In Vitro Activity and Atom Pair Fingerprint Analysis of Potent Hits from Malaria Box against Staphylococcus aureus Isolated from Cows with Clinical Mastitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sampling and Isolation
2.3. Molecular Characterization of S. aureus
2.4. Antimicrobial Susceptibility Assay
2.5. Structural Similarity Measurements
2.6. Microdilution Assays and Minimum Inhibitory Concentration (MIC)
2.7. In Vitro Drug Combination Test
2.8. Determination of Fractional Inhibitory Concentration (FIC)
2.9. Statistical Analysis
3. Results
3.1. Detection of S. aureus
3.2. MMV665941, a Potent Hit against S. aureus
3.3. Bioinformatic and Combination Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhandari, V.; Chakraborty, S.; Brahma, U.; Sharma, P. Identification of Anti-staphylococcal and Anti-biofilm Compounds by Repurposing the Medicines for Malaria Venture Pathogen Box. Front. Cell Infect. Microbiol. 2018, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Bihon, A.; Syoum, A.; Assefa, A. Assessment of risk factors and isolation of Staphylococcus aureus and Escherichia coli from bovine subclinical mastitic milk in and around Gondar, Northwest Ethiopia. Trop. Anim. Health Prod. 2019, 51, 939–948. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.P.; Watts, J.L.; Salmon, S.A.; Aarestrup, F.M. Antimicrobial susceptibility of Staphylococcus aureus isolated from bovine mastitis in Europe and the United States. J. Dairy Sci. 2000, 83, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Liao, G.; Wu, Z.; Lv, J.; Chen, W. Prevalence and characterization of Staphylococcus aureus isolates from subclinical bovine mastitis in southern Xinjiang, China. J. Dairy Sci. 2020, 103, 3368–3380. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; Baghdadi, H.B.; El-Sayed, S.A.E.S.; Eltaysh, R.; Igarashi, I. Repurposing of the Malaria Box for Babesia microti in mice identifies novel active scaffolds against piroplasmosis. Parasites Vectors 2022, 15, 329. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.; Alkhoudary, M.S.; Alsharif, K.F.; Abdel-Daim, M.M.; Igarashi, I. Compounds from the Medicines for Malaria Venture Box Inhibit In Vitro Growth of Babesia divergens, a Blood-Borne Parasite of Veterinary and Zoonotic Importance. Molecules 2021, 26, 7118. [Google Scholar] [CrossRef]
- Matyi, S.A.; Dupre, J.M.; Johnson, W.L.; Hoyt, P.R.; White, D.G.; Brody, T.; Odenwald, W.F.; Gustafson, J.E. Isolation and characterization of Staphylococcus aureus strains from a Paso del Norte dairy. J. Dairy Sci. 2013, 96, 3535–3542. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Adams, J.H.; Adelfio, R.; Ahyong, V.; Akabas, M.H.; Alano, P.; Alday, A.; Alemán Resto, Y.; Alsibaee, A.; Alzualde, A.; et al. Open Source Drug Discovery with the Malaria Box Compound Collection for Neglected Diseases and Beyond. PLoS Pathog. 2016, 12, e1005763. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature protocols 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Algharib, S.A.; Dawood, A.; Xie, S. Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.; Igarashi, I. In vivo activity and atom pair fingerprint analysis of MMV665941 against the apicomplexan parasite Babesia microti, the causative agent of babesiosis in humans and rodents. Pathog. Glob. Health 2023, 117, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Backman, T.W.; Cao, Y.; Girke, T. ChemMine tools: An online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011, 39, W486–W491. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.; Salman, D.; Marghani, B.H.; Gadalla, H.E.; Sayed-Ahmed, M.Z. Immunomodulatory Effect of Vitamin C on Proinflammatory Cytokines Production in Ossimi Lambs (Ovis aries) with Pneumonic Pasteurellosis. Animals 2021, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Charisi, A.; Cheng, L.C.; Jiang, T.; Girke, T. ChemmineR: A compound mining framework for R. Bioinformatics 2008, 24, 1733–1734. [Google Scholar] [CrossRef] [PubMed]
- Suleman, N.; Kalhapure, R.S.; Mocktar, C.; Rambharose, S.; Singh, M.; Govender, T. Silver salts of carboxylic acid terminated generation 1 poly (propyl ether imine)(PETIM) dendron and dendrimers as antimicrobial agents against S. aureus and MRSA. RSC Adv. 2015, 5, 34967–34978. [Google Scholar] [CrossRef]
- Rishton, G.M. Reactive compounds and in vitro false positives in HTS. Drug Discov. Today 1997, 2, 382–384. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Spangenberg, L.; Romppel, M.; Bormann, B.; Hofmeister, D.; Brähler, E.; Strauß, B. Psychometrische Überprüfung einer Kurzform des Narcissistic Personality Inventory (NPI-15): Dimensionalität und psychometrische Eigenschaften des NPI-15 in einer repräsentativen Bevölkerungsstichprobe. Psychother. Psychosom. Med. Psychol. 2013, 63, 341–347. [Google Scholar] [CrossRef]
- Gamo, F.J.; Sanz, L.M.; Vidal, J.; de Cozar, C.; Alvarez, E.; Lavandera, J.L.; Vanderwall, D.E.; Green, D.V.; Kumar, V.; Hasan, S.; et al. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar] [CrossRef]
- Guiguemde, W.A.; Shelat, A.A.; Bouck, D.; Duffy, S.; Crowther, G.J.; Davis, P.H.; Smithson, D.C.; Connelly, M.; Clark, J.; Zhu, F.; et al. Chemical genetics of Plasmodium falciparum. Nature 2010, 465, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Meister, S.; Plouffe, D.M.; Kuhen, K.L.; Bonamy, G.M.; Wu, T.; Barnes, S.W.; Bopp, S.E.; Borboa, R.; Bright, A.T.; Che, J.; et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 2011, 334, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Boerlin, P.; Kuhnert, P.; Hussy, D.; Schaellibaum, M. Methods for identification of Staphylococcus aureus isolates in cases of bovine mastitis. J. Clin. Microbiol. 2003, 41, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Brakstad, O.G.; Maeland, J.A.; Tveten, Y. Multiplex polymerase chain reaction for detection of genes for Staphylococcus aureus thermonuclease and methicillin resistance and correlation with oxacillin resistance. APMIS 1993, 101, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Abd-Elghany, S.M.; Elhadidy, M.; Tamura, T. Molecular Characterization and Antimicrobial Resistance Profile of Methicillin-Resistant Staphylococcus aureus in Retail Chicken. J. Food Prot. 2015, 78, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.; El-Khodery, S.; Yokoyama, N.; Igarashi, I. Discovering the in vitro potent inhibitors against Babesia and Theileria parasites by repurposing the Malaria Box: A review. Vet. Parasitol. 2019, 274, 108895. [Google Scholar] [CrossRef]
- Bhat, A.M.; Soodan, J.S.; Singh, R.; Dhobi, I.A.; Hussain, T.; Dar, M.Y.; Mir, M. Incidence of bovine clinical mastitis in Jammu region and antibiogram of isolated pathogens. Vet. World 2017, 10, 984–989. [Google Scholar] [CrossRef]
- Lone, A.M.; Rather, M.A.; Bhat, M.A.; Bhat, Z.S.; Tantry, I.Q.; Prakash, P. Synthesis and in vitro evaluation of 2-(((2-ether)amino)methylene)-dimedone derivatives as potential antimicrobial agents. Microb. Pathog. 2018, 114, 431–435. [Google Scholar] [CrossRef]
- Alemán Resto, Y.; Fernández Robledo, J.A. Identification of MMV Malaria Box inhibitors of Perkinsus marinus using an ATP-based bioluminescence assay. PLoS ONE 2014, 9, e111051. [Google Scholar] [CrossRef] [PubMed]
- Bilsland, E.; Bean, D.M.; Devaney, E.; Oliver, S.G. Yeast-Based High-Throughput Screens to Identify Novel Compounds Active against Brugia malayi. PLoS Neglected Trop. Dis. 2016, 10, e0004401. [Google Scholar] [CrossRef] [PubMed]
- Bessoff, K.; Spangenberg, T.; Foderaro, J.E.; Jumani, R.S.; Ward, G.E.; Huston, C.D. Identification of Cryptosporidium parvum active chemical series by Repurposing the open access malaria box. Antimicrob. Agents Chemother. 2014, 58, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.L.; Avery, V.M. Development and application of a sensitive, phenotypic, high-throughput image-based assay to identify compound activity against Trypanosoma cruzi amastigotes. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Jesudoss Chelladurai, J.R.J.; Bader, C.; Carreiro, E.; Long, K.; Thompson, K.; Brewer, M.T. Repurposing the open access malaria box reveals compounds with activity against Tritrichomonas foetus trophozoites. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 89–93. [Google Scholar] [CrossRef]
- Maley, A.M.; Arbiser, J.L. Gentian violet: A 19th century drug re-emerges in the 21st century. Exp. Dermatol. 2013, 22, 775–780. [Google Scholar] [CrossRef]
- Davis, J.L.; Smith, G.W.; Baynes, R.E.; Tell, L.A.; Webb, A.I.; Riviere, J.E. Update on drugs prohibited from extralabel use in food animals. J. Am. Vet. Med. Assoc. 2009, 235, 528–534. [Google Scholar] [CrossRef]
- Randhawa, M.A. Calculation of LD50 values from the method of Miller and Tainter, 1944. J. Ayub Med. Coll. Abbottabad 2009, 21, 184–185. [Google Scholar] [PubMed]
- El-Sayed, S.A.E.; Rizk, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro and in vivo inhibitory effect of thymoquinone on piroplasm parasites. Parasites Vectors 2019, 12, 37. [Google Scholar] [CrossRef]
- Ingram-Sieber, K.; Cowan, N.; Panic, G.; Vargas, M.; Mansour, N.R.; Bickle, Q.D.; Wells, T.N.; Spangenberg, T.; Keiser, J. Orally active antischistosomal early leads identified from the open access malaria box. PLoS Negl. Trop. Dis. 2014, 8, e2610. [Google Scholar] [CrossRef]

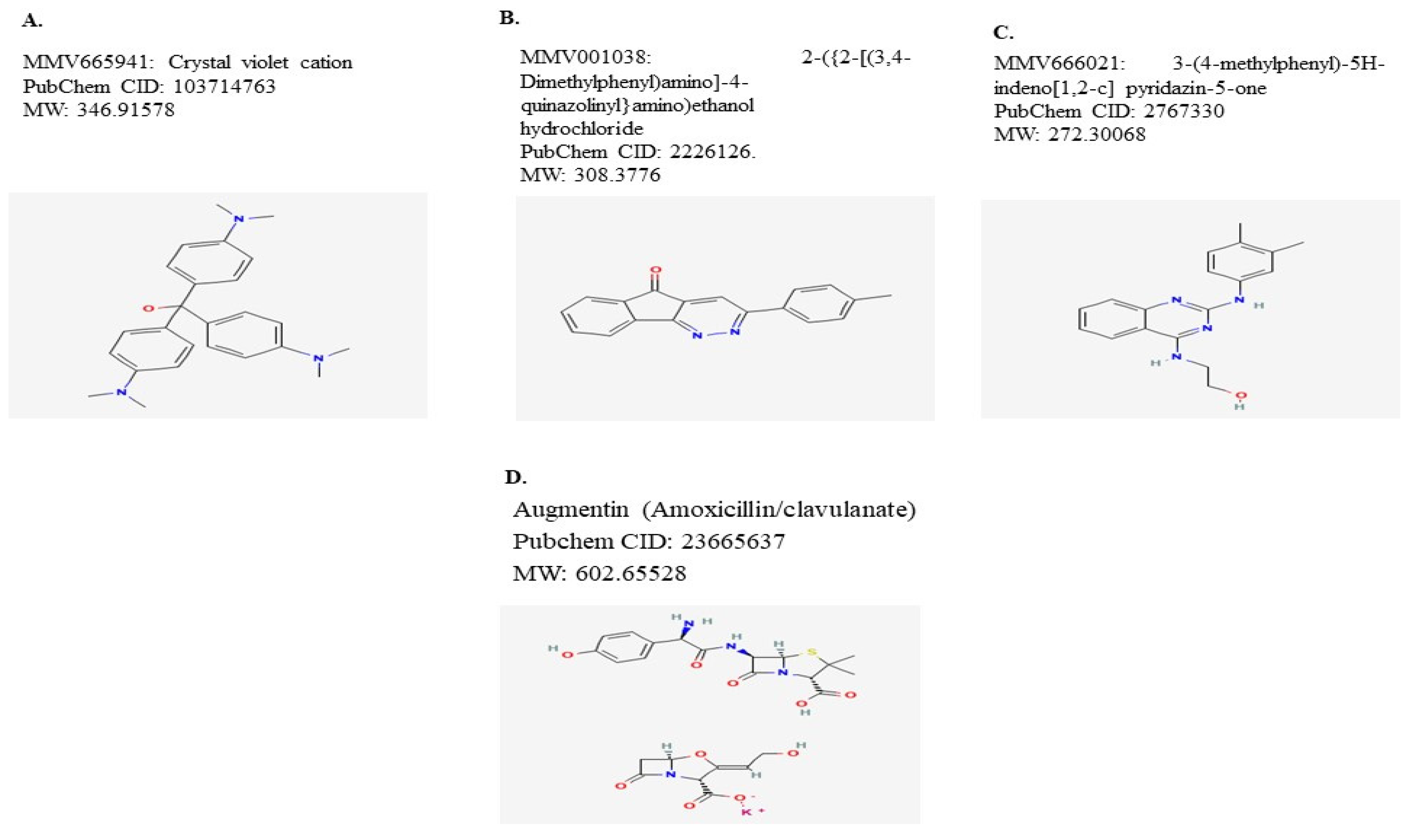
| Compound No. | Compound ID a | Set * | MW * (g/mol) | AlogP * | MIC b |
|---|---|---|---|---|---|
| A-2 | MMV665915 | Drug-like | 420.47 | 3.27 | 0.50 |
| A-3 | MMV666023 | Probe-like | 453.53 | 8.50 | 0.25 |
| A-4 | MMV665876 | Drug-like | 326.43 | 4.29 | 0.50 |
| A-5 | MMV019406 | Probe-like | 367.52 | 4.76 | 0.25 |
| A-6 | MMV019871 | Drug-like | 329.37 | 2.42 | 0.25 |
| A-7 | MMV666596 | Probe-like | 468.09 | 8.66 | 0.5 |
| A-8 | MMV006309 | Probe-like | 292.33 | 3.77 | 0.25 |
| A-9 | MMV000642 | Probe-like | 468.99 | 5.09 | 0.25 |
| A-10 | MMV396672 | Drug-like | 396.46 | 4.77 | 0.25 |
| A-11 | MMV001038 | Drug-like | 308.37 | 3.13 | 0.125 |
| B-2 | MMV665916 | Drug-like | 353.37 | 1.65 | 0.25 |
| B-3 | MMV666101 | Probe-like | 631.71 | 6.99 | 0.25 |
| B-4 | MMV665841 | Probe-like | 273.32 | 3.13 | 0.25 |
| B-5 | MMV007695 | Probe-like | 657.75 | 7.24 | 0.25 |
| B-6 | MMV020788 | Probe-like | 271.39 | 3.60 | 0.25 |
| B-7 | MMV009063 | Drug-like | 322.44 | 4.48 | 0.50 |
| B-8 | MMV007384 | Probe-like | 460.52 | 6.65 | 0.25 |
| B-9 | MMV006429 | Drug-like | 409.50 | 3.58 | 0.25 |
| B-10 | MMV007116 | Drug-like | 245.27 | 2.92 | 0.25 |
| B-11 | MMV396703 | Drug-like | 317.81 | 3.35 | 0.25 |
| Compound No. | Compound ID a | Set * | MW * (g/mol) | AlogP * | MIC b |
|---|---|---|---|---|---|
| C-2 | MMV665785 | Probe-like | 261.40 | 4.38 | 0.25 |
| C-3 | MMV665805 | Drug-like | 367.39 | 4.06 | 0.25 |
| C-4 | MMV020492 | Drug-like | 309.81 | 3.50 | 0.25 |
| C-5 | MMV006937 | Drug-like | 279.33 | 4.35 | 0.25 |
| C-6 | MMV666693 | Drug-like | 309.31 | 3.58 | 0.25 |
| C-7 | MMV007686 | Probe-like | 334.38 | 3.51 | 0.25 |
| C-8 | MMV019066 | Drug-like | 359.39 | 1.60 | 0.25 |
| C-9 | MMV000662 | Drug-like | 434.55 | 4.43 | 0.25 |
| C-10 | MMV007839 | Drug-like | 312.18 | 2.61 | 0.25 |
| C-11 | MMV396680 | Probe-like | 476.90 | 5.66 | 0.50 |
| D-2 | MMV665782 | Drug-like | 324.41 | 2.62 | 0.25 |
| D-3 | MMV665874 | Drug-like | 341.71 | 4.81 | 0.25 |
| D-4 | MMV011567 | Drug-like | 389.78 | 3.52 | 0.25 |
| D-5 | MMV006278 | Drug-like | 200.27 | 3.11 | 0.25 |
| D-6 | MMV085583 | Probe-like | 592.64 | 7.34 | 0.25 |
| D-7 | MMV020885 | Probe-like | 510.42 | 6.83 | 0.25 |
| D-8 | MMV006457 | Probe-like | 293.31 | 3.93 | 0.25 |
| D-9 | MMV020549 | Drug-like | 398.50 | 4.63 | 0.25 |
| D-10 | MMV011259 | Drug-like | 321.30 | 4.63 | 0.50 |
| D-11 | MMV396679 | Probe-like | 492.90 | 5.16 | 0.50 |
| Compound No. | Compound ID a | Set * | MW * (g/mol) | AlogP * | MIC b |
|---|---|---|---|---|---|
| E-2 | MMV665941 | Probe-like | 389.53 | 4.76 | 0.0078 |
| E-3 | MMV665878 | Drug-like | 367.39 | 2.72 | 0.25 |
| E-4 | MMV000448 | Probe-like | 279.37 | 3.36 | 0.25 |
| E-5 | MMV666691 | Probe-like | 340.39 | 3.75 | 0.25 |
| E-6 | MMV006455 | Drug-like | 370.48 | 3.97 | 0.25 |
| E-7 | MMV666600 | Probe-like | 459.92 | 6.82 | 0.25 |
| E-8 | MMV006558 | Probe-like | 287.78 | 4.91 | 0.5 |
| E-9 | MMV020548 | Drug-like | 413.51 | 3.77 | 0.5 |
| E-10 | MMV011256 | Drug-like | 327.69 | 4.32 | 0.5 |
| E-11 | MMV396678 | Probe-like | 462.87 | 5.18 | 0.25 |
| F-2 | MMV666021 | Probe-like | 272.30 | 4.17 | 0.125 |
| F-3 | MMV665820 | Drug-like | 293.53 | 3.13 | 0.25 |
| F-4 | MMV085203 | Probe-like | 362.42 | 3.72 | 0.50 |
| F-5 | MMV008956 | Drug-like | 377.86 | 4.13 | 0.50 |
| F-6 | MMV007907 | Drug-like | 267.34 | 3.45 | 0.25 |
| F-7 | MMV666601 | Probe-like | 637.71 | 7.03 | 0.25 |
| F-8 | MMV000570 | Probe-like | 278.34 | 5.29 | 0.25 |
| F-9 | MMV001246 | Drug-like | 327.42 | 3.38 | 0.25 |
| F-10 | MMV396797 | Drug-like | 309.32 | 1.54 | 0.50 |
| F-11 | MMV006087 | Drug-like | 277.79 | 3.33 | 0.50 |
| Compound No. | Compound ID a | Set * | MW * (g/mol) | AlogP * | MIC b |
|---|---|---|---|---|---|
| G-2 | MMV666062 | Probe-like | 571.66 | 7.03 | 0.25 |
| G-3 | MMV665827 | Probe-like | 346.39 | 3.36 | 0.25 |
| G-4 | MMV666688 | Probe-like | 476.03 | 7.22 | 0.25 |
| G-5 | MMV008416 | Probe-like | 228.33 | 4.02 | 0.25 |
| G-6 | MMV006172 | Probe-like | 368.47 | 5.02 | 0.25 |
| G-7 | MMV007160 | Probe-like | 621.76 | 8.04 | 0.25 |
| G-8 | MMV006427 | Drug-like | 463.95 | 4.49 | 0.25 |
| G-9 | MMV019258 | Drug-like | 397.49 | 4.16 | 0.25 |
| G-10 | MMV011099 | Drug-like | 275.30 | 3.52 | 0.25 |
| G-11 | MMV019110 | Drug-like | 250.29 | 3.10 | 0.25 |
| H-2 | MMV665977 | Probe-like | 456.50 | 4.32 | 0.25 |
| H-3 | MMV665831 | Probe-like | 426.52 | 4.33 | 0.25 |
| H-4 | MMV006203 | Probe-like | 319.44 | 4.07 | 0.25 |
| H-5 | MMV020500 | Drug-like | 249.73 | 2.15 | 0.25 |
| H-6 | MMV006861 | Probe-like | 355.43 | 4.32 | 0.25 |
| H-7 | MMV008294 | Probe-like | 380.43 | 4.71 | 0.50 |
| H-8 | MMV020439 | Drug-like | 459.60 | 4.45 | 0.25 |
| H-9 | MMV666607 | Probe-like | 281.26 | 2.76 | 0.50 |
| H-10 | MMV008138 | Drug-like | 361.22 | 1.77 | 0.50 |
| H-11 | MMV396693 | Probe-like | 254.30 | 2.81 | 0.50 |
| Compounds | AP Tanimoto | MCS Tanimoto | MCS Size | MCS Min | MCS Max | Chemical Structure |
|---|---|---|---|---|---|---|
| MMV001038 + Augmentin | 0.121951 | 0.1250 | 7 | 0.3043 | 0.1750 |  |
| MMV666021 + Augmentin | 0.0987203 | 0.1296 | 7 | 0.3333 | 0.1750 |  |
| MMV665941 + Augmentin | 0.0855148 | 0.1481 | 8 | 0.3636 | 0.2000 |  |
| MMV665941 + MMV666021 | 0.0450237 | 0.1316 | 5 | 0.2381 | 0.2273 | 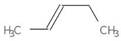 |
| MMV665941 + MMV001038 | 0.102506 | 0.1250 | 5 | 0.2273 | 0.2174 | 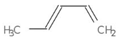 |
| MMV666021 + MMV001038 | 0.357771 | 0.2222 | 8 | 0.3810 | 0.3478 | 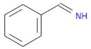 |
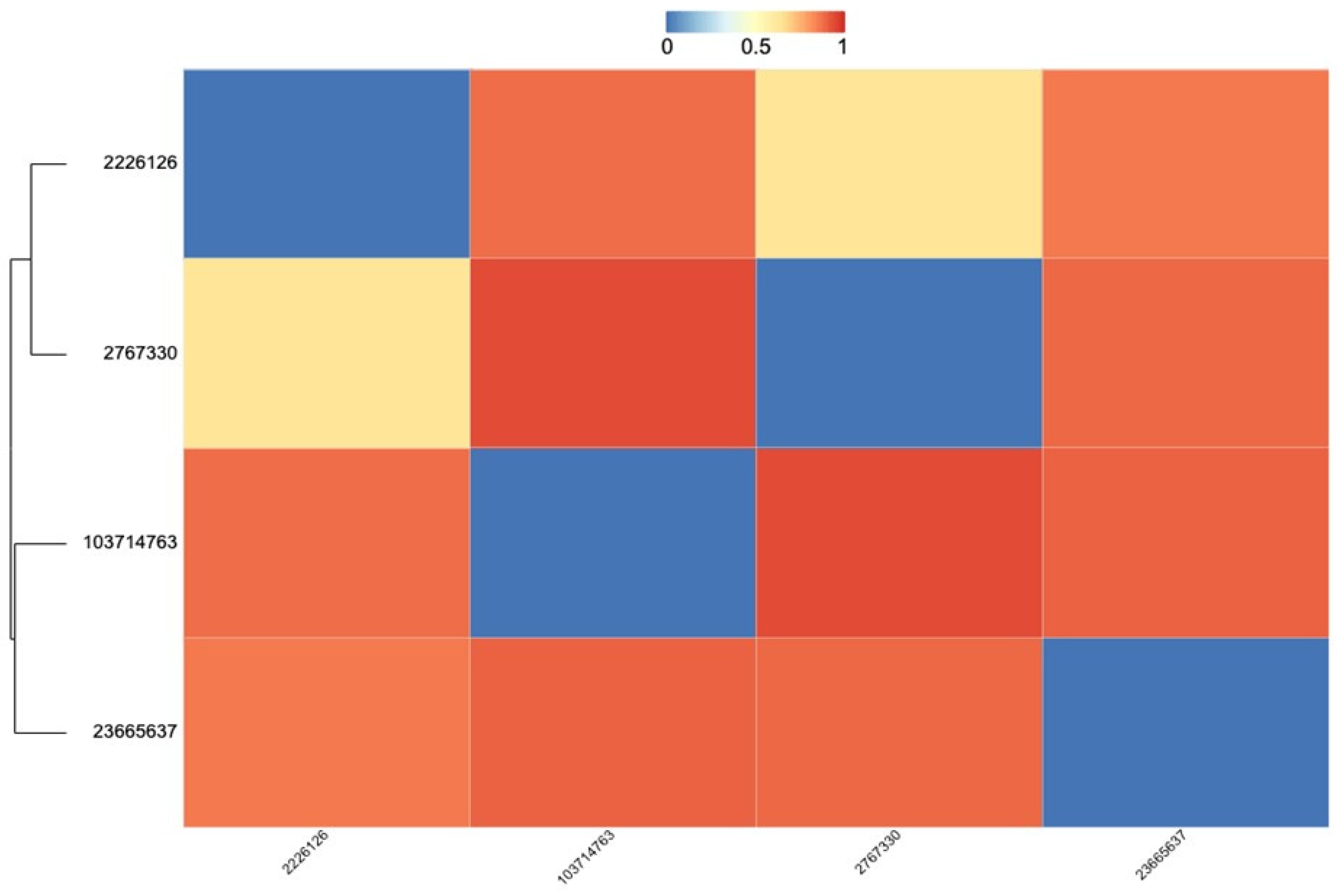
| MMV001038 | MMV665941 | MMV666021 | Augmentin | |
|---|---|---|---|---|
| MMV001038 | 0 | 0.90 | 0.64 | 0.88 |
| MMV666021 | 0.64 | 0.95 | 0 | 0.90 |
| MMV665941 | 0.90 | 0 | 0.95 | 0.91 |
| Augmentin | 0.88 | 0.91 | 0.90 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, A.; Elkenany, R.; Awad, A.; Rizk, M.A. In Vitro Activity and Atom Pair Fingerprint Analysis of Potent Hits from Malaria Box against Staphylococcus aureus Isolated from Cows with Clinical Mastitis. Dairy 2023, 4, 722-734. https://doi.org/10.3390/dairy4040049
Mansour A, Elkenany R, Awad A, Rizk MA. In Vitro Activity and Atom Pair Fingerprint Analysis of Potent Hits from Malaria Box against Staphylococcus aureus Isolated from Cows with Clinical Mastitis. Dairy. 2023; 4(4):722-734. https://doi.org/10.3390/dairy4040049
Chicago/Turabian StyleMansour, Ayat, Rasha Elkenany, Amal Awad, and Mohamed Abdo Rizk. 2023. "In Vitro Activity and Atom Pair Fingerprint Analysis of Potent Hits from Malaria Box against Staphylococcus aureus Isolated from Cows with Clinical Mastitis" Dairy 4, no. 4: 722-734. https://doi.org/10.3390/dairy4040049
APA StyleMansour, A., Elkenany, R., Awad, A., & Rizk, M. A. (2023). In Vitro Activity and Atom Pair Fingerprint Analysis of Potent Hits from Malaria Box against Staphylococcus aureus Isolated from Cows with Clinical Mastitis. Dairy, 4(4), 722-734. https://doi.org/10.3390/dairy4040049








