Abstract
Interest in the human microbiome in terms of mental health has increased with the rise in psychiatric diseases and disorders. The digestive system, the immune system, the brain, and the autonomic nervous system can all suffer from long-term lack of sleep and relaxation brought on by stress. There is little doubt that stress affects the human intestinal microbiota’s health and encourages problems with its composition, according to scientific studies. Chronic stress exposure raises the risk of both physical and mental illnesses. Therefore, this review’s goal was to support the theory that diseases including anxiety and stress are influenced by microbiome patterns. A total of 8600 sources directly relevant to this study’s topic were chosen from the 236,808 records returned by the literature search, and those with the highest scientific value were then selected based on bibliometric impact factors, language, and year of publication. A total of 87 sources, the most recent scientific output, were finally used for the literature review’s final analysis. The small number of studies on the subject indicates that it is still a developing problem, according to the literature study.
1. Introduction
Anxiety and stress disorders have become increasingly prevalent in modern society, affecting individuals of all ages and backgrounds. These disorders can have a significant impact on an individual’s overall well-being, leading to impaired daily functioning and reduced quality of life. While the exact etiology of these disorders remains complex and multifactorial, emerging research suggests that the gut microbiota may play a crucial role in their development and progression. Currently, anxiety disorders are a broad group of disorders that include stress, panic, obsessions, and compulsions in the classification [1]. The diagnostic description of anxiety disorders has been updated under the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the eleventh edition of the International Classification of Diseases (ICD-11). Anxiety disorders include generalized anxiety disorder (GAD), paroxysmal anxiety, agoraphobia, social phobia, specific phobia, separation anxiety, and selective mutism. However, excessive anxiety can coexist with other psychiatric or somatic disorders [2]. Statistical data show an increase in the prevalence of anxiety disorders in society. The Global Burden of Disease Study showed a 47.19% increase in the incidence of anxiety disorders (from 31.13 million in 1990 to 45.82 million in 2019). The disability-adjusted life years (DALY) rate also increased. There was an increase of 53.70% (from 18.66 million in 1990 to 28.68 million). These results show a steady upward trend in the prevalence of anxiety disorders and the need to deepen mental health care and prevention options [3].
The human gut is home to trillions of microorganisms, collectively known as the gut microbiota. These microorganisms consist of a diverse array of bacteria, viruses, fungi, and other microbes that interact with the host’s physiology in numerous ways. Recent studies have highlighted the bidirectional communication between the gut microbiota and the central nervous system, known as the gut–brain axis. This communication occurs through various mechanisms, including the production of neurotransmitters, immune system modulation, and metabolic pathways [4,5,6,7,8].
In recent years, there has been growing interest in understanding the role of the gut microbiota in mental health, particularly in anxiety and stress disorders. Preclinical and clinical studies have provided compelling evidence supporting the notion that alterations in the composition and function of the gut microbiota may contribute to the development of these disorders. Furthermore, emerging evidence suggests that manipulating the gut microbiota through dietary interventions, probiotics, and fecal microbiota transplantation (FMT) may have therapeutic potential in managing anxiety and stress-related symptoms [9,10].
However, despite the growing body of research in this field, the precise mechanisms underlying the gut microbiota’s influence on anxiety and stress disorders remain poorly understood. Therefore, a comprehensive review of the state of knowledge is essential to elucidate the current understanding of the relationship between microbiota patterns and these mental health conditions [11,12].
The aim of this systematic review is to critically evaluate and synthesize the existing literature on the role of microbiota patterns in anxiety and stress disorders. By examining and analyzing the available evidence, we seek to identify common themes, key findings, and potential avenues for future research. Additionally, we aim to highlight the limitations of the current knowledge and propose potential directions for further investigation.
Through this review, we hope to contribute to a better understanding of the complex interplay between the gut microbiota and anxiety and stress disorders. Ultimately, such insights may pave the way for novel therapeutic strategies targeting the gut microbiota to improve the management and treatment of these debilitating mental health conditions.
2. Materials and Methods
2.1. Methodological Introduction
The aim of the present study was to investigate if the hypothesis that the intestinal microbiota contributes to the occurrence of anxiety and stress-related disorders. The microbiota plays a key role in human health and research findings in recent years have emphasized its importance in the development of various medical conditions. Due to the absence of official guidelines for microbiota-based prevention and therapy in anxiety and stress-related disorders, the authors reviewed the scientific literature in this area.
2.2. Review Structure and Search Criteria
This study was conducted in accordance with established guidelines and good practices commonly used in this type of research. The authors began this study by defining its scope. To achieve this, they conducted a comprehensive search of multiple databases to ensure a thorough examination of the available literature. In addition to searching PubMed, the authors also used other commonly used academic search engines, such as Google Scholar, Embase, Scopus or PsycINFO, which are well known in the biomedical and psychological literature, to supplement the search and minimize the possibility of missing important studies. To ensure a rigorous and comprehensive search, the literature items were reviewed not only by this study’s authors, but also by a qualified library specialist. Their combined experience and expertise helped ensure comprehensive coverage of relevant publications.
The search strategy used by the authors included the use of relevant keywords related to the topic of interest, such as “gut microbiota”, “microbiome”, “dysbiosis”, “anxiety disorders”, “stress”, “separation anxiety syndrome” and “post-traumatic stress disorder.” The authors used Boolean operators and their combinations and configurations to refine the search and increase its specificity.
By including multiple databases and using a systematic approach to literature search and selection, the authors sought to gather comprehensive evidence on the role of the gut microbiota in anxiety and stress disorders. This rigorous methodology enhances the validity and reliability of this study’s findings, providing a solid foundation for the research and its conclusions.
2.3. Final Methodological Scheme
A search of the literature generally related to stress, anxiety and microbiota resulted in 236,808 records, while 8600 sources were selected for the hypothesis under discussion. The sources with the highest research quality were further selected based on bibliometric evaluation, language and year of publication. In the end, 87 sources were used for the final review of the literature, representing mainly scientific output from recent years (Figure 1).
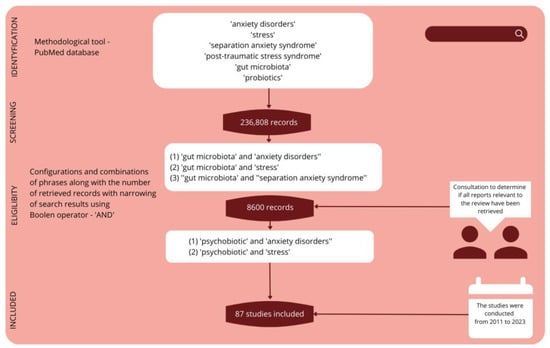
Figure 1.
Methodological overview.
The GRADE system (The Grading of Recommendations Assessment, Development, and Evaluation) allowed the accuracy, reliability and relevance of the study to be assessed, as it aims to avoid inaccuracies caused by the presence of various evaluation approaches for findings in the literature. GRADE is a commonly used tool in systematic reviews that allows researchers to assess the quality of scientific evidence and strength of recommendations for various interventions or health issues. GRADE takes into account several important factors, such as the quality of the research, consistency of results, safety, patient value and preference, and the benefits and costs of the intervention. The GRADE system classifies recommendations as strong or weak, and rates the quality of evidence as high, moderate, low or very low. With this rating system, GRADE-based systematic reviews can provide reliable and transparent information that supports decision-making in clinical practice and public health. A review article based on 87 scientific studies from the years 2011–2023 was prepared, with the most significant focus on results from the last five years, which represent over 80% of the quoted sources.
3. Results
3.1. The Role of the Microbiome in Anxiety and Stress-Related Disorders
With the increasing prevalence of psychiatric diseases and disorders, there has been a growing interest in the human microbiota in terms of mental health. The physiological gut microbiota consists of bacteria, viruses, fungi, protozoa, and archaeons, which together can reach a mass of up to 1.5 kg. Among the bacteria implicated in the pathogenesis of mental disorders are species of Firmicutes, Actinobacteria, Bacteroides, and Bifidobacterium genera. The gut microbiota has been shown to affect immune processes, neuroendocrine signaling, and enteroendocrine stress response, as well as the tightness of the intestinal barrier. In addition, some of the microbiota can produce neurotransmitters [5]. Neurotransmitters play a fundamental role in the development of mood disorders. Decreased dopamine neurotransmission promotes both chronic pain and depression [13]. Serotonin also plays an important role in depression and anxiety disorders—the serotonin theory of depression has been challenged in recent years, but serotonin undoubtedly has an impact on mood. Modulation of the serotonin system is one possible pathway through which the circadian system regulates vulnerability to depression [14,15].
Glutamate systems in cortico-limbic circuits interact with GABAergic, serotonin, dopaminergic and other systems involved in the stress response, and thus has a potential role in the development of anxiety disorders. Unlike glutamate, gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter of the nervous system. Anxiety is thought to result from an imbalance between excitatory and inhibitory systems, leading to dysregulation [16]. Glutamate synthesis depends on the bacteria Lactobacillus plantarum, Bacteroides vulgatus, and Campylobacter jejuni, while gamma-aminobutyric acid synthesis depends on Bifidobacterium, Bacteroides fragilis, Parabacteroides, Eubacterium. Choline, involved in the synthesis of acetylcholine, is a precursor of the bacteria Lactobacillus plantarum, Bacillus acetylcholini, Bacillus subtilis, Escherichia coli, Staphylococcus aureus [6]. Bacteria of the genus Staphylococcus are involved in the synthesis of dopamine and serotonin, in the case of serotonin, Clostridium species can also be distinguished [6]. The entire interplay between the microbiota and its metabolites and functions on the brain is referred to as the brain–gut–microbiome axis. This axis is also mediated by the gut, the vagus nerve, and the immune system [5]. A prolonged lack of relaxation induced by stress can disrupt the functioning of the digestive system, the immune system, or the functioning of the brain and autonomic nervous system. Scientific studies indicate that stress also affects the state of the human gut microbiota and promotes disorders in the composition of the microbiota [1,2,3,4,5,6].
3.1.1. HPA Axis
The role of the hypothalamic-pituitary-adrenal (HPA) axis in regulating the body’s response to stress is crucial in understanding the relationship between microbiota and anxiety/stress disorders. Abnormalities in the HPA axis have been associated with psychiatric conditions, including those characterized by anxiety and stress. Chronic stress can have detrimental effects on the integrity of the intestinal barrier. This can lead to changes in the functioning of the HPA axis, triggered by the release of cytokines and chemokines by gut bacteria [5,17]. In individuals with a propensity for anxiety, there is an increase in the expression of glucocorticoid receptors and lower levels of dehydroepiandrosterone (DHEA), a hormone with anti-anxiety properties. Moreover, anxious and traumatized individuals exhibit reduced production of the anti-inflammatory cytokine IL-10 [18]. Anxiety symptoms often co-occur with deficits in emotional attention control. Individuals with lower levels of emotional attention control show higher diurnal cortisol secretion and a slower decline in cortisol levels throughout the day. Factors influencing emotional attention control can contribute to chronic hyperactivity of the HPA axis, particularly in situations of prolonged stress exposure [19]. On the other hand, lower salivary cortisol awakening response (CAR) has been observed in individuals with post-traumatic stress disorder (PTSD) [20]. These findings suggest that dysregulation of the HPA axis, influenced by factors such as gut microbiota and inflammatory responses, plays a significant role in the development and manifestation of anxiety and stress disorders. Understanding the intricate interactions between microbiota, HPA axis functioning, and associated physiological responses can provide valuable insights into potential targets for interventions and therapeutic approaches aimed at alleviating anxiety and stress-related symptoms. Further research in this area is necessary to unravel the underlying mechanisms and establish effective treatment strategies.
Additionally, studies have shown that the gut–brain axis plays a crucial role in the bidirectional communication between the gut microbiota and the central nervous system. This axis involves intricate pathways, including neural, immune, and endocrine signaling, which collectively influence emotional and behavioral responses.
The dysbiosis of gut microbiota, characterized by an imbalance in microbial composition and diversity, has been associated with increased vulnerability to anxiety and stress-related disorders. Alterations in the gut microbiota can lead to changes in the production and release of neurotransmitters, such as serotonin and gamma-aminobutyric acid (GABA), which are crucial in regulating mood and anxiety. Moreover, the gut microbiota plays a vital role in the metabolism of neuroactive compounds and the synthesis of various neuroactive substances, including short-chain fatty acids (SCFAs), which have been implicated in modulating brain function and behavior [7,21].
Furthermore, the immune system also plays a significant role in the interaction between the gut microbiota and anxiety/stress disorders. Dysregulated immune responses, such as increased production of pro-inflammatory cytokines, can contribute to chronic low-grade inflammation, which has been linked to the pathogenesis of anxiety and stress-related conditions [8,22].
Understanding the complex interplay between the gut microbiota, HPA axis, immune system, and neurotransmitter signaling is crucial for unraveling the mechanisms underlying anxiety and stress disorders. This knowledge can pave the way for the development of novel therapeutic interventions, including targeted probiotic treatments, prebiotics, or dietary interventions aimed at modulating the gut microbiota and restoring its balance [9,23,24,25].
In conclusion, the role of the microbiota pattern in anxiety and stress disorders is multifaceted and involves intricate interactions between the gut microbiota, HPA axis, immune system, and neurotransmitter signaling. Further research is needed to elucidate the underlying mechanisms and to explore the potential of microbiota-targeted interventions as a novel approach for preventing and treating anxiety and stress-related conditions.
3.1.2. Microbiota and Anxiety Disorders
Mental health is linked to the state and diversity of the gut microbiota and its metabolites. It is indicated that in anxiety-related disorders, including generalized anxiety disorder, there are disorders of the gut microbiota—gut dysbiosis. In depression and anxiety, changes can include an abundance of gut bacteria at every taxonomic level, including 15 genera and 18 groups [26]. A reduction in short-chain fatty acid (SCFA)-producing bacteria and an overgrowth of Escherichia-Shigella, Fusobacterium, and Ruminococcus gnavus have been noted among GAD patients. These changes remained despite the remission of GAD [27]. In active GAD, Escherichia-Shigella, and Bacteroides abundance affect the severity of anxiety [28]. A correlation has been noted between the presence of C-reactive protein (CRP) in GAD patients and dysbiosis in Bacteroidales, Selenomonadales, Clostridiales, and Holdemanella. For both GAD and depression, differences have been noted in the genus Holdemanella, Desulfovibrio, Barnesiella, and the family Coriobacteriaceae [29]. In depression and GAD, intestinal dysbiosis shows many similarities, but there are also opportunities to differentiate these disorders in terms of gut bacteria content. In GAD, there is a noticeable decrease in the abundance of Firmicutes while an increase in Bacteroides, which is the opposite situation to that in depression [28,30]. In addition, Proteobacteria abundance increases in GAD. Differences are also noticeable in terms of bacterial abundance. In GAD, the abundance of Fusicatenibacter and Christensenellaceae R7group is reduced compared to the control group. On the other hand, comparing the abundance of GAD and depression there is an increase in Fusobacteria, Tenericutes, Verrucomicrobia, and Bacteroidetes and a decrease in Proteobacteria, Actinobacteria, and Firmicutes. These differences offer the possibility of creating targeted probiotic therapy [30]. A positive correlation between the abundance of the Eubacterium coprostanoligenes group, Ruminococcaceae UCG-014, and Prevotella 9 with the reduction in anxiety has been shown [28]. One possible modulation of the gut microbiome is the use of probiotics. Dysbiosis associated with an increased abundance of Fusobacterium, and Clostridium and decreased abundance of Prevotella, Streptococcus was initially reported among students exhibiting exam anxiety. The applied probiotic therapy containing Bifidobacterium longum subsp., Longum BAMA-B-05/Bau-B1024, B. lactis BAMA-B06/Bau-B0111, B. adolescentis, Streptococcus thermophiles, Lactobacillus acidophilus, L. delbrueckii subsp. bulgarium was beneficial in reducing anxiety and improving the composition of the microbiome. The composition of the microbiota showed a reduction in Fusobacterium and Clostridium and an increase in Streptococcus, and Akkermansia [31]. In the case of anxiety, of particular interest is L. delbrueckii, supplementation of which reduces anxiety while increasing the expression of glutamate decarboxylase 1 (GAD1). In addition, it changes the composition of the intestinal bacterial flora—increasing the abundance of Lactobacillus, Verrucomicrobium, and Gordonia and decreasing the abundance of Legionella, Planctomyces, Flavobacterium, and Prevotella [32]. Probiotic therapy also reduces anxiety severity in carriers of the IL-1β rs16944 gene polymorphism, which is a risk factor for GAD. A probiotic suspension containing Bifidobacterium animalis subsp. Lactis, Bifidobacterium bifidum, S. thermophiles, L. bulgaricus, Lactococcus lactis subsp. Lactis, L. acidophilus, L. plantarum, L. reuteri were used to benefit anxiety after 12 weeks [17].
3.1.3. Microbiota and Stress
Chronic stress induces many adverse changes in the gastrointestinal tract such as the development of colitis, an increase in IL-6, a decrease in IL-10, damage to the intestinal mucosa, and dysbiosis. In terms of microbiota modulation, chronic stress results in increased levels of Helicobacter, Peptostreptococcaceae, Streptococcus, and Enterococcus faecalis. In turn, Rikenella, Roseburia, and Lachnospiraceae are decreased [33]. Adequate probiotic therapy can have positive effects on people living under chronic stress (Figure 2).
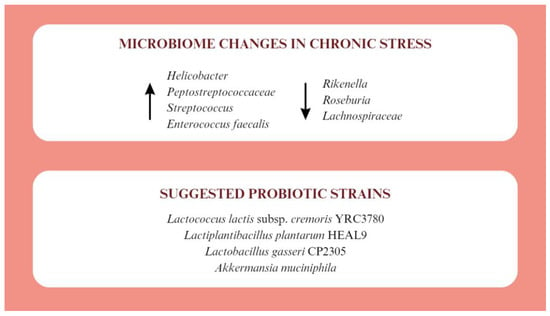
Figure 2.
Microbiotic changes in chronic stress and proposed probiotic therapy [33,34,35,36,37,38].
The use of Lactococcus lactis subsp. cremoris YRC3780 affects the HPA axis response to acute psychological stress. It also lowers morning salivary cortisol concentrations and promotes a decrease in cortisol after a stressful situation [34]. In acute stress, it may also be beneficial to take Lactiplantibacillus plantarum HEAL9, which, despite not affecting cortisol levels, reduces plasma inflammatory markers such as soluble fractalkine and CD163 [35]. Lactobacillus gasseri CP2305 has been shown to have broad support for people living under chronic stress. Its supplementation lowers salivary levels of the stress marker CgA and improves sleep quality. At the level of intestinal microflora, it mitigated the decrease in Bifidobacterium counts and the increase in Streptococcus, which are noticeable during stress. It also increases the concentration of n-valeric acid, which belongs to SCFAs [36]. For stress-induced disorders with symptoms resembling depression, benefits have been reported after using a probiotic containing Akkermansia muciniphila. This probiotic up-regulates corticosterone, dopamine, and brain-derived neurotrophic factor (BDNF) levels. At the same time, increases in β-alanyl-3-methyl-1-histidine and edaravone have been reported to contribute to symptom relief [37]. In addition to probiotics, supplementation with short-chain fatty acids, such as butyric acid, propionate or acetate, which reduce the cortisol response to acute stress, has shown beneficial effects [38].
3.1.4. Separation Anxiety Syndrome
The topic of gut microbiota in the context of separation anxiety syndrome is poorly understood. Available studies mainly involve animal models. Separation from the mother promotes the development of defects in Paneth cells and intestinal dysbiosis, associated with E. coli among others, which lead to the development of visceral hypersensitivity [39,40]. Abnormalities of the HPA axis in separation anxiety are indicated, which may also be involved in the development of intestinal barrier dysfunction and exaggerated stress response [40]. Separation from the mother reduces microbial diversity. Lachnospiraceae, Parasutterella, Prevotellaceae, Oscillibacter, Ruminislostridium, and Bacteroidia are increased [41]. Difficult maternal experiences in early childhood may contribute to dysbiosis of Lachnospiraceae, Porphyromonadaceae, Firmicutes, Bacteroides, Lactobacillus, Alloprevotella in men and Lactobacillus and Mucispirillum in women [42]. Appropriate probiotic therapy can provide mental health benefits to people with separation anxiety syndrome (Figure 3).
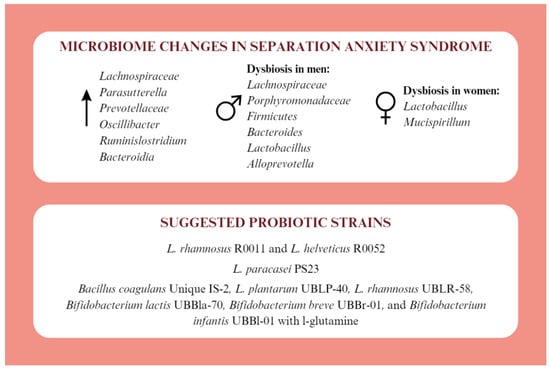
Figure 3.
Microbiotic changes in separation anxiety syndrome and proposed probiotic therapy [39,40,41,42,43,44,45].
The use of a probiotic containing L. rhamnosus R0011 and L. helveticus R0052 counteracts the effects of stress on the neural circuitry of fear expression [43]. Supplementation with L. paracasei PS23 reduces symptoms of anxiety and depression, blood corticosterone levels, and increases IL-10 levels. Its immunomodulatory and neurotransmitter-regulating effects may have a beneficial effect on mental health for separation anxiety [41,44]. Benefits are also noted after supplementation with multistrain probiotics. The combination of Bacillus coagulans Unique IS-2, L. plantarum UBLP-40, L. rhamnosus UBLR-58, Bifidobacterium lactis UBBla-70, Bifidobacterium breve UBBr-01, and Bifidobacterium infantis UBBl-01 with l-glutamine has benefits on anxiety and depression after 6 weeks of use. Furthermore, reduced levels of CRP, TNF-α and dopamine and increased levels of BDNF and serotonin were noted. A stabilisation of plasma levels of l-kynirenin, kynurenic acid, l-tryptophan and 3-hudroxyanthranilic acid was also observed. The normal intestinal function was restored by normalizing the Firmicutes to Bacteroides ratio, acetate, butyrate, and propionate concentrations, and the number of cup cells [45].
3.1.5. Post-Traumatic Stress Disorder
Stressful and psychologically taxing events promote post-traumatic stress disorder (PTSD), as observed among healthcare workers during the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic. Along with PTSD symptoms, co-occurring intestinal dysbiosis was demonstrated within the abundance of gut bacteria associated with stress. Differences were noted in the abundance of Faecalibacterium, Eubacterium eligens, Bacteroides, Lachnospiraceae, and Roseburia [46]. Considering the functions of these bacteria among the subjects, there was impaired production of gamma-aminobutyric acid, butyric acid, and serotonin within the gut, and increased permeability of the intestinal barrier [46,47]. A reduction in the abundance of Actinobacteria, Lentisphaerae, and Verrucomicrobia has also been noted among those with PTSD symptoms [5]. A major contribution of the HPA axis and pro-inflammatory cytokines is indicated in the development of PTSD [48]. Disorders of the gut microbiome and intestinal barrier permeability can induce changes in HPA axis function [5,17].
3.1.6. Beneficial Factors for the Microbiome in Anxiety and Stress
The intestinal microbiome is determined by a variety of factors. It is possible to use lifestyle interventions to improve the diversity of the gut microbial flora. One option is the use of probiotics containing strains of bacteria beneficial to mental health, known as psychobiotics. Clinical studies show the benefits of including probiotic therapy among patients with mental health disorders. The use of Bifidobacterium breve CCFM1025 affects tryptophan metabolism, alleviating depressive symptoms and gastrointestinal complaints caused by the mental state [49]. A reduction in cinurenine levels and improvement in cognitive function was observed among patients with major depression after treatment with Lactobacillus plantarum 299v [50]. The L. plantarum strain has also shown beneficial effects in acute stress and anxiety [17,35,45]. Anti-anxiety effects have also been attributed to the Lactobacillus rhamnosus CNCM I-3690 strain [51]. The use of a probiotic containing Streptococcus thermophiles, Bifidobacterium animalis subsp. Lactis, Bifidobacterium bifidum, Streptococcus thermophiles, Lactobacillus bulgaricus, Lactococcus lactis subsp. Lactis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus reuteri alleviates anxiety symptoms [17]. A reduction in anxiety symptoms was noted after four weeks of Lactobacillus reuteri NK33 in combination with Bifidobacterium adolescentis NK98 [52]. Probiotics consisting of multiple strains have shown considerable potential in alleviating and treating anxiety [53,54]. However, meta-analyses of studies on the impact of probiotics on the treatment of anxiety indicate insufficient scientific evidence and the need for further research development [55,56]. The use of probiotics can alleviate subjective feelings of stress among healthy individuals [57]. Benefits in perceived stress among subjects supplementing Lactobacillus gasseri CP2305 after 24 weeks of use have been reported [36]. Supplementation with Lactobacillus plantarum DR7 promotes a reduction in stress, plasma cortisol levels, and improved cognitive function and memory after 12 weeks of use [58]. Similar benefits have been reported with the Lactobacillus plantarum P8 strain for 12 weeks [59]. A probiotic containing Bifidobacterium longum R0175, Lactobacillus helveticus R0052, and Lactiplantibacillus plantarum R1012 after 4 weeks of use affects the activity of brain areas regulating emotions and stress responses [60]. Among multistrain formulations, a combination of Bacillus coagulans Unique IS2, Lactobacillus rhamnosus UBLR58, Bifidobacterium lactis UBBla70, Lactobacillus plantarum UBLP40, Bifidobacterium breve UBBr01, and Bifidobacterium infantis UBBI01 showed a reduction in stress after 28 days of use among students [61]. Bifidobacterium longum 1714 is also a strain worth considering when selecting supplementation in people at risk for severe stress [62]. The benefits of supplementation with Saccharomyces boulardii, Lactobacillus rhamnosus HN001, Lacticaseibacillus rhamnosus HN001 have not been demonstrated in individuals at risk of acute and chronic stress [63,64,65]. Further research in the context of the potential of selected microorganisms to reduce anxiety and stress is needed to obtain some data useful for the development of targeted probiotic therapy.
3.1.7. Dietary Interventions in Reducing Stress and Anxiety
In addition to probiotic therapy, appropriate diet therapy may be widely used. A psychobiotic diet that takes into account the probiotics and prebiotics found in products is worth considering among people under stress. The inclusion of whole grains, vegetables, and fruits rich in prebiotic fiber, legumes, and fermented foods while reducing the intake of processed foods reduces perceived stress by 32% [66]. It may be beneficial to include products in the diet that are sources of vitamin B6, a deficiency of which is noted in anxiety symptoms, especially in women [67]. A low-energy diet to reduce anxiety is also worth considering, although available recommendations for this intervention are conflicting and uncertain [68,69]. In addition, an anti-anxiety diet model that includes fish, fish oil, and fresh fruit has long-term benefits in preventing the onset of anxiety [70]. In addition, a diet rich in fruits and vegetables reduces the risk of anxiety disorders among adolescents living in low- and middle-income countries. Including fruit and vegetable intake may therefore be a universal recommendation for mental health support [71]. Increasing dietary fiber supply has beneficial effects on the microbiome and emotional mood. As a result of the dietary intervention of increasing fiber intake, an increase in Lactobacillus, Bifidobacterium, and Akkermansia and a decrease in Desulfovibrio, Klebsiella, and other opportunistic microorganisms have been noted [72]. Worth adding to the diet of anxious people is curcumin [73]. There is no evidence for the validity of a low-carbohydrate diet, intermittent fasting, or Omega-3 fatty acid supplementation [74,75,76]. Benefit in terms of anxiety has also not been reported following a diet rich in polyphenols, a vegetarian or vegan diet, and the DASH (Dietary Approaches to Stop Hypertension) diet [77,78,79,80]. Adequate education of people with stress-related disorders is particularly important, as stress is an important predictor of eating behavior. It may activate relevant reward (pleasure) center signaling pathways in the brain, which in turn may be associated with increased consumption of unhealthy snacks at the expense of adequate fruit and vegetable intake [81]. Given the importance of an adequate diet for mental health, the care of both a psychologist and a nutritionist may be highly beneficial.
In physiologically healthy individuals, the gut microbiota is diverse and strains that increase the body’s resistance to external factors, including stress and inflammation, predominate. In contrast, when dysbiosis is present, the microbiota is less diverse, making it difficult or impossible to maintain internal homeostasis. Attempting to improve the gut microbiome in people with anxiety and stress disorders is particularly worth considering, given that the state of the microbiota is extremely important for mental health [82] (Table 1).

Table 1.
Practical use of probiotics in patients with anxiety and stress disorders—a review of selected studies.
Psychopharmacotherapy and psychotherapy are not always successful in treating anxiety disorders. As a result, other interventions are receiving increasing attention. In recent years, there has been a surge in research on the effects of nutrition and targeted probiotic therapy on mental status, which may be an important aspect of the prevention of many mental disorders, and at the same time may lead to a reduction in the percentage of people with mental disorders [86,87].
3.2. Strengths and Limitations
In the scientific space, there are still few articles summarizing key findings in relation to the impact of the microbiota on anxiety and stress disorders, and the existing studies do not always highlight this link, in the way this review does. We emphasized the importance of homeostasis maintenance in preventing these disorders. The primary limitation of the presented review of studies on the link between the microbiota and stress- and anxiety-related disorders is the small number of studies directly addressing selected anxiety disorders. Larger-scale and longer-term studies are needed to obtain precise insights into the long-term effects of interventions, including possible risks and complications. The authors have made every effort to ensure that this review is conducted fairly. Multicentre research projects have been considered and mainstream studies highlighted. This review also found no adverse reactions to probiotics, perhaps reflecting a positive attitude towards reporting due to the lack of systematic reports of adverse events associated with probiotic interventions.
The study of the role of microbiota patterning in anxiety and stress disorders has many practical implications for improving the diagnosis, treatment and prevention of these disorders. First, understanding the relationship between microbiota pattern and anxiety and stress disorders may lead to the development of new diagnostic biomarkers. Studies can identify specific bacterial species or combinations of microorganisms that differ in people with anxiety and stress disorders compared to healthy individuals. This could help develop simple diagnostic tests based on the microbiota profile. Second, learning more about the role of the microbiota in anxiety and stress disorders could open the door to new forms of therapy. Manipulating the microbiota through supplementation with probiotics, prebiotics, diet or gut microbiota transplantation therapy (FMT) could be a new strategy for treating these disorders. Research into the relevant bacterial strains and their effects on improving mental status could lead to the development of innovative microbiota therapies. Third, knowledge of the link between the microbiota and anxiety and stress disorders can help with public education and prevention. People at increased risk or already affected by these disorders can be made aware of the impact of their lifestyle and diet on the microbiota. Education about healthy eating habits, physical activity and stress reduction can help maintain a healthy microbiota and reduce the risk of developing or exacerbating anxiety and stress disorders. Finally, the study on this topic reveals gaps in knowledge and provides directions for future research. There is a need to further explore the mechanisms and interactions between the microbiota and the nervous system, as well as to conduct intervention studies to evaluate the effectiveness of microbiota therapies. Future research may focus on identifying specific factors, such as diet, lifestyle and environment, that shape the microbiota and may be relevant to the prevention and treatment of anxiety and stress disorders. Research may also focus on evaluating the long-term effects of microbiota therapies and identifying potential interactions between microbiota therapy and other therapies, such as pharmacotherapy or behavioral therapy. This review’s conclusions about the role of microbiota patterning in anxiety and stress disorders may have important implications for clinical practice and public health. Understanding these implications may contribute to the development of innovative diagnostic, therapeutic and preventive strategies that can improve care for those affected by these disorders. However, there is still a need for further research to better understand the complex interactions between the microbiota and the nervous system and to evaluate the efficacy and safety of microbiota therapies in the context of anxiety and stress disorders.
4. Conclusions
The relationship between gut microbiota and general health is increasingly discussed in the scientific space. The present study showed that probiotic interventions in specific cases can be effective in mental health disorders related to anxiety and stress. This review outlines the importance of the gut microbiota for anxiety and stress-related disorders, particularly due to its effects on neurotransmission and HPA axis. Specific gut dysbiosis has been shown to be present in psychiatric disorders such as GAD, depression, chronic stress, separation anxiety syndrome and PTSD, which is an important indication for recommendations of targeted probiotic therapy. Probiotic strains proven to improve the symptoms of the aforementioned disorders are also indicated. The importance of diet for mental health was also pointed out. A diet based on whole grains, vegetables and fruits rich in prebiotic fiber, legumes and fermented foods while limiting the intake of processed foods can reduce perceived stress. The supply of vitamin B6 and curcumin in people with anxiety is worth considering. The importance of mental health is consistently a major public health concern and further research is needed into the importance of microbiota and nutrition in this area. Additionally, there is a need for universal and clarified preventive recommendations against stress-related disorders.
Author Contributions
Conceptualization, K.K.-K.; methodology, K.K.-K. and W.G.; investigation, K.K.-K. and S.N.; writing—original draft preparation, K.K.-K., S.N. and M.G.; writing—review and editing, K.K.-K. and W.G.; supervision, K.K.-K. and M.G.; project administration, K.K.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research complies with the provisions of the Helsinki Declaration.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Crocq, M.A. A history of anxiety: From Hippocrates to DSM. Dialogues Clin. Neurosci. 2015, 17, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety disorders. Lancet 2021, 397, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Liu, M.; Liu, B.; Hall, B.J. Trends in the incidence and DALYs of anxiety disorders at the global, regional, and national levels: Estimates from the Global Burden of Disease Study 2019. J. Affect. Disord. 2022, 297, 83–93. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef] [PubMed]
- Halverson, T.; Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota regulation of the mammalian gut-brain axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Serafini, R.A.; Pryce, K.D.; Zachariou, V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol. Psychiatry 2020, 87, 64–73. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Daut, R.A.; Fonken, L.K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocrinol. 2019, 54, 100746. [Google Scholar] [CrossRef]
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate Systems in DSM-5 Anxiety Disorders: Their Role and a Review of Glutamate and GABA Psychopharmacology. Front. Psychiatry 2020, 11, 548505. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Cioccoloni, G.; De Lorenzo, A.; Romano, L.; Cammarano, A.; Colica, C.; Condò, R.; Di Renzo, L. Psychobiotics Regulate the Anxiety Symptoms in Carriers of Allele A of IL-1β Gene: A Randomized, Placebo-Controlled Clinical Trial. Mediat. Inflamm. 2020, 2020, 2346126. [Google Scholar] [CrossRef]
- Viljoen, M.; Benecke, R.M.; Martin, L.; Adams, R.C.M.; Seedat, S.; Smith, C. Anxiety: An overlooked confounder in the characterisation of chronic stress-related conditions? PLoS ONE 2020, 15, e0230053. [Google Scholar] [CrossRef]
- Lenaert, B.; Barry, T.J.; Schruers, K.; Vervliet, B.; Hermans, D. Emotional attentional control predicts changes in diurnal cortisol secretion following exposure to a prolonged psychosocial stressor. Psychoneuroendocrinology 2016, 63, 291–295. [Google Scholar] [CrossRef]
- Rauch, S.A.M.; King, A.; Kim, H.M.; Powell, C.S.; Rajaram, N.; Venners, M.R.; Simon, N.M.; Hamner, M.B.; Liberzon, I. Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology 2020, 118, 104714. [Google Scholar] [CrossRef]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2020, 13, 100239. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2017, 358, 465–466. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.P.; Zeng, L.; Chen, J.C.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G.; Strodl, E.; Parham, S.; Bambling, M.; Cramb, S.; Vitetta, L. An exploratory study of the gut microbiota in major depression with anxious distress. J. Affect. Disord. 2023, 320, 595–604. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Chen, Y.H.; Bai, J.; Wu, D.; Yu, S.-F.; Qiang, X.; Bai, H.; Wang, H.; Peng, Z. Association between fecal microbiota and generalized anxiety disorder: Severity and early treatment response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, P.; Cheng, S.; Jia, Y.; Wen, Y.; Yang, X.; Yao, Y.; Pan, C.; Li, C.; Zhang, H.; et al. Assessing the effect of interaction between C-reactive protein and gut microbiome on the risks of anxiety and depression. Mol. Brain 2021, 14, 133. [Google Scholar] [CrossRef]
- Dong, Z.; Shen, X.; Hao, Y.; Li, J.; Li, H.; Xu, H.; Yin, L.; Kuang, W. Gut Microbiome: A Potential Indicator for Differential Diagnosis of Major Depressive Disorder and General Anxiety Disorder. Front. Psychiatry 2021, 12, 651536. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, H.; Yang, Y.; Yifei, W.; Xia, C.; Tian, P.; Wei, J.; Li, S.; Chen, T. Probiotic Supplement Preparation Relieves Test Anxiety by Regulating Intestinal Microbiota in College Students. Dis. Markers 2021, 2021, 5597401. [Google Scholar] [CrossRef] [PubMed]
- Olorocisimo, J.P.; Diaz, L.A.; Co, D.E.; Carag, H.M.; Ibana, J.A.; Velarde, M.C. Lactobacillus delbrueckii reduces anxiety-like behavior in zebrafish through a gut microbiome—Brain crosstalk. Neuropharmacology 2023, 225, 109401. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.T.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Motoshima, H.; Uchida, K.; Yamanaka, Y. Effects of Lactococcus lactis subsp. cremoris YRC3780 daily intake on the HPA axis response to acute psychological stress in healthy Japanese men. Eur. J. Clin. Nutr. 2022, 76, 574–580. [Google Scholar] [CrossRef]
- Önning, G.; Hillman, M.; Hedin, M.; Montelius, C.; Eriksson, J.; Ahrné, S.; Jönsson, P. Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress: A randomized, double-blind, placebo-controlled study. Physiol. Behav. 2020, 225, 113083. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.P.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Dalile, B.; Vervliet, B.; Bergonzelli, G.; Verbeke, K.; Van Oudenhove, L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: A randomized, placebo-controlled trial. Neuropsychopharmacology 2020, 45, 2257–2266. [Google Scholar] [CrossRef]
- Riba, A.; Olier, M.; Lacroix-Lamandé, S.; Lencina, C.; Bacquie, V.; Harkat, C.; Gillet, M.; Baron, M.; Sommer, C.; Mallet, V.; et al. Paneth Cell Defects Induce Microbiota Dysbiosis in Mice and Promote Visceral Hypersensitivity. Gastroenterology 2017, 153, 1594–1606.e2. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef]
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus paracasei Supplementation Prevents Early Life Stress-Induced Anxiety and Depressive-Like Behavior in Maternal Separation Model-Possible Involvement of Microbiota-Gut-Brain Axis in Differential Regulation of MicroRNA124a/132 and Glutamate Receptors. Front. Neurosci. 2021, 15, 719933. [Google Scholar] [CrossRef] [PubMed]
- Rincel, M.; Aubert, P.; Chevalier, J.; Grohard, P.; Basso, L.; De Oliveira, C.P.; Helbling, J.C.; Lévy, É.; Chevalier, G.; Leboyer, M.; et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav. Immun. 2019, 80, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.S.M.; Stylianakis, A.A.; Richardson, R. Early-life stress, microbiota, and brain development: Probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev. Cogn. Neurosci. 2019, 37, 100627. [Google Scholar] [CrossRef]
- Liao, J.F.; Hsu, C.C.; Chou, G.T.; Hsu, J.S.; Liong, M.T.; Tsai, Y.C. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef. Microbes 2019, 10, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, M.P.; Palepu, M.S.K.; Satti, S.; Jaiswal, Y.; Singh, A.P.; Dash, S.P.; Gajula, S.N.R.; Sonti, R. Multi-strain Probiotic Formulation Reverses Maternal Separation and Chronic Unpredictable Mild Stress-Generated Anxiety- and Depression-like Phenotypes by Modulating Gut Microbiome-Brain Activity in Rats. ACS Chem. Neurosci. 2022, 13, 1948–1965. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guo, R.; Ma, Q.; Li, Y.; Wang, W.; Fan, Y.; Ju, Y.; Zhao, B.; Gao, Y.; Qian, L.; et al. Stressful events induce long-term gut microbiota dysbiosis and associated post-traumatic stress symptoms in healthcare workers fighting against COVID-19. J. Affect. Disord. 2022, 303, 187–195. [Google Scholar] [CrossRef]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 2022, 55, 2851–2894. [Google Scholar] [CrossRef]
- Cai, M.; Park, H.R.; Yang, E.J. Nutraceutical Interventions for Post-Traumatic Stress Disorder in Animal Models: A Focus on the Hypothalamic-Pituitary-Adrenal Axis. Pharmaceuticals 2022, 15, 898. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Huiyue, Z.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.X.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Wauters, L.; Van Oudenhove, L.; Accarie, A.; Geboers, K.; Geysen, H.; Toth, J.; Luypaerts, A.; Verbeke, K.; Smokvina, T.; Raes, J.; et al. Lactobacillus rhamnosus CNCM I-3690 decreases subjective academic stress in healthy adults: A randomized placebo-controlled trial. Gut Microbes 2022, 14, 2031695. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.K.; Kim, D.H.; Jang, S.P.; Han, S.H.; Yoon, I.Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Zhebrak, M.; Yacoub, C.; Pelletier, J.; Hawley, D. The gut-brain relationship: Investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J. Affect. Disord. 2019, 252, 271–277. [Google Scholar] [CrossRef]
- El Dib, R.; Periyasamy, A.G.; de Barros, J.L.; França, C.N.; Senefonte, F.L.; Vesentini, G.; Alves, M.G.O.; Da Silva Rodrigues, J.V.; Gomaa, H.; Júnior, J.O.C.A.; et al. Probiotics for the treatment of depression and anxiety: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 75–90. [Google Scholar] [CrossRef]
- Cohen Kadosh, K.; Basso, M.; Knytl, P.; Johnstone, N.; Lau, J.Y.F.; Gibson, G.R. Psychobiotic interventions for anxiety in young people: A systematic review and meta-analysis, with youth consultation. Transl. Psychiatry 2021, 11, 352. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Wang, M.; Liu, J.; Ju, Y.; Zhang, Y.; Liu, T.B.; Li, L.; Li, Q. Efficacy of probiotics on anxiety-A meta-analysis of randomized controlled trials. Depress. Anxiety 2018, 35, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Li, M.L.; Wang, W.; Liu, Z.; Xi, C.; Huang, X.; Liu, J.T.; Huang, J.W.; Tian, D.; et al. Efficacy of probiotics on stress in healthy volunteers: A systematic review and meta-analysis based on randomized controlled trials. Brain Behav. 2020, 10, e01699. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.S.; Choi, S.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.B.; Zakaria, N.H.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomized, double-blind, placebo-controlled study. Benef. Microbes. 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Edebol Carlman, H.M.T.; Rode, J.; König, J.; Repsilber, D.; Hutchinson, A.; Thunberg, P.; Persson, J.; Kiselev, A.; Pruessner, J.C.; Brummer, R.J.M. Probiotic Mixture Containing Lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum Affects Brain Responses to an Arithmetic Stress Task in Healthy Subjects: A Randomised Clinical Trial and Proof-of-Concept Study. Nutrients 2022, 14, 1329. [Google Scholar] [CrossRef]
- Venkataraman, R.; Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Vinay, H.R.; Lal, A.; Thomas, G.; Stephen, S. Effect of Multi-strain Probiotic Formulation on Students Facing Examination Stress: A Double-Blind, Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2021, 13, 12–18. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Karbownik, M.S.; Kręczyńska, J.; Kwarta, P.; Cybula, M.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pietras, T.; Szemraj, J. Effect of Supplementation with Saccharomyces Boulardii on Academic Examination Performance and Related Stress in Healthy Medical Students: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 1469. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Li, E. A randomized trial of probiotic supplementation in nurses to reduce stress and viral illness. Sci. Rep. 2022, 12, 14742. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Li, E.; Mitchell, E.A. Probiotics for Reduction of Examination Stress in Students (PRESS) study: A randomized, double-blind, placebo-controlled trial of the probiotic Lacticaseibacillus rhamnosus HN001. PLoS ONE 2022, 17, e0267778. [Google Scholar] [CrossRef]
- Berding, K.; Bastiaanssen, T.F.S.; Moloney, G.M.; Boscaini, S.; Strain, C.R.; Anesi, A.; Long-Smith, C.M.; Mattivi, F.; Stanton, C.; Clarke, G.; et al. Feed your microbes to deal with stress: A psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol. Psychiatry 2023, 28, 601–610. [Google Scholar] [CrossRef]
- Kafeshani, M.; Feizi, A.; Esmaillzadeh, A.; Keshteli, A.H.; Afshar, H.; Roohafza, H.; Adibi, P. Higher vitamin B6 intake is associated with lower depression and anxiety risk in women but not in men: A large cross-sectional study. Int. J. Vitam. Nutr. Res. 2020, 90, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozada, C.; Cuervo, M.; Martínez, J.A.; Goni, L.; Riezu-Boj, J.I.; Navas-Carretero, S.; Milagro, F.I.; Martínez, J.A. Changes in Anxiety and Depression Traits Induced by Energy Restriction: Predictive Value of the Baseline Status. Nutrients 2019, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Ein, N.; Armstrong, B.; Vickers, K. The effect of a very low calorie diet on subjective depressive symptoms and anxiety: Meta-analysis and systematic review. Int. J. Obes. 2019, 43, 1444–1455. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Yao, Z.; Zhan, T.; Li, Z. Dietary inflammatory potential and the incidence of depression and anxiety: A meta-analysis. J. Health Popul. Nutr. 2022, 41, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.W.; Chen, Q.T.; Towne, S.D., Jr.; Zhang, J.; Yu, H.; Tang, R.; Gasevic, D.; Wang, P.; He, Q.-Q. Fruit and vegetable intake in relation to depressive and anxiety symptoms among adolescents in 25 low- and middle-income countries. J. Affect. Disord. 2020, 261, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, B.; Ren, L.; Du, H.; Fei, C.; Qian, C.; Li, B.; Zhang, R.; Liu, H.; Li, Z.; et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front. Cell. Infect. Microbiol. 2023, 13, 1069954. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Varaee, H.; Darand, M.; Hassanizadeh, S.; Hosseinzadeh, M. Effect of low-carbohydrate diet on depression and anxiety: A systematic review and meta-analysis of controlled trials. J. Affect. Disord. 2023, 325, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, E.; Etchecopar-Etchart, D.; Thellier, D.; Lancon, C.; Boyer, L.; Fond, G. Fasting Interventions for Stress, Anxiety and Depressive Symptoms: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3947. [Google Scholar] [CrossRef]
- Deane, K.H.O.; Jimoh, O.F.; Biswas, P.; O’Brien, A.; Hanson, S.; Abdelhamid, A.; Fox, C.; Hooper, L. Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: Systematic review and meta-analysis of randomised trials. Br. J. Psychiatry 2021, 218, 135–142. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.M.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12, 2445. [Google Scholar] [CrossRef]
- Askari, M.; Daneshzad, E.; Darooghegi Mofrad, M.; Bellissimo, N.; Suitor, K.; Azadbakht, L. Vegetarian diet and the risk of depression, anxiety, and stress symptoms: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 261–271. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Arab, A.; Khorvash, F.; Kazemi, M.; Heidari, Z.; Askari, G. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on clinical, quality of life and mental health outcomes in women with migraine: A randomised controlled trial. Br. J. Nutr. 2022, 128, 1535–1544. [Google Scholar] [CrossRef]
- Gwioździk, W.; Helisz, P.; Grajek, M.; Krupa-Kotara, K. Psychobiotics as an Intervention in the Treatment of Irritable Bowel Syndrome: A Systematic Review. Appl. Microbiol. 2023, 3, 465–475. [Google Scholar] [CrossRef]
- Krupa-Kotara, K.; Helisz, P.; Gwioździk, W.; Grajek, M. The Importance of the Microbiota in Shaping Women’s Health-The Current State of Knowledge. Appl. Microbiol. 2023, 3, 11–34. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled study. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Grajek, M.; Krupa-Kotara, K.; Białek-Dratwa, A.; Sobczyk, K.; Grot, M.; Kowalski, O.; Staśkiewicz, W. Nutrition and mental health: A review of current knowledge about the impact of diet on mental health. Front. Nutr. 2022, 9, 943998. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).