Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Fungal Strain and Edible Insects
2.1.2. Cultivation of C. militaris VCCM 34117
2.2. Reagents
2.3. Adenosine and Cordycepin Content in C. militaris
2.4. Total Phenolic Content and Evaluation of Total Flavonoid Content
2.5. Antioxidant and Xanthine Oxidase Inhibition (XOD) Activities
2.6. α-Amylase Inhibition (AAI) Assay and α-Glucosidase Inhibition (AGI) Assay
2.7. Statistical Analysis
3. Results
3.1. Analysis of Adenosine and Cordycepin Concentrations in C. militaris Cultivars
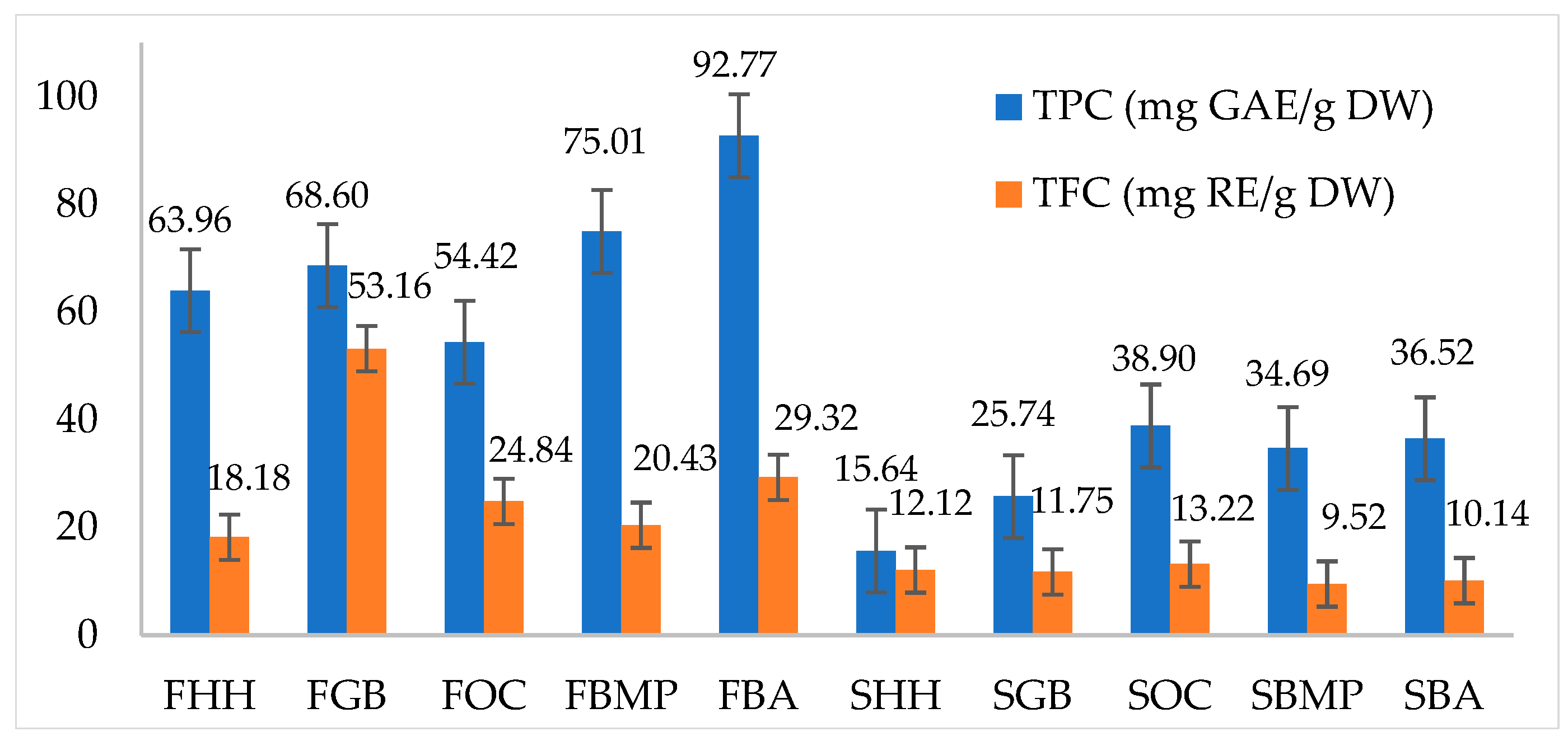
3.2. Variations in Phenolic and Flavonoid Contents across Different C. militaris Hosts
3.3. Antioxidant and Xanthine Oxidase Inhibitory Activities of Cordyceps militaris Cultivations
3.4. Analysis of Enzymatic Inhibition by C. militaris Cultivations in Type 2 Diabetes Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, S.; Shrestha, B.; Park, J.H.; Lee, D.Y.; Cho, J.G.; Baek, N.I. Chemical constituents of Yarsagumba (Ophiocordyceps sinensis Berk.), a valued traditional Himalayan medicine. Nep. J. Sci. Technol. 2012, 13, 43–58. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, C.M.; Lee, S.W.; Seo, S.Y.; Seo, M.J.; Kang, B.W.; Jo, W.S. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol. Rep. 2013, 30, 1996–2002. [Google Scholar] [CrossRef]
- Liu, J.Y.; Feng, C.P.; Li, X.; Chang, M.C.; Meng, J.L.; Xu, L.J. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int. J. Biol. Macromol. 2016, 86, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, K.S.; Shaw, J.F.; Chen, J.R.; Kuo, W.S.; Shen, G.; Yang, C.H. Trends in the immunomodulatory effects of Cordyceps militaris: Total extracts, polysaccharides and cordycepin. Front. Pharmacol. 2020, 11, 575704. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D. Superfoods: The Food and Medicine of the Future; North Atlantic Books: Berkeley, CA, USA, 2009. [Google Scholar]
- Hanser, M.; Hudson, S.L. Alternative Therapies: Growing Options in Nursing Practice; National Center of Continuing Education, Inc.: Lakeway, TX, USA, 2006. [Google Scholar]
- Munch, A.H.A.B.G. Neuroprotective Mechanisms: Oxidative Stress as a Target for Neuroprotective Therapies in Alzheimer’s and Parkinson’s. Handb. Neurochem. Mol. Neurobiol. Degener. Dis. Nerv. Syst. 2007, 84, 77–102. [Google Scholar]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from mushroom: Health attributes and food industry applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef] [PubMed]
- Jedrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Zieba, P.; Marzec, K.; Sekara, A.; Muszynska, B. Cordyceps militaris—Fruiting Bodies, Mycelium, and Supplements: Valuable Component of Daily Diet. Antioxidants 2022, 11, 1861. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi. 2021, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products. Int. J. Med. Mushrooms 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Phoungthong, K.; Aiphuk, W.; Maneerat, T.; Suwunwong, T.; Choto, P.; Chomnunti, P. Utilization of corncob biochar in cultivation media for cordycepin production and biomass of C. militaris. Sustainability 2022, 14, 9362. [Google Scholar]
- Zeng, Z.; Mou, D.; Luo, L.; Zhong, W.; Duan, L.; Zou, X. Different cultivation environments affect the yield, bacterial community and metabolites of Cordyceps cicadae. Front. Microbiol. 2021, 12, 669785. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N.; Ratanavaraporn, J.; Nakpheng, T.; Srichana, T. An anticancer cordycepin produced by Cordyceps militaris growing on the dead larva of Bombyx mori silkworm. J. Agric. Sci. 2014, 6, 41. [Google Scholar]
- Li, Y.T.; Yao, H.T.; Huang, Z.L.; Gong, L.C.; Herman, R.A.; Wu, F.A.; Wang, J. Silkworm Pupae globulin promotes Cordyceps militaris fermentation: Regulation of metabolic pathways enhances cordycepin synthesis and extends the synthesis phase. Food Biosci. 2024, 59, 103971. [Google Scholar] [CrossRef]
- Kaewkam, A.; Sornchai, P.; Chanprame, S.; Iamtham, S. Utilization of Spirulina maxima to enhance yield and cordycepin content in Cordyceps militaris artificial cultivation. Antioxidants 2021, 11, 1861. [Google Scholar]
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Guo, S.; Wang, W.; Liu, X. Cordyceps industry in China. Mycology 2015, 6, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.H.; Cheng, Z.; Yang, X.L.; Li, S.; Ding, Z.Q.; Zhou, T.S.; Chen, J.K. Genetic diversity and structure of Cordyceps sinensis populations from extensive geographical regions in China as revealed by inter-simple sequence repeat markers. J. Microbiol. 2008, 46, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Thananusak, R.; Laoteng, K.; Raethong, N.; Koffas, M.; Vongsangnak, W. Dissecting metabolic regulation in mycelial growth and fruiting body developmental stages of Cordyceps militaris through integrative transcriptome analysis. Biotechnol. Bioprocess Eng. 2023, 28, 406–418. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Dong, T.T.; Tsim, K.W. Determination of Nucleosides in Natural Cordyceps sinensis and Cultured Cordyceps mycelia by Capillary Electrophoresis. Electrophoresis 2001, 22, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of theTotal Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Khanh, T.D.; Chung, I.M. Comparative Efficacies In Vitro of Antibacterial, Fungicidal, Antioxidant, and Herbicidal Activities of Momilatones A and B. J. Plant Interact. 2007, 2, 245–251. [Google Scholar] [CrossRef]
- Minh, T.N.; Van, T.M.; Andriana, Y.; Hau, D.V.; Duyen, D.H.; Guzman-Gelani, C.D. Antioxidant, Xanthine oxidase, α-Amylaseand α-Glucosidase Inhibitory Activities of Bioactive Compounds from Rumex crispus L. Root. Molecules 2019, 24, 3899. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Liang, C.H.; Wu, C.Y. Various grain substrates for the production of fruiting bodies and bioactive compounds of the medicinal caterpillar mushroom, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2014, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.X.; Xue, D.; Lu, Z.H.; Huang, H.L. Effects of substrates on the production of fruiting bodies and the bioactive components by different Cordyceps militaris strains (ascomycetes). Int. J. Med. Mushrooms 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, P.D.; Loomis-Powers, M.; Patel, D. Analysis of quality and techniques for hybridization of medicinal fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes). Int. J. Med. Mushrooms 2004, 6, 2. [Google Scholar]
- Li, Y.; Yang, H.; Yang, H.; Wang, J.; Chen, H. Assessment of drying methods on the physiochemical property and antioxidant activity of Cordyceps militaris. J. Food Meas. Charact. 2019, 13, 513–520. [Google Scholar] [CrossRef]
- Yu, J.; Sun, M.; Wang, X.; Qi, D.; Han, C. Poly-pathways metabolomics for high-yielding cordycepin of Cordyceps militaris. Biomed. Chromatogr. 2023, 37, e5551. [Google Scholar] [CrossRef] [PubMed]
- Turk, A.; Abdelhamid, M.A.; Yeon, S.W.; Ryu, S.H.; Lee, S.; Ko, S.M.; Kim, B.S.; Pack, S.P.; Hwang, B.Y.; Lee, M.K. Cordyceps mushroom with increased cordycepin content by the cultivation on edible insects. Front. Microbiol. 2022, 13, 1017576. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.V.; Balasubramanian, B.; Park, S.; Bhattacharya, S.; Kadanthottu Sebastian, J.; Liu, W.C.; Malaviya, A. Conservation of Endangered Cordyceps sinensis Through Artificial Cultivation Strategies of Cordyceps militaris, an Alternate. Mol. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nephale, L.E.; Moyo, N.A.; Rapatsa, M.M. Dietary Full-fat Stinkbug (Encosternum delegorguei) Meal Effects on Growth Performance, Blood Chemistry, Liver and Intestinal Histology of Juvenile Mozambique tilapia (Oreochromis mossambicus). Cogent Food Agric. 2023, 9, 2253717. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From Farm to Fork: Crickets as Alternative Source Of Protein, Minerals, and Vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef] [PubMed]
- Woolley, V.C.; Teakle, G.R.; Prince, G.; de Moor, C.H.; Chandler, D. Cordycepin, a metabolite of Cordyceps militaris, reduces immune-related gene expression in insects. J. Invertebr. Pathol. 2020, 177, 107480. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Ahmed, M.; Park, H.J. Cordyceps militaris as a biofunctional food source: Pharmacological potential, anti-inflammatory actions and related molecular mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.N.; Anh, L.V.; Trung, N.Q.; Minh, B.Q.; Xuan, T.D. Efficacy of Green Extracting Solvents on Antioxidant, Xanthine Oxidase, and Plant Inhibitory Potentials of Solid-Based Residues (SBRs) of Cordyceps militaris. Stresses 2023, 3, 11–21. [Google Scholar] [CrossRef]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.C.; Kang, C.; Meng, Z.B.; Qi, Y.B.; Hyde, K.D.; Kang, J.C. Enhanced production of cordycepin by solid state fermentation of Cordyceps militaris using additives. Chiang Mai J. Sci. 2016, 43, 972–984. [Google Scholar]
- Sripilai, K.; Chaicharoenaudomrung, N.; Phonchai, R.; Chueaphromsri, P.; Kunhorm, P.; Noisa, P. Development of an animal-free nitrogen source for the liquid surface culture of Cordyceps militaris. Lett. Appl. Microbiol. 2023, 76, ovad053. [Google Scholar] [CrossRef] [PubMed]
- Zu, Z.; Wang, S.; Zhao, Y.; Fan, W.; Li, T. Integrated enzymes activity and transcriptome reveal the effect of exogenous melatonin on the strain degeneration of Cordyceps militaris. Front. Microbiol. 2023, 14, 1112035. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, Q.; Wang, Y.; Li, L.; Zhu, L.; Kuang, X.; Dai, H. Design, synthesis, antibacterial/antitumor activity and in vitro stability of novel cordycepin derivatives with unsaturated fatty acid chain. Eur. J. Pharm. Sci. 2023, 187, 106466. [Google Scholar] [CrossRef]
- Hoang, C.Q.; Duong, G.H.; Tran, M.H.; Vu, T.X.; Tran, T.B.; Pham, H.T. Molecular mechanisms underlying phenotypic degeneration Cordyceps militaris: Insights from transcriptome reanalysis and osmotic stress studies. Sci. Rep. 2024, 14, 2231. [Google Scholar] [CrossRef] [PubMed]
- Soraksa, N.; Heebkaew, N.; Promjantuek, W.; Kunhorm, P.; Kaokean, P.; Chaicharoenaudomung, N.; Noisa, P. Cordycepin, a bioactive compound from Cordyceps spp., moderates Alzheimer’s disease-associated pathology via anti-oxidative stress and autophagy activation. J. Asian Nat. Prod. Res. 2024, 26, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, J.; Zhou, Z.; Li, Z.; Zhou, Y.; Xu, S.; Zou, X. Positive effects of Cordyceps cateniannulata colonization in tobacco: Growth promotion and resistance to abiotic stress. Front. Microbiol. 2023, 14, 1131184. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Huang, R.Y.; Wei, Y.J.; Tsai, G.J.; Huang, C.H. Influence of C. militaris-fermented grain substrate extracts on alleviating food allergy in mice. Heliyon 2023, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Valado, A. Algae-Derived Natural Products in Diabetes and Its Complications—Current Advances and Future Prospects. Life 2023, 13, 1831. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Malek, R.A.; Elmarzugi, N.A.; Mahomoodally, M.F.; Uy, D.; Leng, O.M.; El-Enshasy, H.A. Cordycepin: A biotherapeutic molecule from medicinal mushroom. In Biology of Macrofungi; Springer: Berlin/Heidelberg, Germany, 2018; pp. 319–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.J. Cordyceps as potential therapeutic agents for atherosclerosis. J. Integr. Med. 2024, 22, 102–114. [Google Scholar] [CrossRef] [PubMed]


| Insect | Nutritional Characteristics | Contribution to C. militaris Cultivation | Part | Weight (g) | Code |
|---|---|---|---|---|---|
| Bombyx mori Pupae (Silkworm Pupae) | Proteins (51–55%) Fats (25–30%) Essential amino acids Vitamins (B) Minerals (K, Ca, Mg, Fe) | Provides robust nutrients for fungal growth and bioactive compound synthesis | Fruiting Body | 1.32 ± 0.08 | FBMP |
| Solid-Based Residue | 4.28 ± 0.12 | SBMP | |||
| Brihaspa atrostigmella (Chit Worm) | High in protein Fats Essential Micronutrients Bioactive antioxidant compounds | Supports production of cordycepin and adenosine | Fruiting Body | 1.50 ± 0.10 | FBA |
| Solid-Based Residue | 4.50 ± 0.15 | SBA | |||
| Halyomorpha halys (Brown Stink Bug) | Proteins (up to 70%) Healthy fats Vitamins (riboflavin, niacin) Minerals (Fe, Mg, Zn) | Contributes to antioxidant and enzymatic activities | Fruiting Body | 1.24 ± 0.06 | FHH |
| Solid-Based Residue | 4.50 ± 0.15 | SHH | |||
| Oxya chinensis (Grasshoppers) | Proteins (60–70%) Unsaturated fats Vitamins (B12, E) Minerals (Fe, Zn, Mg) | Promotes synthesis of phenols and flavonoids | Fruiting Body | 1.15 ± 0.05 | FOC |
| Solid-Based Residue | 4.50 ± 0.15 | SOC | |||
| Gryllus bimaculatus (Cricket) | Proteins (65–70%) Unsaturated fats Vitamins (B12, riboflavin) Minerals (Fe, Ca, Mg) | Enhances xanthine oxidase inhibitory activity | Fruiting Body | 1.30 ± 0.07 | FGB |
| Solid-Based Residue | 4.25 ± 0.13 | SGB |
| Code | Adenosine | Cordycepin |
|---|---|---|
| (mg/g) | (mg/g) | |
| FHH | 0.984 ± 0.015 b | 1.818 ± 0.012 b |
| FGB | 0.810 ± 0.009 c | 2.658 ± 0.006 a |
| FOC | 0.774 ± 0.007 c | 1.242 ± 0.004 b |
| FBMP | 0.572 ± 0.011 d | 2.554 ± 0.010 a |
| FBA | 1.062 ± 0.014 a | 2.932 ± 0.011 a |
| SHH | 0.164 ± 0.015 e | 0.303 ± 0.012 c |
| SGB | 0.135 ± 0.006 e | 0.443 ± 0.007 c |
| SOC | 0.129 ± 0.008 e | 0.207 ± 0.005 c |
| SBMP | 0.053 ± 0.004 f | 0.238 ± 0.006 c |
| SBA | 0.130 ± 0.010 e | 0.338 ± 0.011 c |
| HPLC | Standards | |
| Retention time (min) | 10.828 ± 0.108 | 11.236 ± 0.122 |
| LOD (µg/mL) | 0.274 | 0.366 |
| LOQ (µg/mL) | 0.831 | 1.11 |
| Code | IC50 (µg/mL) | IC50 (µg/mL) | |
|---|---|---|---|
| DPPH | ABTS | XOD | |
| FHH | 128.1 ± 9.2 de | 1219. ± 20.9 b | 755.0 ± 12.9 f |
| FGB | 119.4 ± 8.5 de | 1264. ± 21.6 b | 415.7 ± 11.2 g |
| FOC | 150.6 ± 10.8 d | 421.7 ± 30.3 f | 727.9 ± 12.4 f |
| FBMP | 109.2 ± 7.8 de | 305.9 ± 22.0 g | 932.1 ± 15.9 e |
| FBA | 88.34 ± 6.3 e | 247.3 ± 17.7 g | 427.0 ± 7.3 g |
| SHH | 513.9 ± 20.2 a | 1429.8 ± 40.5 a | 1522.9 ± 26.1 b |
| SGB | 318.3 ± 22.9 b | 961.7 ± 27.3 c | 1703.8 ± 29.2 a |
| SOC | 210.6 ± 15.1 c | 526.6 ± 37.8 e | 909.1 ± 15.5 e |
| SBMP | 236.2 ± 16.9 c | 646.7 ± 21.9 d | 1142.0 ± 19.5 c |
| SBA | 224.4 ± 16.1 c | 612.3 ± 17.4 d | 1084.8 ± 18.6 d |
| BHT * | 18.78 ± 1.3 f | 40.4 ± 0.6 h | - |
| Allopurinol * | - | - | 21.0 ± 0.3 h |
| Code | IC50 (µg/mL) | |
|---|---|---|
| α-Amylase Inhibition | α-Glucosidase Inhibition | |
| FHH | 631.1 ± 6.1 b | 450.7 ± 18.4 c |
| FGB | 895.4 ± 5.7 b | 746.2 ± 21.0 c |
| FOC | 887.9 ± 7.2 b | 591.9 ± 14.5 c |
| FBMP | 979.1 ± 5.2 b | 815.9 ± 28.9 c |
| FBA | 504.6 ± 4.2 c | 336.4 ± 16.0 d |
| SHH | 3538.7 ± 25.1 a | 2722.1 ± 27.8 b |
| SGB | 3867.4 ± 15.2 a | 2578.3 ± 34.3 b |
| SOC | 3808.8 ± 10.0 a | 2539.2 ± 29.1 b |
| SBMP | 3924.3 ± 11.3 a | 3018.7 ± 11.6 a |
| SBA | 3614.8 ± 10.7 a | 2780.6 ± 44.9 b |
| Acarbose * | 90.7 ± 0.6 d | 143.2 ± 2.1 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trung, N.Q.; Dat, N.T.; Anh, H.N.; Tung, Q.N.; Nguyen, V.T.H.; Van, H.N.B.; Van, N.M.N.; Minh, T.N. Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications. Chemistry 2024, 6, 517-530. https://doi.org/10.3390/chemistry6040030
Trung NQ, Dat NT, Anh HN, Tung QN, Nguyen VTH, Van HNB, Van NMN, Minh TN. Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications. Chemistry. 2024; 6(4):517-530. https://doi.org/10.3390/chemistry6040030
Chicago/Turabian StyleTrung, Nguyen Quang, Nguyen Tien Dat, Ho Ngoc Anh, Quach Ngoc Tung, Vu Thi Hanh Nguyen, Ho Ngoc Bich Van, Nguyen Minh Nhat Van, and Truong Ngoc Minh. 2024. "Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications" Chemistry 6, no. 4: 517-530. https://doi.org/10.3390/chemistry6040030
APA StyleTrung, N. Q., Dat, N. T., Anh, H. N., Tung, Q. N., Nguyen, V. T. H., Van, H. N. B., Van, N. M. N., & Minh, T. N. (2024). Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications. Chemistry, 6(4), 517-530. https://doi.org/10.3390/chemistry6040030








