Effect of Processing on Cow’s Milk Protein Microstructure and Peptide Profile After In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples
2.3. Proximate Analysis by Lactoscope
2.4. Particle Size Measurement by Dynamic Light Scattering
2.5. Free Thiol Group Content by Ellman’s Reagent
2.6. Milk Microstructure Evaluation by Confocal Laser Microscopy
2.7. In Vitro Digestion of Milk
2.8. Peptide Profile Analysis
2.9. Identification of Bioactive Peptides
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.2. Particle Size Analysis
3.3. Free Thiol Groups
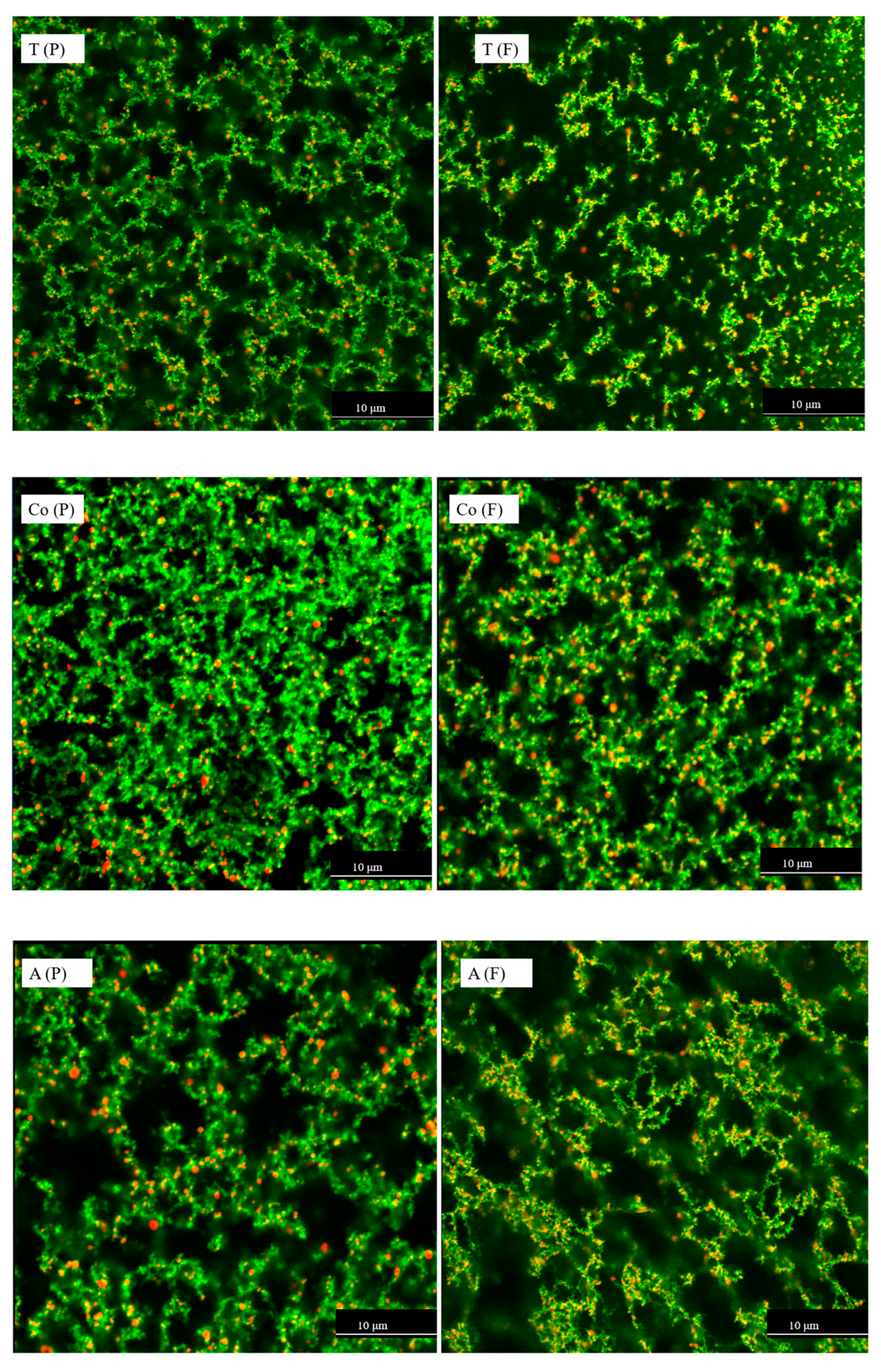
3.4. Milk Microstructure
3.5. Percentage of Protein Digestion
3.6. Peptides After the In Vitro Intestinal Stage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, L.; Tavares, G.M. Thermal treatments and emerging technologies: Impacts on the structure and techno-functional properties of milk proteins. Trends Food Sci. Technol. 2019, 90, 88–99. [Google Scholar] [CrossRef]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Papliński, R.; Kapłan, M.; Chawla, P. Influence of ultra-heat treatment on properties of milk proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.-D.A.; Kumar, S.; Bhat, H.F. Processing technologies for improved digestibility of milk proteins. Trends Food Sci. Technol. 2021, 118, 1–16. [Google Scholar] [CrossRef]
- van Lieshout, G.A.; Lambers, T.T.; Bragt, M.C.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef]
- Lopez, C.; Briard-Bion, V.; Ménard, O.; Beaucher, E.; Rousseau, F.; Fauquant, J.; Leconte, N.; Robert, B. Fat globules selected from whole milk according to their size: Different compositions and structure of the biomembrane, revealing sphingomyelin-rich domains. Food Chem. 2011, 125, 355–368. [Google Scholar] [CrossRef]
- Ding, M.; Huang, Z.; Jin, Z.; Zhou, C.; Wu, J.; Zhao, D.; Shan, K.; Ke, W.; Zhang, M.; Nian, Y. The effect of fat content in food matrix on the structure, rheological properties and digestive properties of protein. Food Hydrocoll. 2022, 126, 107464. [Google Scholar] [CrossRef]
- Iqbal, S.; Zhang, P.; Wu, P.; Ge, A.; Kirk, T.V.; Chen, X.D. Impact of fat content on the modulation of viscosity, microstructure and enzymatic hydrolysis of UHT milk during simulated gastrointestinal digestion. Int. J. Dairy Technol. 2024, 77, 59–70. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. J. Dairy Sci. 2017, 100, 36–47. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Egger, L. Impact of milk processing on the generation of peptides during digestion. Int. Dairy J. 2014, 35, 130–138. [Google Scholar] [CrossRef]
- Elwell, M.W.; Barbano, D.M. Use of microfiltration to improve fluid milk quality. J. Dairy Sci. 2006, 89, 20–30. [Google Scholar] [CrossRef]
- Cheryan, M. Membranes in food processing. In Effective Industrial Membrane Processes: Benefits and Opportunities; Springer: Berlin/Heidelberg, Germany, 1991; pp. 157–180. [Google Scholar]
- Maubois, J. Membrane microfiltration: A tool for a new approach in dairy technology. Aust. J. Dairy Technol. 2002, 57, 92. [Google Scholar]
- Dinkçi, N.; Sirbu, A. Quality assessment of extended shelf life (ESL) milk in comparison with other kinds of pasteurised milk commercially available on the market. Sci. Study Res.-Chem. Chem. Eng. Biotechnol. Food Ind. 2024, 25, 35–48. [Google Scholar]
- Wang, D.; Fritsch, J.; Moraru, C.I. Shelf life and quality of skim milk processed by cold microfiltration with a 1.4-μm pore size membrane, with or without heat treatment. J. Dairy Sci. 2019, 102, 8798–8806. [Google Scholar]
- Kelly, S.T.; Zydney, A.L. Protein fouling during microfiltration: Comparative behavior of different model proteins. Biotechnol. Bioeng. 1997, 55, 91–100. [Google Scholar] [PubMed]
- Owusu-Apenten, R. Colorimetric analysis of protein sulfhydyl groups in milk: Applications and processing effects. Crit. Rev. Food Sci. Nutr. 2005, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Interactions of whey proteins with milk fat globule membrane proteins during heat treatment of whole milk. Le Lait 2004, 84, 269–283. [Google Scholar]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar]
- Miciński, J.; Kowalski, I.M.; Zwierzchowski, G.; Szarek, J.; Pierożyński, B.; Zabłocka, E. Characteristics of cow’s milk proteins including allergenic properties and methods for its reduction. Pol. Ann. Med. 2013, 20, 69–76. [Google Scholar]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Conformational changes of β-lactoglobulin induced by shear, heat, and pH—Effects on antigenicity. J. Dairy Sci. 2015, 98, 4255–4265. [Google Scholar]
- Wilson, S.; Martinez-Villaluenga, C.; De Mejia, E. Purification, thermal stability, and antigenicity of the immunodominant soybean allergen P34 in soy cultivars, ingredients, and products. J. Food Sci. 2008, 73, T106–T114. [Google Scholar]
- Wu, X.; Lu, Y.; Xu, H.; Lin, D.; He, Z.; Wu, H.; Liu, L.; Wang, Z. Reducing the allergenic capacity of β-lactoglobulin by covalent conjugation with dietary polyphenols. Food Chem. 2018, 256, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Li, Q.; Zhu, D.; Chen, G.; Wu, L. Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: A review. Food Sci. Hum. Wellness 2024, 13, 1135–1151. [Google Scholar]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [PubMed]
- Argov, N.; Lemay, D.G.; German, J.B. Milk fat globule structure and function: Nanoscience comes to milk production. Trends Food Sci. Technol. 2008, 19, 617–623. [Google Scholar]
- Anderson, M.; Brooker, B. Loss of material during the isolation of milk fat globule membrane. J. Dairy Sci. 1975, 58, 1442–1448. [Google Scholar]
- Jhanwar, A.; Ward, R. Particle size distribution and lipid composition of skim milk lipid material. Int. Dairy J. 2014, 36, 110–117. [Google Scholar]
- Bellassi, P.; Cappa, F.; Fontana, A.; Morelli, L. Phenotypic and genotypic investigation of two representative strains of Microbacterium species isolated from micro-filtered milk: Growth capacity and spoilage-potential assessment. Front. Microbiol. 2020, 11, 554178. [Google Scholar]
- García, L.F.; Rodríguez, F.R. Combination of microfiltration and heat treatment for ESL milk production: Impact on shelf life. J. Food Eng. 2014, 128, 1–9. [Google Scholar]
- Hoffmann, W.; Kiesner, C.; Clawin-Rädecker, I.; Martin, D.; Einhoff, K.; Lorenzen, P.C.; Teufel, P. Processing of extended shelf life milk using microfiltration. Int. J. Dairy Technol. 2006, 59, 229–235. [Google Scholar]
- Michalski, M.-C.; Cariou, R.; Michel, F.; Garnier, C. Native vs. damaged milk fat globules: Membrane properties affect the viscoelasticity of milk gels. J. Dairy Sci. 2002, 85, 2451–2461. [Google Scholar] [CrossRef]
- Berton, A.; Rouvellac, S.; Robert, B.; Rousseau, F.; Lopez, C.; Crenon, I. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized milk fat globules. Food Hydrocoll. 2012, 29, 123–134. [Google Scholar]
- Mootse, H.; Pisponen, A.; Pajumägi, S.; Polikarpus, A.; Tatar, V.; Sats, A.; Poikalainen, V. Investigation of casein micelle particle size distribution in raw milk of Estonian Holstein dairy cows. Agron. Res. 2014, 12, 753–758. [Google Scholar]
- Guingamp, M.-F.; Humbert, G.; Linden, G. Determination of sulfhydryl groups in milk using Ellman’s procedure and clarifying reagent®. J. Dairy Sci. 1993, 76, 2152–2155. [Google Scholar]
- ThermoScientific. Ellman’s Reagen; ThermoScientific: Waltham, MA, USA, 2011. [Google Scholar]
- Gallier, S.; Gragson, D.; Jiménez-Flores, R.; David, E. Using confocal laser scanning microscopy to probe the milk fat globule membrane and associated proteins. J. Agric. Food Chem. 2010, 58, 4250–4257. [Google Scholar]
- Gallier, S.; Ye, A.; Singh, H. Structural changes of bovine milk fat globules during in vitro digestion. J. Dairy Sci. 2012, 95, 3579–3592. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, G.; Song, S.; Xu, X.; Voglmeir, J.; Liu, L.; Zhao, F.; Li, M.; Li, L.; Yu, X. Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish. Proteomics 2015, 15, 3688–3698. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry method for protein quantitation. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 7–10. [Google Scholar]
- Martínez-Maqueda, D.; Hernández-Ledesma, B.; Amigo, L.; Miralles, B.; Gómez-Ruiz, J.Á. Extraction/fractionation techniques for proteins and peptides and protein digestion. In Proteomics in Foods: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 21–50. [Google Scholar]
- Sinha, R.; Radha, C.; Prakash, J.; Kaul, P. Whey protein hydrolysate: Functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 2007, 101, 1484–1491. [Google Scholar]
- Nielsen, S.D.-H.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.-J.; Dallas, D.C. Bioactive milk peptides: An updated comprehensive overview and database. Crit. Rev. Food Sci. Nutr. 2024, 64, 11510–11529. [Google Scholar] [CrossRef]
- Lay, H.T.; Yeow, R.J.E.; Ma, Y.; Zydney, A.L.; Wang, R.; Chew, J.W. Internal membrane fouling by proteins during microfiltration. J. Membr. Sci. 2021, 637, 119589. [Google Scholar]
- Fox. The Major Constituents of Milk; Woodhead Publishing Limited: Sawston, UK, 2003. [Google Scholar]
- Han, T.; Wang, M.; Wang, Y.; Tang, L. Effects of high-pressure homogenization and ultrasonic treatment on the structure and characteristics of casein. LWT 2020, 130, 109560. [Google Scholar] [CrossRef]
- Loveday, S.M. Protein digestion and absorption: The influence of food processing. Nutr. Res. Rev. 2023, 36, 544–559. [Google Scholar] [CrossRef]
- Qi, P.X.; Ren, D.; Xiao, Y.; Tomasula, P.M. Effect of homogenization and pasteurization on the structure and stability of whey protein in milk. J. Dairy Sci. 2015, 98, 2884–2897. [Google Scholar] [PubMed]
- Zamora, A.; Ferragut, V.; Guamis, B.; Trujillo, A. Changes in the surface protein of the fat globules during ultra-high pressure homogenisation and conventional treatments of milk. Food Hydrocoll. 2012, 29, 135–143. [Google Scholar]
- Fox, F.; Uniacke-Lowe, T.; McSweeney, L.; O’Mahony, J. Heat-Induced Changes in Milk. In Dairy Chemistry and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 345–375. [Google Scholar]
- Augustin, M.A.; Udabage, P. Influence of processing on functionality of milk and dairy proteins. Adv. Food Nutr. Res. 2007, 53, 1–38. [Google Scholar] [PubMed]
- Olson, D.; White, C.; Richter, R. Effect of pressure and fat content on particle sizes in microfluidized milk. J. Dairy Sci. 2004, 87, 3217–3223. [Google Scholar] [PubMed]
- Anema, S.G.; Lowe, E.K.; Stockmann, R. Particle size changes and casein solubilisation in high-pressure-treated skim milk. Food Hydrocoll. 2005, 19, 257–267. [Google Scholar] [CrossRef]
- Cosio, M.; Mannino, S.; Buratti, S. Electrochemical sensor detecting free sulfhydryl groups: Evaluation of milk heat treatment. J. Dairy Sci. 2000, 83, 1933–1938. [Google Scholar]
- Pofahl, T.; Vakaleris, D. Effects of heat on sulfhydryl and disulfide groups of milk proteins as measured by the spectrofluorometric method. J. Dairy Sci. 1968, 51, 1345–1348. [Google Scholar]
- Wijesinha-Bettoni, R.; Gao, C.; Jenkins, J.A.; Mackie, A.R.; Wilde, P.J.; Mills, E.C.; Smith, L.J. Heat treatment of bovine α-lactalbumin results in partially folded, disulfide bond shuffled states with enhanced surface activity. Biochemistry 2007, 46, 9774–9784. [Google Scholar]
- Verruck, S.; Sartor, S.; Marenda, F.B.; da Silva Barros, E.L.; Camelo-Silva, C.; Canella, M.M.; Prudencio, E.S. Influence of heat treatment and microfiltration on the milk proteins properties. Adv. Food Technol. Nutr. Sci. 2019, 5, 54–66. [Google Scholar]
- Hansen, S.F.; Nielsen, S.D.; Rasmusen, J.T.; Larsen, L.B.; Wiking, L. Disulfide bond formation is not crucial for the heat-induced interaction between β-lactoglobulin and milk fat globule membrane proteins. J. Dairy Sci. 2020, 103, 5874–5881. [Google Scholar] [PubMed]
- Alzagtat, A.A.; Alli, I. Protein-lipid interactions in food systems: A review. Int. J. Food Sci. Nutr. 2002, 53, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, S.; Sun, N. How does food matrix components affect food allergies, food allergens and the detection of food allergens? A systematic review. Trends Food Sci. Technol. 2022, 127, 280–290. [Google Scholar]
- Monaci, L.; Tregoat, V.; van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar]
- Capuano, E.; Janssen, A.E. Food matrix and macronutrient digestion. Annu. Rev. Food Sci. Technol. 2021, 12, 193–212. [Google Scholar] [PubMed]
- Egger, L.; Ménard, O. Update on bioactive peptides after milk and cheese digestion. Curr. Opin. Food Sci. 2017, 14, 116–121. [Google Scholar]
- Cui, Q.; Zhang, Z.; Li, M.; Zhou, M.; Sun, X. Peptide profiles and allergy-reactivity of extensive hydrolysates of milk protein. Food Chem. 2023, 411, 135544. [Google Scholar]
- Graversen, K.B.; Larsen, J.M.; Pedersen, S.S.; Sørensen, L.V.; Christoffersen, H.F.; Jacobsen, L.N.; Halken, S.; Licht, T.R.; Bahl, M.I.; Bøgh, K.L. Partially hydrolysed whey has superior allergy preventive capacity compared to intact whey regardless of amoxicillin administration in brown Norway rats. Front. Immunol. 2021, 12, 705543. [Google Scholar] [CrossRef]
- Huang, M.; Tan, H.; Xiong, Z.; Hu, W.; Wu, Y.; Meng, X.; Chen, H.; Li, X. Allergenicity evaluation of peptides from milk and yogurt after gastrointestinal digestion based on epitopes. Food Funct. 2022, 13, 10769–10789. [Google Scholar]
- Van Hekken, D.; Tunick, M.; Ren, D.; Tomasula, P. Comparing the effect of homogenization and heat processing on the properties and in vitro digestion of milk from organic and conventional dairy herds. J. Dairy Sci. 2017, 100, 6042–6052. [Google Scholar]
- Xu, Y.; Zhang, F.; Mu, G.; Zhu, X. Effect of lactic acid bacteria fermentation on cow milk allergenicity and antigenicity: A review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13257. [Google Scholar] [PubMed]
- Fan, S.; Ma, J.; Liu, Z.; Ning, Y.; Cao, M.; Li, Q.; Zhang, Y. Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard. Food Sci. Hum. Wellness 2023, 12, 728–736. [Google Scholar]
- Villa, C.; Costa, J.; Oliveira, M.B.P.; Mafra, I. Bovine milk allergens: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [PubMed]




| Sample Code | Brand | Process | Label Information (g/100 mL) | ||
|---|---|---|---|---|---|
| Fat | Protein | Sugar | |||
| A | Asda | F | 1.8 | 3.6 | 4.8 |
| P | 1.8 | 3.6 | 4.8 | ||
| Co | Co-op | F | 1.8 | 3.6 | 4.8 |
| P | 1.8 | 3.4 | 5.0 | ||
| DM | Dairy Manor/Aldi | F | 1.8 | 3.6 | 4.8 |
| P | 1.7 | 3.5 | 4.7 | ||
| CB | Cow Belle/Lidl | F | 1.8 | 3.6 | 4.8 |
| P | 1.8 | 3.6 | 4.8 | ||
| T | Tesco | F | 1.8 | 3.3 | 4.9 |
| P | 1.8 | 3.6 | 4.8 | ||
| S | Sainsbury’s | F | 1.6 | 3.1 | 4.9 |
| P | 1.8 | 3.6 | 4.8 | ||
| W | Waitrose | F | 1.6 | 3.3 | 4.9 |
| P | 1.8 | 3.6 | 4.8 | ||
| Samples | Process | pH | (g/100 mL Milk) | ||
|---|---|---|---|---|---|
| Protein | Lactose | Solids | |||
| A | F | 6.77 ± 0.04 | 3.44 ± 0.18 | 4.86 ± 0.25 | 10.50 ± 0.25 |
| P | 6.78 ± 0.05 | 3.53 ± 0.27 | 4.84 ± 0.36 | 10.64 ± 0.36 | |
| Co | F | 6.73 ± 0.04 | 3.41 ± 0.25 | 4.91 ± 0.47 | 10.70 ± 0.47 |
| P | 6.76 ± 0.03 | 3.55 ± 0.36 | 4.88 ± 0.36 | 10.77 ± 0.53 | |
| DM | F | 6.75 ± 0.06 | 3.45 ± 0.34 | 4.94 ± 0.29 | 10.50 ± 0.22 |
| P | 6.76 ± 0.04 | 3.61 ± 0.27 | 4.87 ± 0.47 | 10.76 ± 0.39 | |
| CB | F | 6.72 ± 0.03 | 3.33± 0.33 | 4.89 ±0.54 | 10.42± 0.31 |
| P | 6.73 ± 0.04 | 3.73 ± 0.54 | 4.87 ± 0.38 | 10.77 ± 0.59 | |
| T | F | 6.77 ± 0.05 | 3.43 ± 0.38 | 4.92 ± 0.26 | 10.59 ± 0.29 |
| P | 6.76 ± 0.06 | 3.47 ± 0.27 | 4.89 ± 0.34 | 10.66 ± 0.37 | |
| S | F | 6.75 ± 0.03 | 3.31 ± 0.28 | 4.90 ± 0.47 | 10.59 ± 0.34 |
| P | 6.78 ± 0.05 | 3.53 ± 0.45 | 4.98 ± 0.55 | 10.79 ± 0.47 | |
| W | F | 6.76 ± 0.04 | 3.43 ± 0.36 | 4.88 ± 0.38 | 10.71 ± 0.21 |
| P | 6.77 ± 0.06 | 3.57 ± 0.28 | 4.91 ± 0.25 | 10.84 ± 0.41 | |
| Samples | Process | Fat Content (g/100 mL) | Percentage of Fat Retention (1) | |
|---|---|---|---|---|
| Semi-Skimmed * | After Centrifugation ** (1) | |||
| A | F | 1.51 ± 0.08 | 1.22 ± 0.05 | 19.21 ± 2.1 |
| P | 1.52 ± 0.07 | 1.07 ± 0.06 | 29.60 ± 3.2 | |
| Co | F | 1.54 ± 0.05 | 1.03 ± 0.07 | 32.57 ± 3.0 |
| P | 1.48 ± 0.06 | 0.69 ± 0.06 | 52.91 ± 4.4 | |
| DM | F | 1.45 ± 0.04 | 1.12 ± 0.09 | 23.28 ± 2.2 |
| P | 1.41 ± 0.07 | 1.03 ± 0.07 | 26.59 ± 2.9 | |
| CB | F | 1.45 ± 0.03 | 1.03 ± 0.04 | 28.57 ± 3.1 |
| P | 1.44 ± 0.04 | 0.72 ± 0.08 | 49.84 ± 4.5 | |
| T | F | 1.45 ± 0.08 | 1.19 ± 0.06 | 17.59 ± 1.9 |
| P | 1.42 ± 0.07 | 0.87 ± 0.04 | 38.66 ± 3.3 | |
| S | F | 1.45 ± 0.08 | 1.06 ± 0.07 | 27.02 ± 2.8 |
| P | 1.48 ± 0.05 | 0.66 ± 0.05 | 55.23 ± 3.9 | |
| W | F | 1.49 ± 0.06 | 1.14 ± 0.08 | 23.15 ± 2.0 |
| P | 1.45 ± 0.08 | 0.77 ± 0.05 | 46.81 ± 4.3 | |
| Milk Brand | Process | Z-Average (nm) | Free Thiol (μM) |
|---|---|---|---|
| A | F | 196 ± 10.2 | 0.91 ± 0.10 |
| P | 185 ± 9.9 | 1.31 ± 0.15 | |
| Co | F | 188 ± 11.5 | 1.01 ± 0.11 |
| P | 168 ± 8.7 | 1.29 ± 0.09 | |
| DM | F | 194 ± 9.2 | 0.81 ± 0.07 |
| P | 167 ± 11.2 | 1.15 ± 0.12 | |
| CB | F | 197 ± 12.3 | 0.88 ± 0.10 |
| P | 169 ± 9.1 | 1.20 ± 0.18 | |
| T | F | 198 ± 14.1 | 1.00 ± 0.11 |
| P | 159 ± 11.5 | 1.22 ± 0.98 | |
| S | F | 189 ± 9.5 | 0.95 ± 0.08 |
| P | 165 ± 15.2 | 1.24 ± 0.20 | |
| W | F | 195 ± 13.9 | 0.93 ± 0.10 |
| P | 172 ± 10.1 | 1.15 ± 0.11 |
| Milk Brand | Process | % Dig After Gastric Stage | % Dig After Intestinal Stage |
|---|---|---|---|
| A | F | 39.1 ± 5.1 | 85.2 ± 6.3 |
| P | 43.9 ± 6.5 | 86.8 ± 5.5 | |
| Co | F | 26.2 ± 3.2 | 84.5 ± 5.0 |
| P | 26.5 ± 2.0 | 85.0 ± 6.1 | |
| DM | F | 32.5 ± 6.1 | 83.3 ± 4.3 |
| P | 27.3 ± 5.9 | 84.6 ± 5.7 | |
| CB | F | 31.2 ± 5.2 * | 84.1 ± 5.2 |
| P | 23.1 ± 5.6 | 82.6 ± 4.1 | |
| T | F | 32.9 ± 5.5 * | 86.3 ± 4.5 |
| P | 21.1 ± 4.7 | 87.2 ± 3.1 | |
| S | F | 15.1± 4.3 * | 84.2 ± 5.6 |
| P | 29.5 ± 6.3 | 85.7 ± 4.4 | |
| W | F | 29.4 ± 3.3 | 83.4 ± 3.7 |
| P | 32.1 ± 4.5 | 82.6 ± 4.2 |
| Peptide | Milk Sample | Bioactivity of the Peptide | |||||
|---|---|---|---|---|---|---|---|
| F | P | ACE-Inhibitory | Antimicrobial | DPP-IV Inhibitory | Antioxidant | IgE-Binding | |
| AMKPW | √ | √ | √ | √ | |||
| AYFYPE | √ | √ | √ | √ | |||
| DVENLHLPLPL | √ | √ | √ | ||||
| EMPFPK | √ | √ | √ | √ | |||
| EQLTK | √ | √ | √ | ||||
| FFVAP | √ | √ | √ | ||||
| FVAPFPEVFG | √ | √ | √ | ||||
| FYPEL | √ | √ | √ | √ | |||
| GLDIQK | √ | √ | √ | √ | |||
| GVSLPEW | √ | √ | √ | √ | |||
| HLPLP | √ | √ | |||||
| IPAV | √ | ||||||
| IVP | √ | √ | √ | ||||
| LHLPLP | √ | √ | √ | ||||
| LIVTQTMK | √ | √ | |||||
| LNVPGEIVE | √ | √ | √ | ||||
| LPQ | √ | √ | |||||
| LVYPFPGP | √ | √ | √ | ||||
| LVYPFPGPI | √ | √ | √ | √ | |||
| MPFPKYPVEP | √ | √ | |||||
| NVPGEIVESL | √ | √ | √ | ||||
| PIVLNP | √ | √ | |||||
| PMHIR | √ | √ | |||||
| PVVVPPFLQPE | √ | √ | √ | √ | |||
| RELEEL | √ | √ | √ | √ | |||
| SDIPNPIGSENSEK | √ | √ | √ | √ | |||
| TEDELQDKIHPF | √ | √ | √ | ||||
| TPEVDDEALEK | √ | √ | √ | √ | |||
| TTMPLW | √ | √ | √ | √ | |||
| VLDTDY | √ | √ | √ | √ | |||
| VLPVPQ | √ | √ | |||||
| VPSERYL | √ | √ | √ | √ | |||
| VSLPEW | √ | √ | √ | ||||
| VYPFPGPI | √ | √ | √ | ||||
| VYPFPGPIPN | √ | √ | √ | √ | √ | ||
| YFYPEL | √ | √ | √ | √ | |||
| YPFPGPIP | √ | √ | √ | √ | |||
| YPVEPF | √ | √ | √ | √ | √ | ||
| YQEPVLGPVRGPFPI | √ | √ | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buatig, R.; Clegg, M.E.; Michael, N.; Oruna-Concha, M.-J. Effect of Processing on Cow’s Milk Protein Microstructure and Peptide Profile After In Vitro Gastrointestinal Digestion. Dairy 2025, 6, 15. https://doi.org/10.3390/dairy6020015
Buatig R, Clegg ME, Michael N, Oruna-Concha M-J. Effect of Processing on Cow’s Milk Protein Microstructure and Peptide Profile After In Vitro Gastrointestinal Digestion. Dairy. 2025; 6(2):15. https://doi.org/10.3390/dairy6020015
Chicago/Turabian StyleBuatig, Raja, Miriam E. Clegg, Nicholas Michael, and Maria-Jose Oruna-Concha. 2025. "Effect of Processing on Cow’s Milk Protein Microstructure and Peptide Profile After In Vitro Gastrointestinal Digestion" Dairy 6, no. 2: 15. https://doi.org/10.3390/dairy6020015
APA StyleBuatig, R., Clegg, M. E., Michael, N., & Oruna-Concha, M.-J. (2025). Effect of Processing on Cow’s Milk Protein Microstructure and Peptide Profile After In Vitro Gastrointestinal Digestion. Dairy, 6(2), 15. https://doi.org/10.3390/dairy6020015







