 26.57 ± 1.73 in C, 32.68 ± 2.89 in B and 26.32 ± 2.63 g/100 g in G), followed by Mz, Ro, and St, which fell into an intermediate group. Overall, cheeses derived from B milk tended to have higher dry matter content compared to those from C and G milk.
Fat content followed a similar pattern. Ca again had the highest values ranging from 27.15 ± 1.49 g/100 g (G) to 32.29 ± 3.91 g/100 g (B). St and Ro showed intermediate fat levels, with B samples often presenting higher values. Among the fresh cheeses, Ri and Mz had the lowest fat contents, especially in C-Ri (14.33 ± 1.25 g/100 g) and G-Mz (16.78 ± 0.74 g/100 g). These differences are largely attributable to the product structure and processing methods, particularly moisture retention during curd handling.
The same considerations can also be made for proteins. Ca cheese again exhibited the highest levels across all species (21.60 g/100 g on average). Mz, St, and Ri had similar intermediate protein values, with significantly higher values for B-Mz (12.92 ± 0.98). Ro presented the lowest overall protein values, particularly in C-Ro and G-Ro (9.23 g/100 g on average).
The concentrations of Neu5Gc, Neu5Ac, and their ratio (Neu5Ac/Neu5Gc) in dairy products differed significantly across species (
Table 1).
G-derived products showed a higher concentration of Neu5Gc (
p < 0.001) compared to both C and B samples (about 5.21 µg/g on average), which did not differ significantly from each other. The high levels of Neu5Gc in G dairy products may be attributed to species-specific glycosylation pathways. Neu5Ac levels also varied significantly across species (
p < 0.05), although to a lesser extent. C dairy products showed the highest Neu5Ac concentration (118.94 ± 18.28 µg/g), followed by B (106.15 ± 16.02 µg/g) and G (99.45 ± 9.69 µg/g), which were not statistically different from each other (
Table 1).
The Neu5Ac/Neu5Gc ratio revealed a striking inter-species distinction (
p < 0.001), with C and B products presenting very high values (24.11 ± 2.41 and 21.57 ± 2.31, respectively), reflecting a predominant presence of Neu5Ac. In contrast, the Neu5Ac/Neu5Gc ratio in G dairy products was markedly lower (1.29 ± 0.11), indicating a near balance between the two forms or even a predominance of Neu5Gc in some products (
Table 1). These results emphasize the species-specific sialylation patterns in dairy matrices.
Significant differences (
p < 0.001) were observed among species and dairy products for both individual sialic acids (Neu5Gc and Neu5Ac) and their ratio (
Table 2).
Across all species and product types, Ri consistently showed the highest concentrations of both Neu5Gc and Neu5Ac but also the lowest Neu5Ac/Neu5Gc ratios (except for G). In C-derived dairy products, excluding Ri, Neu5Gc concentrations did not differ significantly among the remaining products, with an average value of 4.26 µg/g across Mz, St, Ca, and Ro. In B milk products, significant differences among the cheeses (excluding Ri, which showed the highest value) were observed only between Ro and St, with the latter presenting the lowest Neu5Gc level (3.54 ± 0.43 µg/g). As previously reported in
Table 1, Neu5Gc concentrations in G dairy products were markedly higher than those of C and B. Among G products, Ri again exhibited the highest Neu5Gc content, followed by Mz and Ro, which had an average of 84.07 µg/g. The lowest values were observed in Ca and St, with a mean of 59.34 µg/g between the two, significantly lower than the other G products.
Regarding Neu5Ac, C-Ri presented the highest concentration among all cheeses and species (163.87 ± 21.29 µg/g). Significantly lower values were found in St and Ca (97.54 µg/g on average), while Ro and Mz showed intermediate values. In B milk products, Neu5Ac concentrations followed a similar trend to that observed for Neu5Gc, ranging from 150.26 ± 14.15 µg/g in Ri to 77.69 ± 12.75 µg/g in St, with intermediate values in the other cheeses. Similarly, in G milk cheeses, Neu5Ac levels ranged from 134.12 ± 5.03 µg/g in Ri to 81.22 µg/g as average in St and Ca. Stracchino and caciotta cheeses showed significantly lower levels compared to Mz and Ro, which exhibited intermediate values.
Regarding the Neu5Ac/Neu5Gc ratio, a consistent trend was observed in both C and B dairy products: Mz showed the highest ratio (28.24 ± 1.87 and 26.49 ± 2.52 for C and B, respectively), while Ri showed the lowest (19.06 ± 2.12 and 15.53 ± 1.98 for the same species). The remaining products presented intermediate values. In contrast, the trend for this ratio was markedly different in G milk products, where values were overall much lower. St showed the highest Neu5Ac/Neu5Gc ratio (1.44 ± 0.16), while Ro had the lowest (1.18 ± 0.17), with the other cheeses falling in between.
3.2. Proximate Composition and Sialic Acid Content in Matrices of Mini-Cheesemaking Trial
In the mini-cheesemaking trial, 1000 g of milk from each species yielded 205 g of C cheese, 250 g of B cheese, and 167 g of G cheese. The yield was higher for B milk compared to C milk (25% vs. 21%) and lower for goat milk (17%). Consequently, the volume of whey produced was lower for B and higher for G.
Significant differences were observed in the chemical composition of milk and whey among species for most parameters (
Figure 4). B milk showed the highest values for fat (7.28 ± 0.24 g/100 g), protein (4.67 ± 0.13 g/100 g), caseins (3.68 ± 0.11 g/100 g), and dry matter (16.4 ± 0.56 g/100 g), significantly exceeding the values observed in C and G milk (
p < 0.05). C milk had the lowest fat percentage (3.87 ± 0.15 g/100 g) and dry matter content (11.75 ± 0.38 g/100 g).
Regarding whey composition, fat and protein levels were highest in goat whey (1.76 ± 0.11 and 1.17 ± 0.06 g/100 g, respectively), while C whey showed the lowest values (0.62 ± 0.04 g/100 g fat and 0.72 ± 0.03 g/100 g protein). Casein content was very low in all whey samples, as expected. Lactose content did not differ significantly among species in either milk or whey.
As a result, the B cheese exhibited the highest dry matter (45.77 ± 4.21 g/100g), fat (26.15 ± 0.19 g/100 g), and protein (15.94 ± 0.12 g/100 g) content compared to G and C cheeses.
In C, Neu5Gc levels in milk and whey were not significantly different and showed an average of 2.83 µg/g, which was significantly lower than the concentration found in fresh cheese (4.02 ± 0.48 µg/g). Similarly, Neu5Ac values in milk and whey did not differ but were significantly lower than in cheese (116.52 ± 8.13 µg/g). The Neu5Ac/Neu5Gc ratio followed an opposite trend, indicating a greater relative accumulation of Neu5Gc in cheese (
Table 3).
In B, the same trend was observed with Neu5Gc concentrations in milk and whey significantly lower than in cheese. Neu5Ac also increased from milk and whey to cheese, with a significant difference between the two extremes. The Neu5Ac/Neu5Gc ratio was significantly higher in milk and whey (mean: 31.20) than in cheese (27.34 ± 2.81), confirming the preferential retention of Neu5Gc in the cheese matrix, as observed also in C (
Table 3).
In G, both Neu5Gc and Neu5Ac concentrations increased significantly from milk and whey to cheese. However, unlike C and B, the Neu5Ac/Neu5Gc ratio remained stable across all matrices, with values ranging from 1.06 ± 0.07 to 1.11 ± 0.09 (
Table 3). This indicated a proportional retention of both sialic acids in G cheese.
When comparing species overall, Neu5Gc concentrations were significantly higher in G than in C and B across all matrices. Conversely, Neu5Ac was more abundant in C and B than in G. As a result, the Neu5Ac/Neu5Gc ratio was markedly lower in G than in C and B combined (
Table 3), confirming the species-specific differences in sialic acid composition.
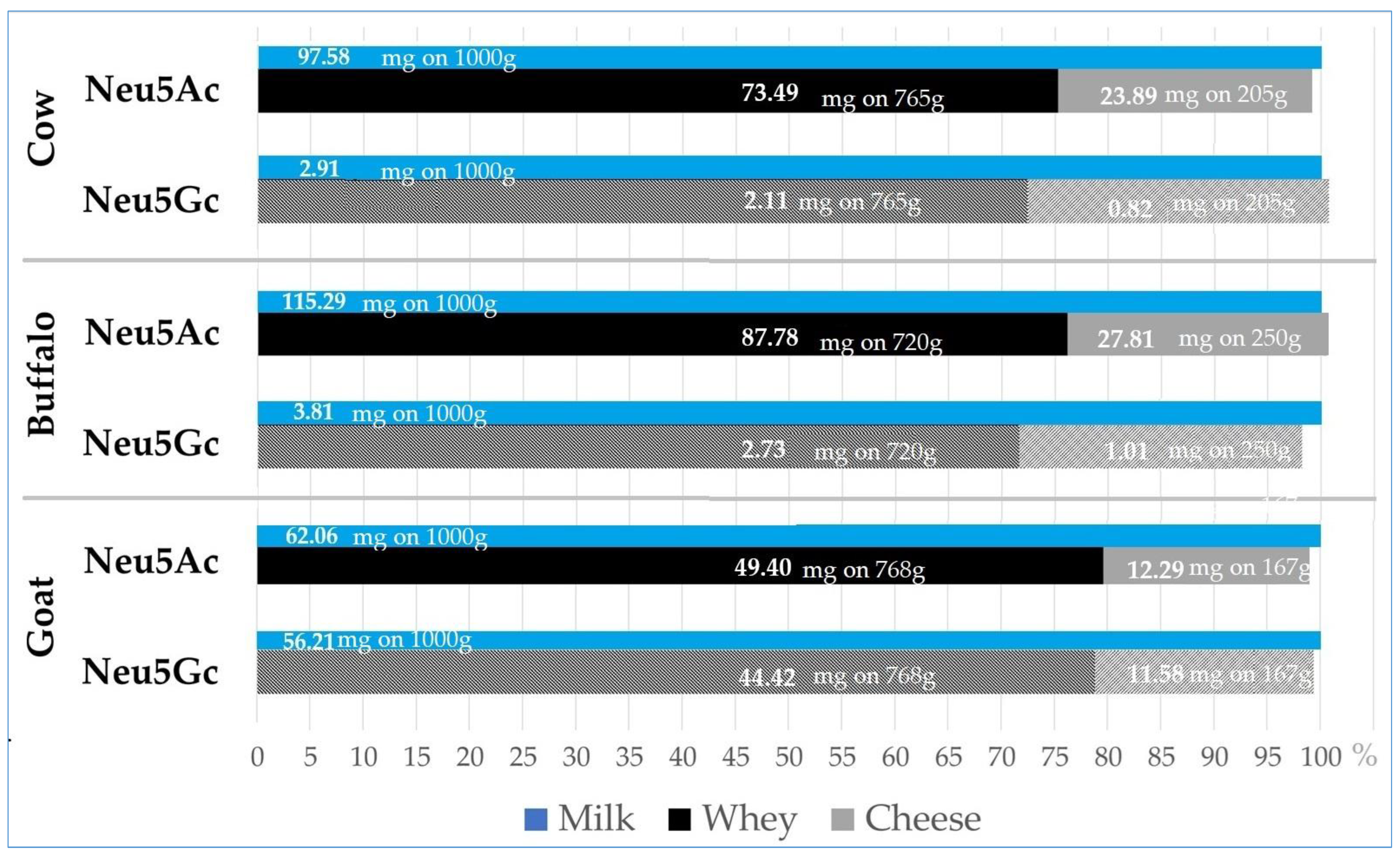
Figure 5 shows the absolute content (in mg) of both sialic acids (Neu5Gc and Neu5Ac) measured in 1000 g of milk, as well as the corresponding amounts (in mg) recovered in the whey and cheese obtained from milk. The figure also illustrates the percentage distribution of Neu5Gc and Neu5Ac across whey and cheese fractions from cow (C), buffalo (B), and goat (G) milk. For each species, the total amount of sialic acid present in the initial milk was considered as 100%, and the relative proportions recovered in the whey and cheese were represented accordingly.
In C milk, 2.91 mg of Neu5Gc were present in 1000 g of milk, of which 2.11 mg (72.4%) were recovered in the whey and 0.82 mg (28.3%) in the cheese. Neu5Ac showed a similar distribution, with 97.58 mg in 1000 g of milk, of which 73.49 mg (75.3%) was transferred to the whey, and 23.89 mg (24.5%) to the cheese.
In 1000 g of B milk, Neu5Gc reached 3.81 mg, with 2.73 mg (71.6%) recovered in the whey and 1.01 mg (26.6%) in the cheese. Neu5Ac content was higher (115.29 mg), with 87.78 mg (76.1%) transferred to the whey and 27.81 mg (24.1%) to the cheese.
In G milk, Neu5Gc content was substantially higher (56.21 mg in 1000 g), with 44.42 mg (79.0%) transferred to the whey and 11.58 mg (20.6%) to the cheese. Similarly, Neu5Ac in G milk was 62.06 mg, with 49.40 mg (79.6%) recovered in the whey and 12.29 mg (19.8%) in the cheese.
Overall, the data showed that both Neu5Gc and Neu5Ac predominantly partitioned into the whey during cheesemaking, regardless of species, with only a limited fraction retained in the cheese matrix.
4. Discussion
Buffalo dairy products exhibited higher fat and protein contents compared to those from the other two species. This emphasizes the role of the starting milk as a key factor in determining the chemical composition of the final product [
3,
18]. Nevertheless, the cheesemaking process itself plays an equally important role in shaping compositional outcomes. Among the technological factors, whey drainage during processing and the extent of ripening are particularly influential in determining total solids retention [
18,
22,
27]. Indeed, among the cheeses considered, Ca, a semi-ripened cheese, consistently showed the highest concentration of solids across all three species. The other cheeses, classified as soft, displayed intermediate total solids levels [
28]. Ricotta, which showed the lowest solid content among the cheeses analysed, was also characterized by high variability, probably because some producers enrich the whey used for ricotta production by adding up to 25% whole milk [
29]. This practice increases the variability of the final product and significantly alters its fat and protein composition, so much so that buffalo ricotta may not differ substantially from mozzarella in terms of these components. Despite the technological variability involved, the values observed in this study fall within the range reported in the literature for such products [
24].
The main chemical characteristics of milk used in the cheesemaking trial reflected the differences among species, with a greater content of fat and protein in buffalo milk [
30,
31]. In contrast, the chemical characteristics of whey after curd separation depended only partially on the type of milk, as shown in
Figure 4. Whey represents the liquid fraction obtained after the coagulation of casein proteins and contains part of the soluble nutrients from milk [
32]. As is well known, whey is rich in lactose and whey proteins such as lactoferrin, lactalbumin, and lactoglobulin, along with smaller amounts of other components, while the curd primarily retains caseins and milk fat [
33]. This explains the chemical characteristics of the whey and fresh cheese reported in
Figure 4.
Concerning the content of SIA in milk reported in
Table 3, the existing literature highlights a wide variability in SIA composition across milk from different species, including human milk. Reported values for Neu5Ac in bovine milk vary greatly, ranging from about 218 mg/L [
34] to 18.6 mg/L, with 0.5 mg/L of Neu5Gc [
35]. However, most of the data reported in the literature show intermediate values, such as those described by Li et al. [
36], who found 93.75 μg/mL of Neu5Ac and 3.51 μg/mL of Neu5Gc. This variability may be attributed to some factors that affect milk composition, such as stage of lactation, animal nutrition, farming practices, and breed differences [
26]. In the case of buffalo milk, data are limited, but the few available studies suggest concentrations similar to or slightly higher than those in bovine milk [
37,
38].
About goat milk, the literature confirms our findings, showing a higher proportion of Neu5Gc compared to Neu5Ac [
35,
39], with Neu5Gc representing about 64% of total sialic acids [
40] reaching about 90 μg/g [
13]. Goat milk contains higher levels of glycolylated sialic acids compared to cow milk, possibly due to its higher content of milk oligosaccharides, which are a significant source of these sialic acids [
35,
39,
40].
Concerning cheese, although available data are still limited, as observed for milk, a wide variability has been reported, also depending on the analytical methods used for quantification. Li et al. [
36] found 231 μg/g of Neu5Ac and 17 μg/g of Neu5Gc in Chinese cheese, while Samraja et al. [
41] reported Neu5Gc levels ranging from 10 to 22 μg/g in cow cheeses and up to 43 μg/g in goat cheeses. Similarly, our goat dairy products showed a higher Neu5Gc content compared to cheeses from cow and buffalo milk, reflecting the same trend observed in milk.
It is important to note, however, that glycosylation in bovine, buffalo, and particularly goat milk also involves the incorporation of Neu5Gc, a form of sialic acid that is completely absent in humans. The presence of this non-human sialic acid in animal-derived products may have potential implications for human health, with a possible pro-inflammatory effect [
15,
16], as also highlighted in red meat, where the high Neu5Gc content has been considered a potential contributing factor to metabolic disorders [
41,
42]. Nevertheless, considering the low concentrations of Neu5Gc detected in all bovine and buffalo cheeses compared with Neu5Ac, it can be reasonably expected that this compound does not exert potential adverse effects on human health [
17]. In contrast, Neu5Ac plays a fundamental role in human health, contributing to a wide range of physiological processes. It is involved in brain development, supports immune function and enhances intestinal maturation. Moreover, Neu5Ac-containing glycoconjugates have been shown to possess anti-adhesive properties against pathogens and promote the development of a beneficial gut microbiota [
9,
11,
12].
Beyond species differences, the sialic acid composition of dairy products is strongly influenced by the nature and fate of glycosylated milk components during cheesemaking. Chen et al. [
13] investigated the distribution of sialic acids in milk from various species, including bovine and caprine, with particular attention to their conjugation with glycoproteins, glycolipids, and oligosaccharides. Their results indicate that sialic acids are predominantly associated with proteins; approximately 55% of sialic acids in cow milk and around 65% in goat milk are bound to glycoproteins. Only about 4–8% are conjugated with lipids, and a similar proportion is found in free form. The remaining fraction is linked to oligosaccharides. In contrast to cow milk, sialic acids in goat milk are more frequently bound to oligosaccharides, although to a lesser extent than in human milk, where 70–80% of sialic acids occur in this form, with the remainder associated with glycoproteins and glycolipids. These findings underscore the important role of glycoproteins as highly sialylated bioactive molecules.
With milk coagulation, the sialic acids present in milk were partly entrapped within the protein matrix of the cheese and partly released into the whey, as they are bound to soluble whey proteins, as shown in
Figure 5. Both Neu5Gc and Neu5Ac are predominantly partitioned into the whey, regardless of species. Moreover, following enzymatic coagulation, as in our trial, chymosin cleaves k-casein, forming para-k-casein, which remains in the curd, and a peptide known as glycomacropeptide (GMP), which becomes soluble in the whey and carries sialic acid residues. Consequently, the sialic acid content of whey largely depends on the presence of GMP, which accounts for approximately 20–25% of total whey proteins [
43,
44].
Furthermore, lactoferrin, immunoglobulins, especially IgA and IgG, and α-Lactalbumin are among the most heavily sialylated glycoproteins in milk and, as in the case of GMP, they remain in the whey during cheesemaking [
45,
46,
47]. These results highlight the crucial role of glycoproteins as highly sialylated bioactive molecules [
48,
49]. In particular, buffalo milk is relatively rich in lactoferrin (~0.8 g/L) [
50], while goat milk tends to contain lower levels (<0.1 g/L), more similar to bovine milk [
51].
Among the various sialylated proteins, the Milk Fat Globule Membrane (MFGM) proteins deserve particular mention. The membrane that surrounds milk fat globules is rich in specific, highly sialylated glycoproteins. A higher fat content in milk corresponds to an increased presence of these glycoproteins [
52,
53]. However, a limited portion of these proteins remains entrapped within the casein network, and, due to their instability under acidic conditions, they are easily denatured during cheesemaking [
54].
These losses in the whey may explain the higher sialic acid concentrations in ricotta compared to cheeses in each species (
Table 2). This is mainly due to the recovery of highly sialylated whey proteins and the presence of GMP in the whey used for its production. Ricotta is obtained by acid rather than enzymatic coagulation, a process that allows the precipitation of whey proteins without their enzymatic cleavage and preserves additional sialylated compounds, particularly when milk starter is added [
24]. Differences in heating temperature, acidification rate, and the proportion of whey and milk added may modulate the degree of whey protein denaturation and thus the retention or loss of sialylated compounds [
25]. This mechanism may help explain the differences in SIA content observed among cheese types.