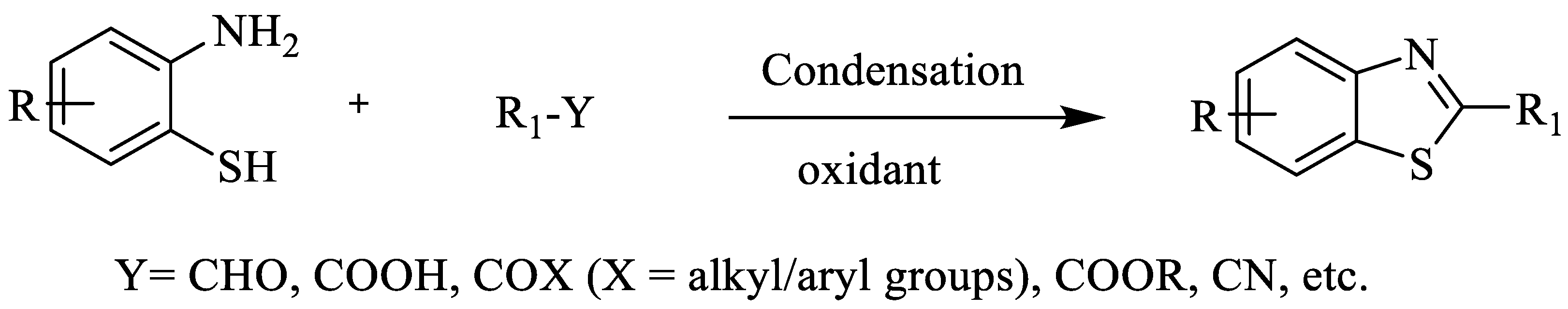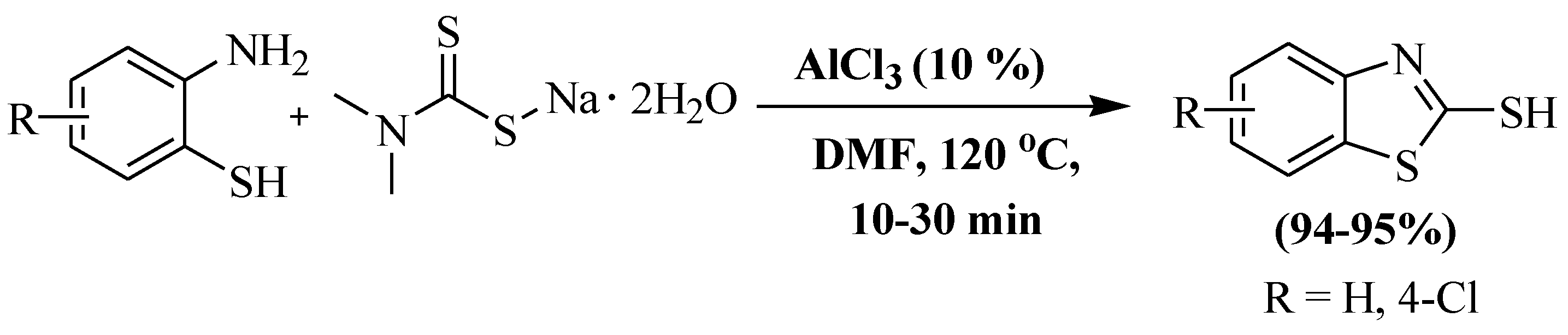Abstract
Heterocycles, compounds featuring heteroatoms like nitrogen, sulfur, and oxygen, are integral in fields such as synthesis, pharmacology, and medicine. Among these, benzothiazoles, formed by fusing thiazole with benzene, hold significant prominence. Their unique reactivity, especially at the carbon position between nitrogen and sulfur, has sparked wide interest. Notably, 2-substituted benzothiazoles exhibit diverse biological activities, including anticonvulsant, antimicrobial, and antioxidant properties, making them valuable in drug discovery. This review unveils an array of mesmerizing methods employed by chemists to prepare these compounds using 2-aminothiophenol as one of the precursors with other varied reactants. From novel strategies to sophisticated methodologies, each section of this review provides a glimpse into the fascinating world of synthetic chemistry of 2-substituted benzothiazoles. Delving into the diverse synthetic applications of 2-substituted benzothiazoles, this paper not only enriches our understanding of their synthesis but also sparks the imagination with the possibilities for future advancements.
1. Introduction
Heterocycles, a fascinating class of organic compounds, form the cornerstone of diverse scientific disciplines [1]. These compounds are defined by their unique ring structures, where at least one of the atoms in the ring is an element other than carbon, commonly nitrogen, sulfur, or oxygen [2,3]. This diversity in composition lends heterocycles remarkable chemical and biological properties, making them central to fields such as pharmaceuticals, materials science, and agrochemicals [4,5,6,7].
The origin of benzothiazoles (BTs) can be traced back to the rich tapestry of organic chemistry [8]. In 1887, A.W. Hoffmann synthesized 2-substituted BT, captivated by its diverse activities and straightforward cyclization mechanism [9]. The fundamental structure of BTs comprises a fusion of a thiazole ring, which consists of a five-membered ring containing sulfur and nitrogen, with a benzene ring, forming a bicyclic structure [10,11,12]. This unique arrangement grants BTs their distinctive properties, making them intriguing subjects of study in both laboratory and industrial applications [13,14].
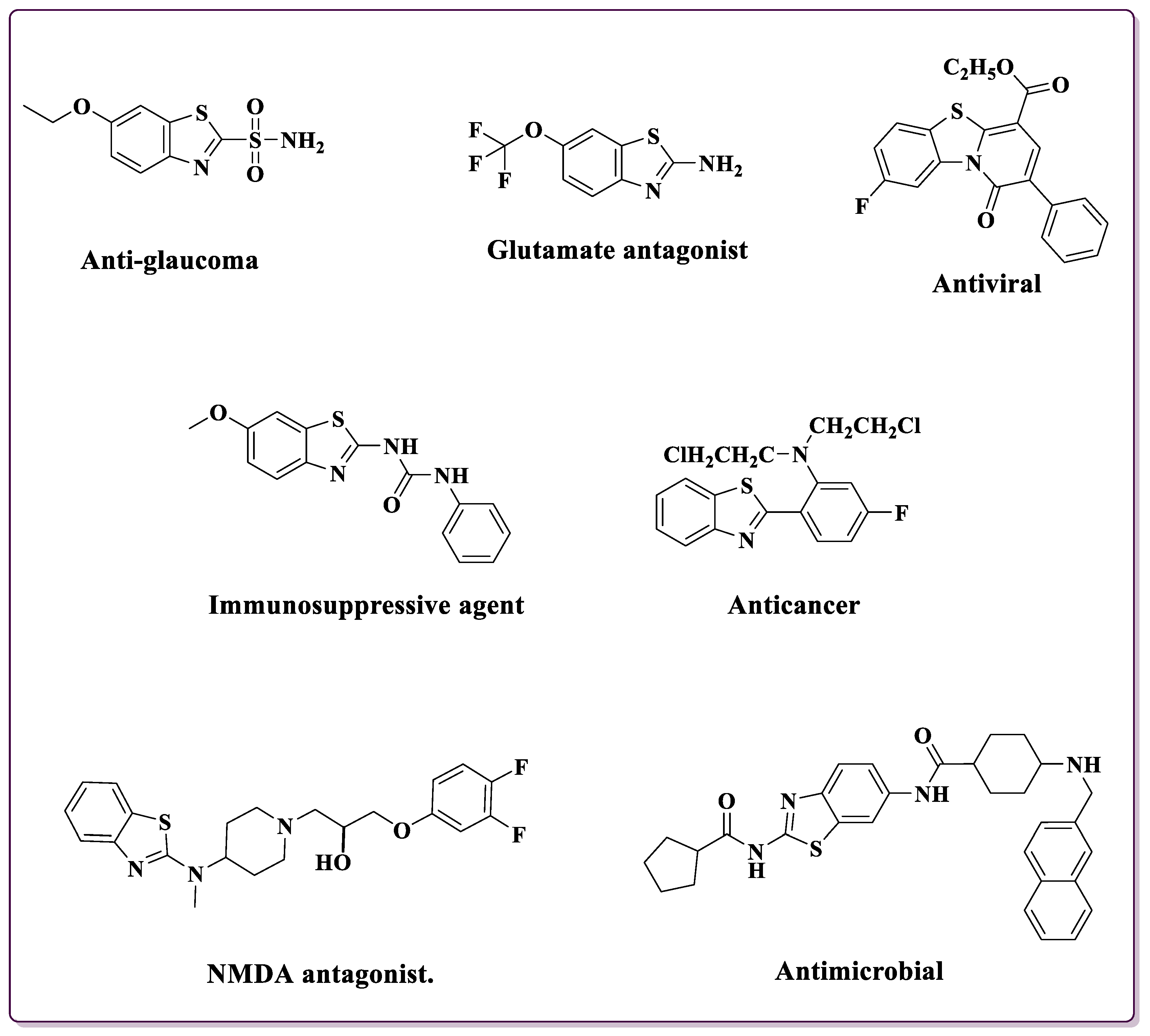
Over the years, the versatility of BTs has been harnessed across various scientific domains [15]. Their presence in medicinal chemistry has led to the development of pharmaceuticals with diverse therapeutic applications. 2-substituted BTs showcase a wide array of biological activities, including antimicrobial [16,17,18], antioxidant [19], anticonvulsant [20], anti-proliferative [21], anti-Parkinson [22], anti-diabetic [23], antimalarial [24], and anti-inflammatory [25] effects. Beyond their pharmacological significance, these compounds find diverse industrial applications such as corrosion inhibition [26], dyes [27], polymer chemistry [28], pesticides [29], textiles [30], and vulcanization acceleration [31]. Categorized in Figure 1 are various drugs incorporating the 2-substituted BT moiety, showcasing the diverse applications and structural variations in this pharmacophore in medicinal chemistry [32,33,34,35,36,37,38].
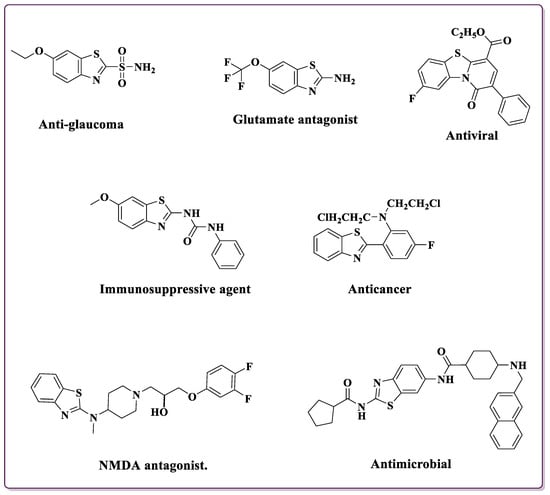
Figure 1.
Compounds ncorporating the 2-substituted BT moiety in pharmaceuticals [32,33,34,35,36,37,38].
The exploration of 2-substituted BTs continues to inspire scientists, offering valuable insights into the intricate world of organic compounds and driving innovations in the fields of medicine, materials, and beyond. This article serves as a gateway to the captivating realm of 2-substituted BTs, showcasing the elegance of diverse synthetic methods that have sculpted these compounds into marvels of chemical ingenuity, with a specific emphasis on strategies employing 2-aminothiophenol (2-ABT) as a starting material.
2. Synthetic Aspects of 2-Substituted BTs
Several synthetic pathways have been developed for 2-substituted BTs. The synthesis involves the reaction of 2-ABT with aldehydes/ketones/cyanides/esters/acids/acyl halides, etc. [39,40] (Scheme 1).

Scheme 1.
Synthesis of 2-substituted BTs [39,40].
2.1. Reaction of 2-ABT and Aldehydes
Enormous synthetic pathways have been developed for 2-substituted BTs using 2-ABT and aldehydes, featuring an array of catalytic systems such as ionic liquids [41,42], nanoparticles [43,44], acid catalysts [45,46,47], visible light [48], metal catalysts [49], biocatalyst [50], etc. Numerous catalytic avenues have been explored for the construction of 2-substituted BTs.
- (a)
- Synthesis of 2-substituted BTs using ionic liquids (ILs)
The development of environmentally benign procedures for the synthesis of heterocyclic scaffolds is a requirement of the current era. For this, the utilization of ILs has increased as they act as catalysts as well as reaction media due to their special properties like thermal stability, recyclability, biodegradability, non-volatility, high catalytic activity, good selectivity, etc.
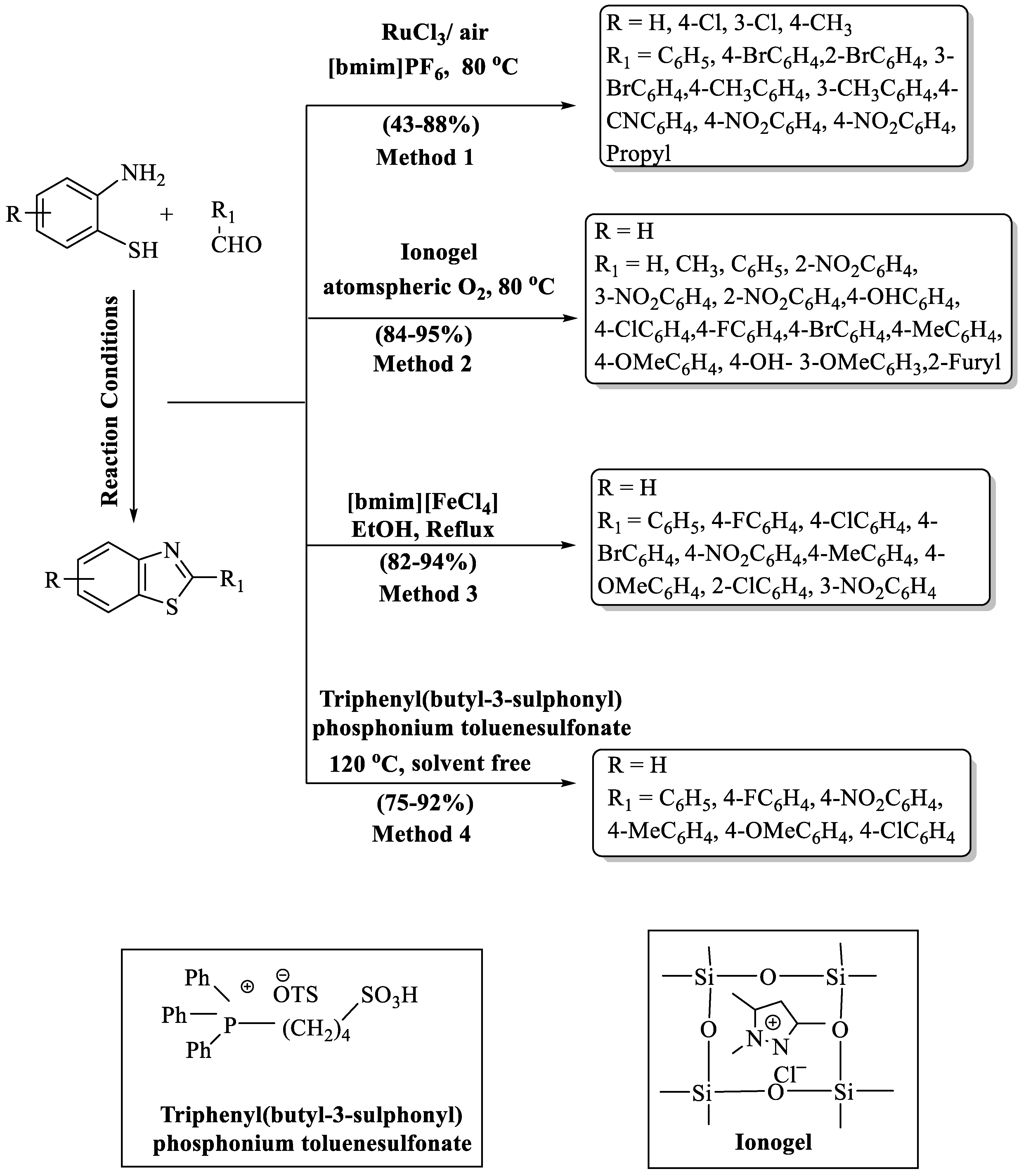
The production of 2-substituted BT analogs through oxidative condensation of 2-ABT and differently substituted aldehydes using RuCl3 operating as a catalytic mediator in [bmim]PF6 (1-butyl-3-methylimidazolium hexafluorophosphate) as a solvent and air as an oxidant was developed by Fan and coauthors [51]. (Scheme 2, Method 1). Moderate to high yields (43–88%) were obtained in 0.5–6 h with 3 instances of recyclability of the catalyst. In this Ru (III)-catalyzed oxidative reaction, it was observed that the presence of electron-withdrawing groups (EWG) on either the aldehyde or 2-ABT resulted in higher yields compared to electron-donating groups (EDG). The EWGs exhibited a favorable impact on the outcome of the reaction, leading to an enhanced yield of the desired product. The method exhibited a limitation in the case of 2-amino-3-chlorobenzenethiol with 2-nitrobenzaldehyde, resulting in a relatively low yield of 43% within a 6 h timeframe.
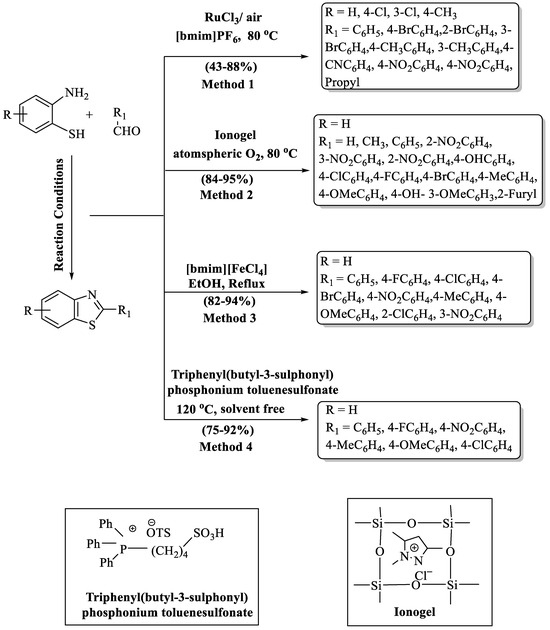
Scheme 2.
Preparation of 2-substituted BT using ionic liquids as catalyst [51,52,53,54].
Sharma and co-authors [52] generated a nature-friendly route to obtain 2-substituted BT derivatives via a nanorod-shaped ionogel (Scheme 2, Method 2). The protocol has various benefits such as its solvent-free, non-toxic catalyst, and the recyclability of the catalyst, which made this strategy highly environmentally benign. A high-to-excellent yield (84–95%) of products was obtained in 10–25 min at ambient reaction conditions (80 °C, solvent-free). The designed pathways were compatible with both EDG and EWG. High yields were obtained with both types of substituents.
A library of new 2-substitutedBTs was innovated by Sayyahi et al. [53] from the reaction involving 2-ABT and a range of aldehydes under the influence of 1-butyl-3-methylimidazolium tetrachloro ferrate (III) [bmim][FeCl4] (Scheme 2, Method 3). The pathway gave high yields (82–94%) of products in 30–90 min. Notably, the procedure demonstrated excellent yields (>90%) with EWG in comparison to EDG. The protocol’s requirement of column chromatography for the purification of synthesized derivatives and the metal catalyst employed were cons of the method.
Hang and coauthors [54] demonstrated a highly proficient, convenient, and environmentally responsible route for 2-substituted BT synthesis, using phosphonium acidic IL as a catalyst through the reaction of 2-ABT with aldehydes (Scheme 2, Method 4). Excellent yields (75–92%) of the desired 2-substituted BTs were obtained at 120 °C using 7 mol% of the catalyst in 25–90 min. The authorized route gave higher yields with EDG compared to the EWG. Despite its notable strengths, such as rapid reaction kinetics, high product yields, and catalyst reusability, the method exhibited limitations in substrate scope and required elevated temperatures, representing its drawbacks.
- (b)
- Synthesis of 2-substituted BTs using nanoparticles
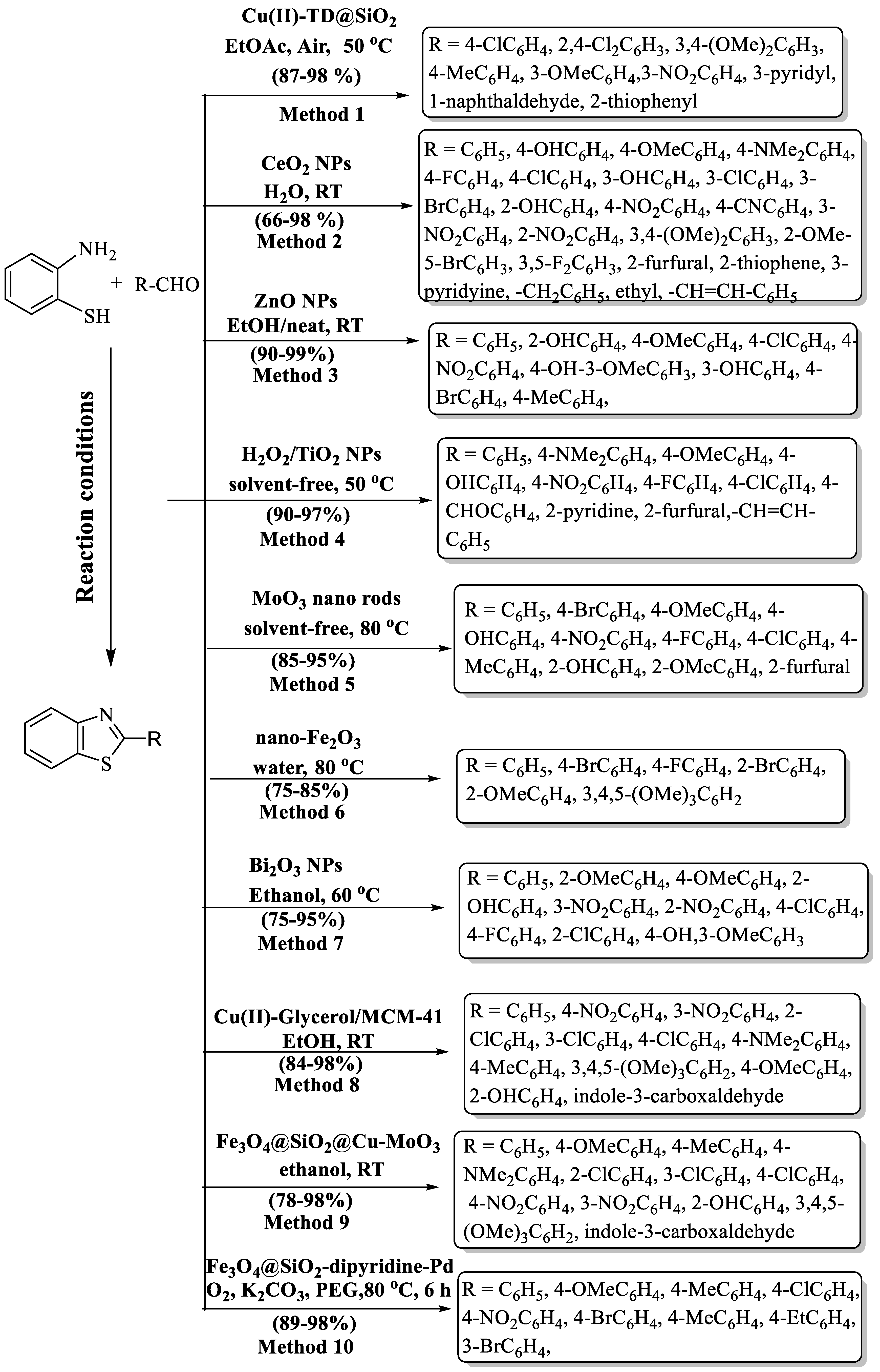
Nasr-Esfahani and colleagues [55] developed a facile route to synthesize 2-substituted BTs using 2-ABT and aryl aldehydes as starting materials and Cu(II)-containing nano-silica triazine dendrimer supported over SiO2 as a catalyst. The desired products were obtained at a yield of 87–98% in 15–90 min (Scheme 3, Method 1). The authors employed a wide substrate scope (disubstituted, EDG, EWG, and heterocycles) and found high yields, irrespective of the nature of the substrate. The only drawback of the process was the use of metal-based catalysts.
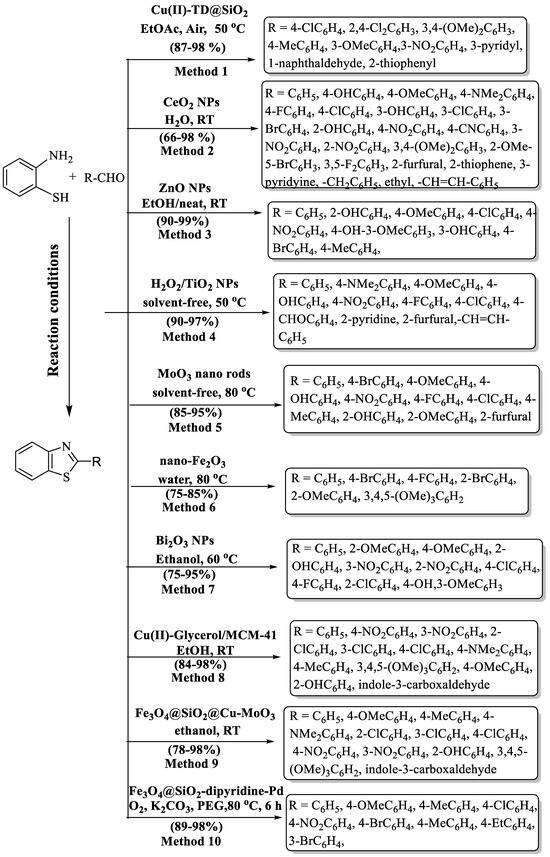
Scheme 3.
Preparation of 2-substituted BTs using nanoparticles (NPs) [44,55,56,57,58,59,60,61,62,63].
Shelkar and coauthors [56] introduced an accessible and green technique for the preparation of 2-substituted BT using nano CeO2 and water solvent at RT using different aldehydes and 2-ABT. The authors examined various catalysts viz. ZnO, MnO2, TiO2, CeO2, SiO2, Al2O3, La2O3, nanoZnO, and nano Cu2O, but nano CeO2 was found to be superior to others because it has a high surface area and small particle size and it provided better catalytic sites (Scheme 3, Method 2). It was observed that 5 mol% and 20–30 min were sufficient for the high yield (76–96%) of the wished products. An excellent yield was achieved with EWG as compared to EDG. Disubstituted aldehydes were also examined, and they exhibited high yields. The aliphatic aldehydes gave lower yields as compared to aromatic aldehydes. The catalyst’s reusability allowed for up to 3 repetitions, with a decrease in activity observed in each subsequent cycle.
Banerjee and coworkers [57] synthesized 2-substituted BTs by the reaction of 2-ABT and diversely substituted aldehydes using ZnO NPs in EtOH/neat at RT (Scheme 3, Method 3). ZnO NPs were very effective catalysts for the synthesis of 2-substituted BTs because they took a very short reaction time (2–8 min), were reusable eight times without significant loss of activity, are cost-effective, eco-friendly, had excellent yields, and possess easy product isolation without column chromatography; however, reliance on a metal catalyst was the limiting aspect of the method. The authors employed diversely substituted aldehydes and they gave an excellent yield, which means the yield of the products was irrespective to the nature of the substituent. The procedure was carried out under solvent-free conditions with liquid aldehydes whereas, for solid aldehydes, ethanol was used as a solvent.
Bahrami and co-workers [58] composed a way to synthesize 2-substituted BT analogs utilizing TiO2 NPs and with the addition of H2O2 under daylight conditions (Scheme 3, Method 4). The devised approach yielded outstanding results, with impressive 90–97% returns achieved in a remarkably brief reaction window of 5–27 min. The yield of the designated pathway was not affected by the presence of any kind of substituents (heterocycles/ EDG/EWG).
Dighore and colleagues [59] developed a green technique for the production of 2-substituted BT via MoO3 NPs. This synthetic route has several advantages such as lower reaction time, recyclability and reusability of the catalyst without loss of activity, solvent-free synthesis, mild reaction conditions, easy workup, green strategy, and stability of MoO3 nanorods (Scheme 3, Method 5). The authors used diversly substituted aldehydes but the yield of 2-aryl BTs was not greatly influenced by the nature of substituents. The method boasts several advantages, but it is constrained by the necessity of employing a transition metal catalyst.
Kommula et al. [44] introduced an ingenious, user-friendly, eco-conscious, and economically viable process for the production of BT derivatives. This innovative two-component reaction included a combination of substituted anilines with aromatic aldehydes, harnessing the recyclable and regenerable properties of a 10% nano-Fe2O3 catalyst in an aqueous solution. The outcome was the attainment of these desired compounds in high-to-excellent yields, between 75% and 85%, in a 2-to-3 h time (Scheme 3, Method 6). The procedure gave high yields with EDG in comparison to EWG. The method has limited substrate scope and, particularly, halo-substituted aldehydes exhibited lower yields.
A hassle-free method for the preparation of 2-substituted BT has been demonstrated by Sharma and colleagues [60] using Bi2O3 NPs as an efficient heterogeneous catalyst at 60 °C. (Scheme 3, Method 7). The designed protocol afforded the envisioned products, yielding a remarkable 75–95% in just 1–2 h. The protocol resulted in higher yields with EWG as compared to EDG.
Pesyan and co-workers [61] engineered a catalyst through the conjugation of Cu(II) with (MeO)3Si(CH2)3Cl, synergized with glycerol, resulting in an exceptionally efficient and resilient catalyst. The reaction proceeded seamlessly at RT, with ethanol as the chosen solvent, delivering remarkable yields (84% to 98%) for the desired products, all accomplished within a concise 2–5 h timeframe (Scheme 3, Method 8). The developed route also gave high yields with EWG, the same as mentioned above in Sharma’s protocol [60]. Moreover, the catalyst exhibited remarkable sustainability, permitting recycling and reuse for up to five successive runs. This intricately designed method boasts a plethora of benefits, including remarkable tolerance for diverse functional groups, the utilization of an environmentally responsible solvent, and outstanding moisture stability of the catalyst. The method involved column chromatography for the purification of derivatives, employed a metal-based catalyst, and experienced extended reaction times for certain compounds.
The uniquely prepared bimetallic particle catalyst (Fe3O4@SiO2@Cu-MoO3) was tailored for synthesizing BTs from 2-ABT and diverse aldehydes [62], demonstrating exceptional catalytic ability. These synthesized nanocomposites display superior catalytic properties, enabling the preparation of BT derivatives with higher efficiency and enhanced selectivity. The protocol obtained excellent yield (83–98%) in 2 to 4 h with both EDG substituents and EWG substituents. However, the protocol exhibited a relatively lower yield (78%) for heterocycles, serving as a limitation in the context of this study. (Scheme 3, Method 9).
A highly fabricated MNPs-phenanthroline-Pd nano catalyst was developed for the synthesis of 2-substituted BT using 2-ABT and substituted aryl aldehydes [63]. The developed catalyst was found to be highly efficient and could be recovered and utilized for seven continuous iterations. Excellent yield was obtained with diversely substituted aldehydes (with both EDG and EWG). The method’s standout features include remarkable yields (89–98%), effortless catalyst recovery using an external magnet, and operational user-friendliness. A clear drawback of this method was its extended reaction time (6 h). (Scheme 3, Method 10).
A comparative analysis of reaction conditions and yields for ILs (part a) and NP catalysts (part b) in 2-substituted BTs synthesis is illustrated in Table 1.

Table 1.
A visual symphony—comparative table of reaction conditions and yields in the synthesis of 2-substituted BTs.
- (c)
- Visible-light-assisted synthesis of 2-substituted BTs
The photocatalyzed pathway is one of the best green methods for the implementation of chemical transformations.
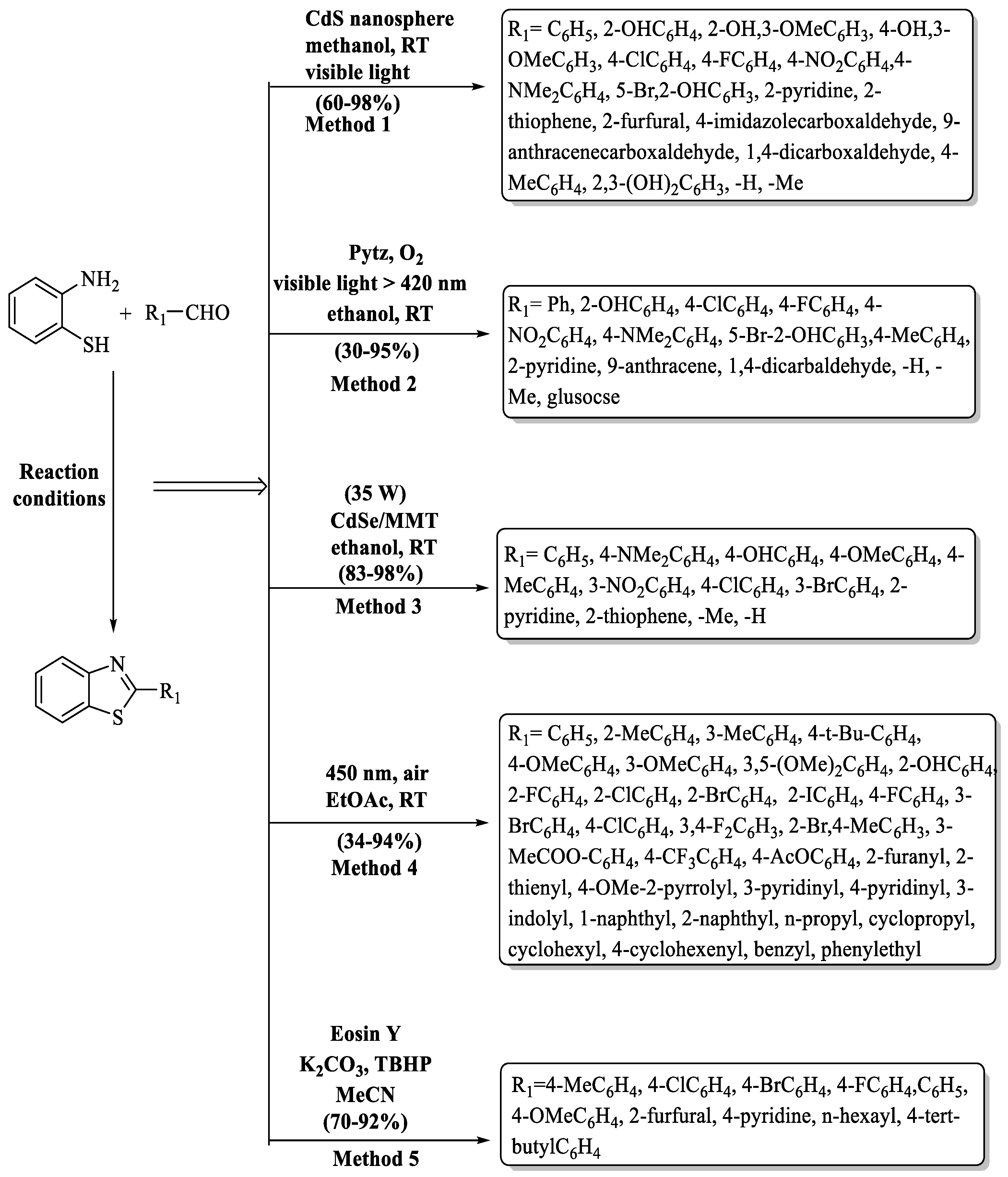
Das and co-workers [64] demonstrated a simple, sustainable, and exceptionally proficient process for synthesizing 2-substituted BT analogs, with CdS nano-spheres serving as a heterogeneous catalyst activated by visible light and methanol was utilized as solvent. This method was eco-friendly because of the photo-induced green pathway and recovery of the catalyst, mild reaction conditions, and moderate-to-high yields (60–98%) in 20–90 min. In the protocol, generally, the aromatic aldehydes containing ED substituents as well as EW substituents exhibited excellent yield. Interestingly, terephthaldehyde gave the 4-benzothiazole-yl-benzaldehyde in methanol selectively, and in chloroform phenyl-1,4-dibenzothiazole was prepared. Furthermore, 3H-imidazole-4-carbaldehyde (60%) and 9-anthracene carboxaldehyde (70%) exhibited lower yields compared to other aldehydes (Scheme 4, Method 1).
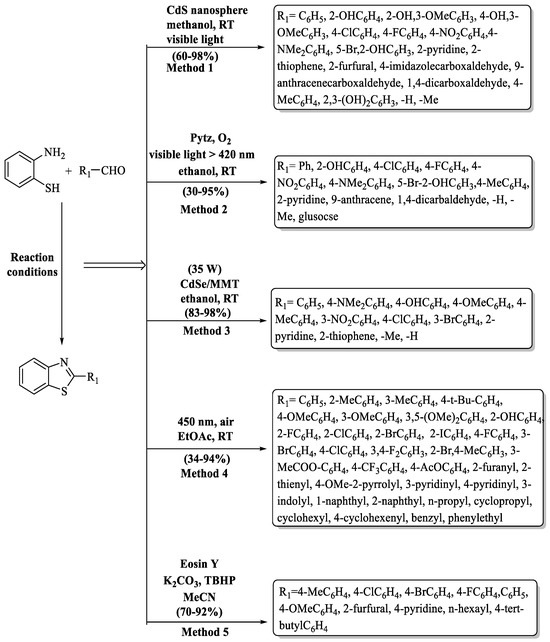
Scheme 4.
Light-activated synthesis of 2-substituted BTs [48,64,65,66,67].
Samanta and colleagues [65] instigated a green synthesis of 2-substituted BTs catalyzed by 3,6-disubstituted-s-tetrazine (pytz) in the presence of visible light (Scheme 4, Method 2). Firstly, cyclic voltammetry and UV spectroscopy were used to study the electrical and optical properties of pytz. It can absorb a maximum wavelength at 535 nm and it is highly electron deficient; thus, it can be reduced easily and this property is used to catalyze the reaction. The desired products were obtained at a yield of 30–95% in 0.5–1 h using ethanol as solvent, and the process exhibited higher yields with EWG as compared to EDG. The methodology encountered difficulties with terephthaldehyde, resulting in a notably low yield (30%) for phenyl-1,4-dibenzothiazole as it was synthesized in tetrahydrofuran instead of ethanol, presenting a challenge to the expected reaction conditions.
Wade and co-authors [66] demonstrated an uncomplicated, easy-to-use, and light-driven method to synthesize 2-substituted BTs in the presence of CdSe NPs dispersed in montmorillonite (MMT). In their quest for the ideal reaction conditions, the authors tested different variables and it was observed that the best outcomes were acquired in ethanol at 10 mol% catalyst concentration. The major benefits of this method are high yields (83–98%), short time (20–55 min), recyclability of catalyst, easy product isolation, and use of solar energy, which makes it cost-effective and environmentally benign. The method offers a speed advantage with heterocyclic aldehydes forming BTs quickly and substituents on aromatic aldehydes also irrespective to yield. However, aliphatic aldehydes required longer reaction time as compared to other substrates, presenting a drawback (Scheme 4, Method 3).
Ye and coworkers [48] unveiled a novel pathway, powered by visible light, for the synthesis of BT motifs. The protocol, meticulously engineered, stands out for its simplicity, efficiency, and the complete absence of metals and additives (Scheme 4, Method 4). It consistently delivered impressive yields of 2-substituted BT derivatives. The coveted 2-phenylbenzo[d]thiazoles were produced consistently, with yields ranging from 34% to 94% in a swift 6 to 12 h. The authors obtained good-to-excellent yields (60–94%) with a wide range of substituted aldehydes (EDG, EWG, heterocycles, disubstituted) and some aldehydes such as 4-methyl-2-pyrrolyl (50%), n-propyl (56%), and 4-cyclohexenyl (59%), phenylethyl (52%) which gave moderate yields. The lowest yield (34%) was exhibited with 4-acetoxyl benzaldehyde.
Le and co-authors [67] developed a light-driven radical cyclization method for 2-substituted BTs, employing 2-substituted anilines and aldehydes, catalyzed by Eosin Y, resulting in product yields ranging from 70% to 92%. While the designed pathway delivered high product yields, the drawbacks of the method involved rigorous reaction conditions, prolonged reaction times (24–36 h), the requirement for flash column chromatography, and non-recoverability of the catalyst. The yield of the products was not much affected by the nature of substituents on aldehydes (Scheme 4, Method 5).
- (d)
- Acid-catalyzed preparation of 2-substituted BTs
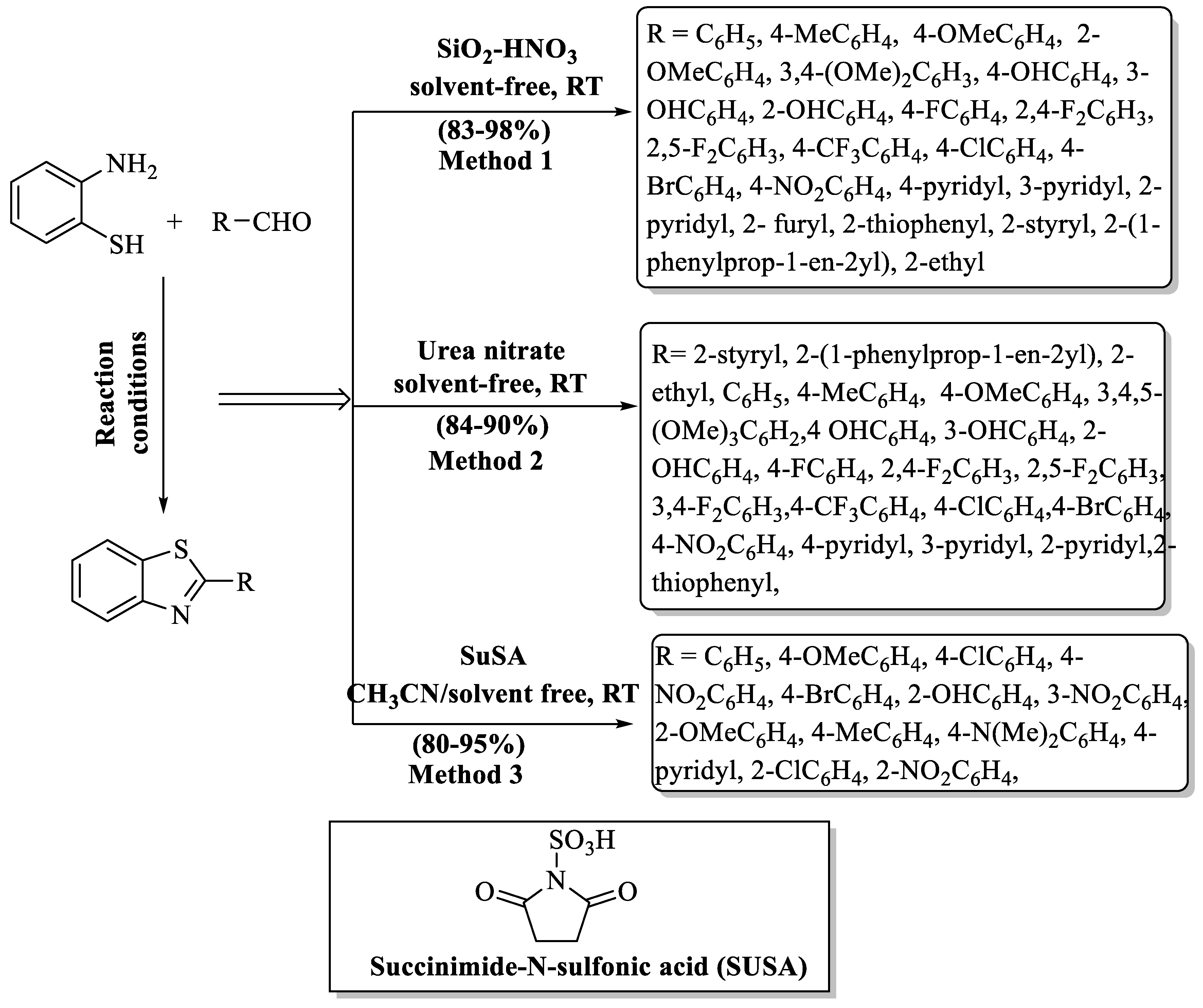
A cost-effective, simple, and solvent-free strategy for the production of 2-aryl BT analogs was devised by Kumar et al. [68] using nitric acid supported on a silica gel (SiO2–HNO3). The desired compounds were obtained at a yield of 83–98% just by shaking the reaction. The authors obtained various 2-substituted BTs (aryl/alkyl/heteroaryl/styryl) using diversely substituted aldehydes with excellent yields, and the yield was irrespective of the nature of substituents. The method exhibits practical utility as demonstrated by gram-scale synthesis, enhancing its applicability. While all derivatives were successfully isolated using recrystallization and column chromatography, it is worth noting that specific compounds (2-ethyl, 2-styryl, and 2-(1-phenylprop-1-en-2yl) derivatives) were only isolated through column chromatography, highlighting a limitation in the isolation method for these particular compounds (Scheme 5, Method 1).
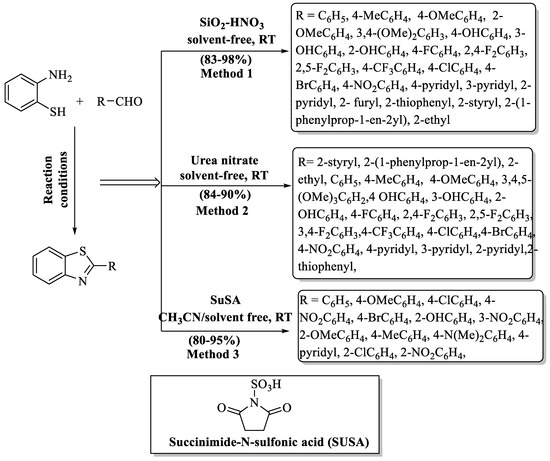
Scheme 5.
Preparation of 2-substituted BT derivatives by different acid catalysts [68,69,70].
Kumar et al. [69] demonstrated a very proficient and green strategy for the synthesis of 2-substituted BTs through the condensation of 2-ABT and aldehydes catalyzed by urea nitrate. Solvent-free, swift, and inexpensive catalyst, as well as simple workup, excellent yields (84–90%), mild reaction conditions, green synthesis, recovery and reuse of catalyst, and gram scale synthesis were the major benefits of this method; however, the requirement of column chromatography for the separation of desired compounds was the major drawback of the method. The yield of the products obtained was irrespective of the nature of substitution (alkyl/aryl/heteroaryl/styryl) (Scheme 5, Method 2).
Shaikh and colleagues [70] developed an easy, green, and effective method for the production of 2-substituted BTs by the treatment of aryl aldehydes with 2-ABT using a succinimide-N-sulfonic acid catalyst in acetonitrile/solvent-free conditions (Scheme 5, Method 3). SuSA being acidic in nature gave a proton, which was attached to the oxygen of aldehyde, thereby making it a good nucleophilic center. Then, the NH2 group of 2-ABT attacked the carbonyl carbon and formed imine as an intermediate stimulated nucleophilic attack to furnish the desired products in 5–15 min. The authors employed the grindstone technique, and a wide range scope of aldehydes gave excellent yields (80–95%) whether containing EDG or EWG as substituent. The use of acetonitrile as a solvent in the method posed a potential drawback since acetonitrile is classified as an air pollutant.
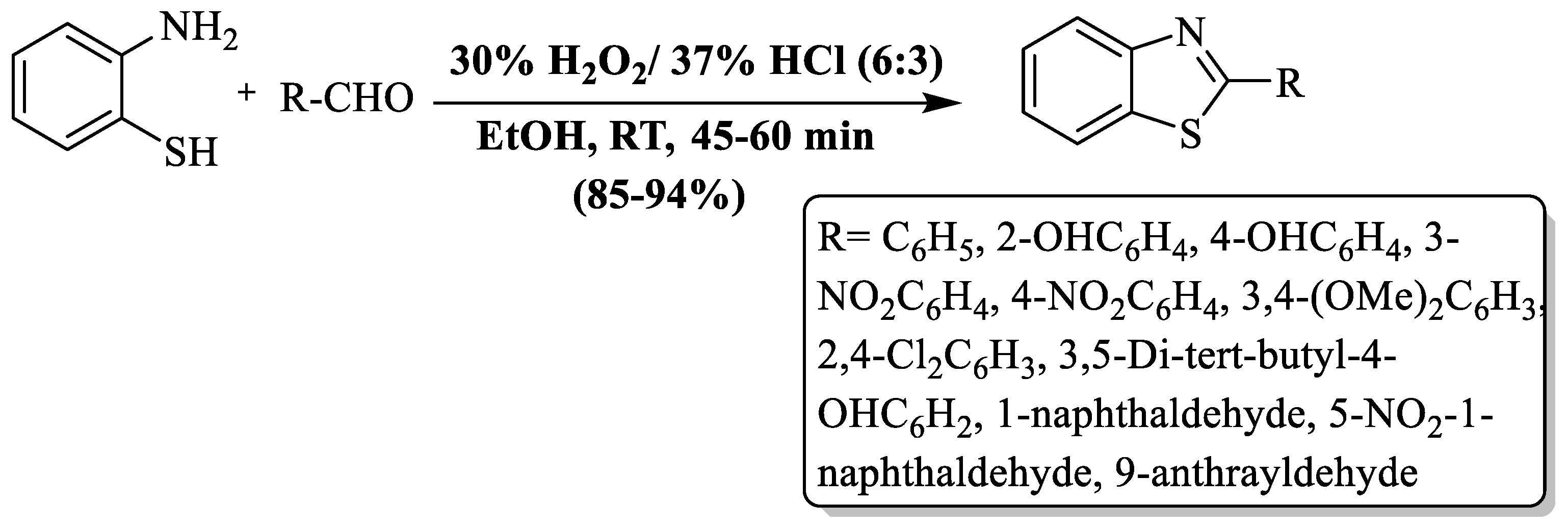
Guo and colleagues [71] synthesized 2-substituted BTs from 2-ABT and aldehydes using H2O2/HCl as a catalyst at RT in ethanol. A ratio of 1:1:6:3 (2-ABT: aldehydes:H2O2:HCl) was found to be the most favorable for the reaction. Excellent yields (85–94%), a short reaction time (45–60 min), easy product isolation, simple setup, etc., are the several benefits of the process. The authors employed diversly substituted aromatic aldehydes and found excellent yields even though substituent was EDG or EWG (Scheme 6).
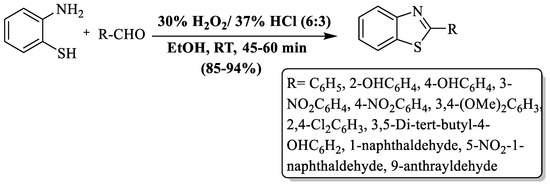
Scheme 6.
Synthesis of 2-substituted BTs using H2O2/HCl [71].
- (e)
- Base catalyzed synthesis of 2-substituted BTs
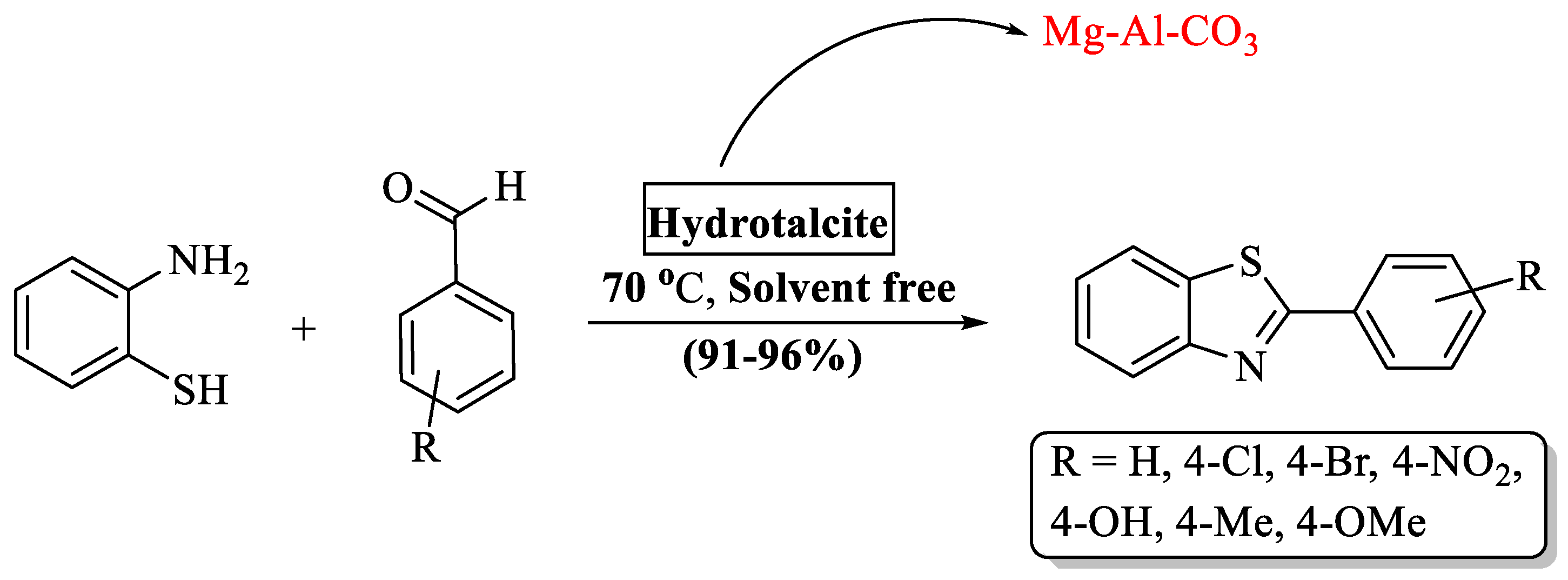
Sahu [72] discovered a green method for the production of 2-substituted BTs analogs by employing hydrotalcite (HT) as a catalyst (Scheme 7). HT is an ionic lamellar solid that has good anion exchange capacity and has a positively charged brucite layer with interlayer space possessing charge compensating anions and water molecules. It has a short O-H bond which increases electrostatic attraction between the layers and provides a good surface area. These properties of (HT) along with its reusability (eight runs) made it a good basic catalyst and afforded the desired products at (91–96%) yields in 0.5–3 h. The outcome of the protocol was excellent with diversely substituted aldehydes whether it was substituted with EDG or EWG. A drawback in this process was its limited substrate scope, as the authors synthesized only seven derivatives.
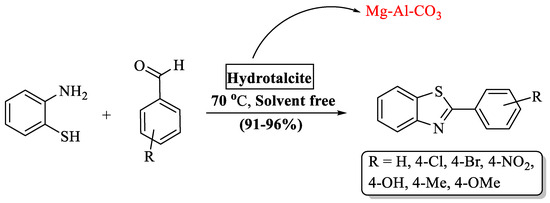
Scheme 7.
Preparation of 2-substituted BTs using hydrotalcite (HT) [72].
- (f)
- Resin-supported or catalyzed synthesis of 2-substituted BTs
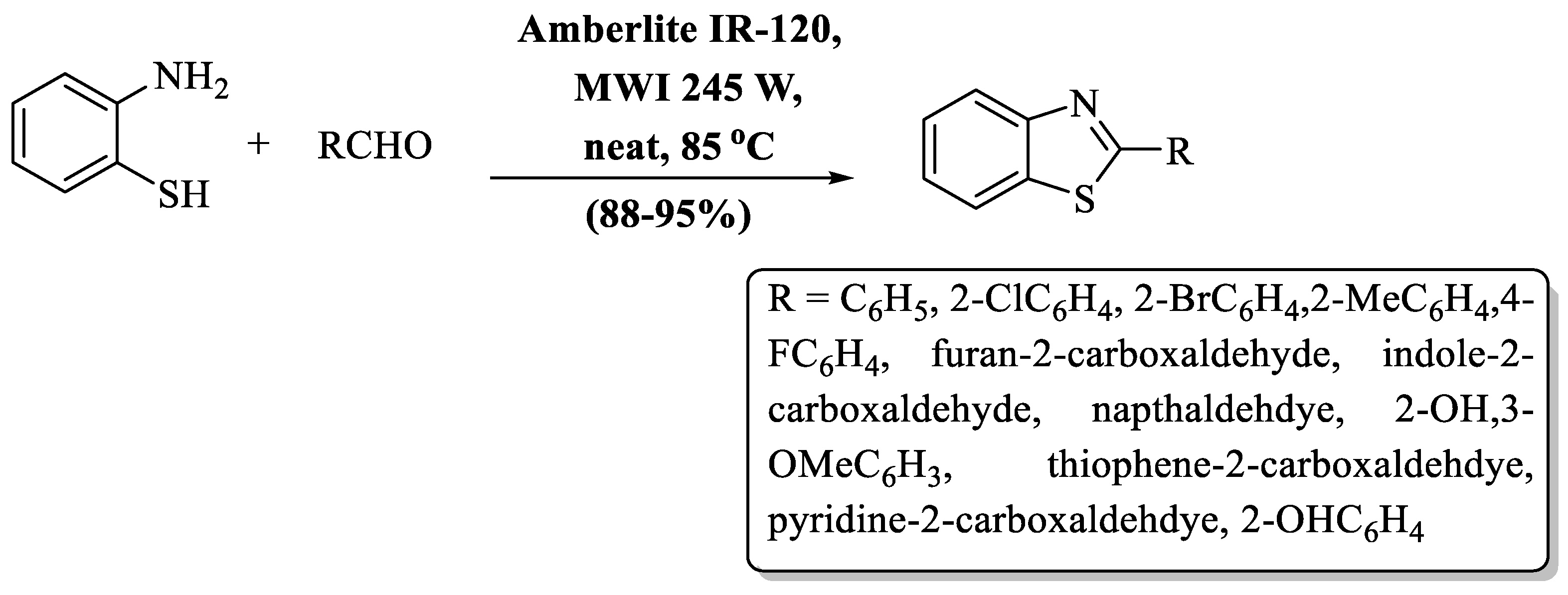
Chhabra and co-workers [73] contrived an eco-friendly pathway for 2-substituted BTs synthesis using aromatic aldehyde, 2-ABT, and amberlite IR120 resin under microwave irradiations. The desired products were obtained in 5–10 min at 85 °C at a yield of 88–95% irrespective of the utilized aryl or heteroaryl aldehydes with diverse substituents. The nature of the substituents did not affect the yield (Scheme 8).
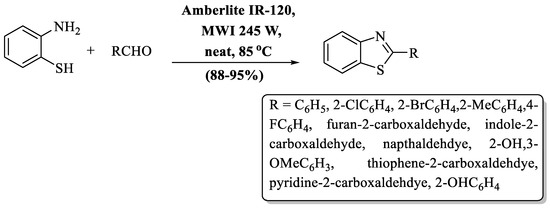
Scheme 8.
Preparation of 2-substituted BTs using resin support [73].
- (g)
- Silica supported synthesis of 2-substituted BTs
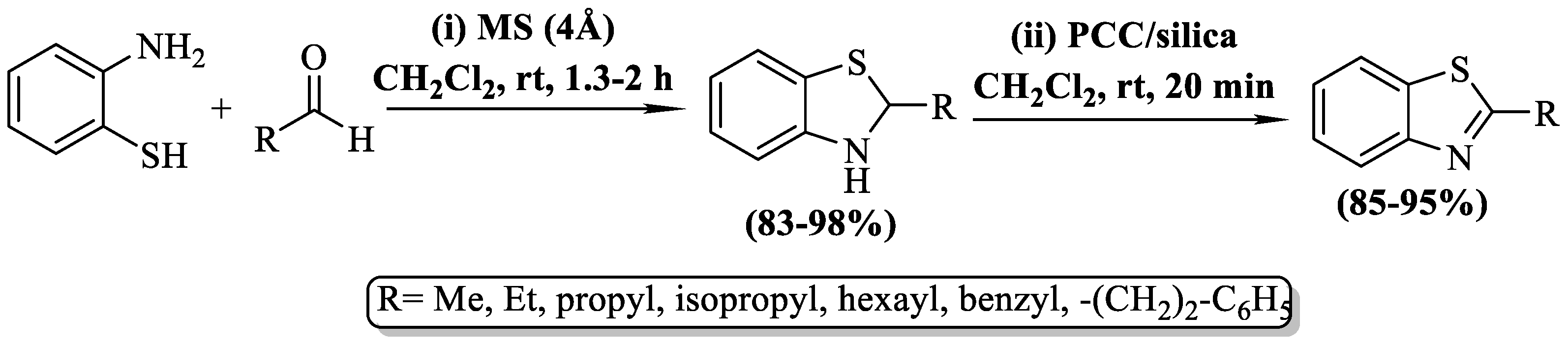
In a study by Waengdongbung et al. [74], 2-substituted BTs were synthesized by employing 2-ABT in conjunction with aldehydes, molecular sieves (MS) 4 Å, and dichloromethane (DCM) solvent. Furthermore, the synthesized 2-alkyl-2,3-dihydrobenzo[d]thiazoles were oxidized with pyridinium chlorochromate (PCC) on silica gel. The synthesized derivatives were obtained at a yield of 85–95%. The approach’s major limitation was its exclusive representation of aliphatic aldehydes, yielding only alkyl BTs. Additionally, the preparation was limited to seven derivatives only (Scheme 9).
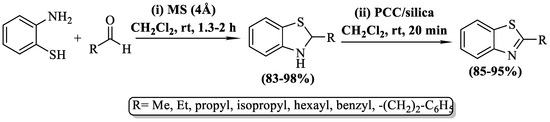
Scheme 9.
Synthesis of 2-substituted BTs by utilizing molecular sieves [74].
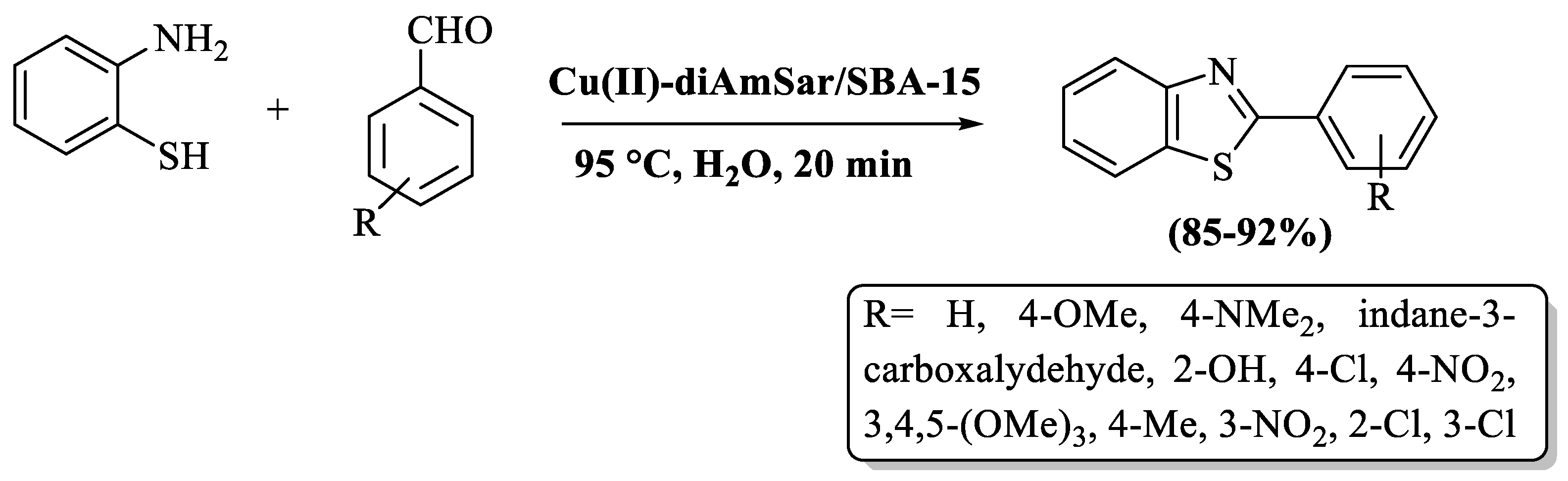
Mohammadi et al. [75] reported an accessible and eco-smart method to synthesize the 2-substituted BTs by the treatment of 2-ABT and different aldehydes employing Cu(II)-diAmSar supported over mesoporous SBA-15 silica as a catalyst in H2O solvent. The catalyst was recycled 6 times. The established pathway gave higher yields (85–92%) with electron-rich substituents in comparison to electron-deficient substituents. The utilization of column chromatography for product isolation served as a limitation of the method (Scheme 10).
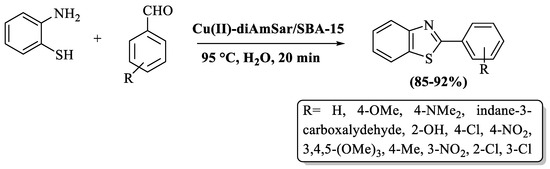
Scheme 10.
Synthesis of 2-substituted BTs via silica as a catalyst [75].
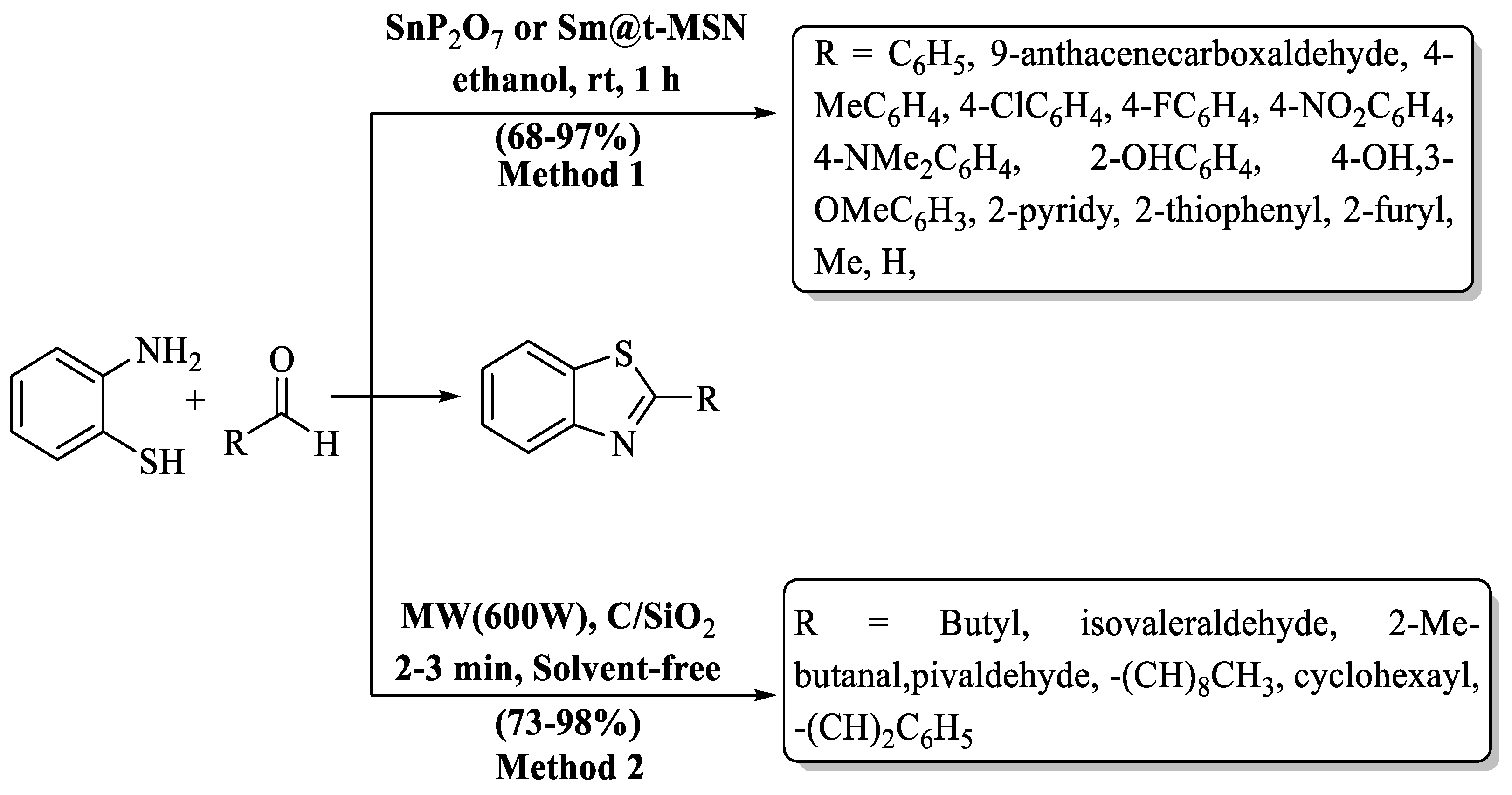
Samanta et al. [76] synthesized 2-substituted BTs at a yield of 68–97% using 2-ABT with varied aldehydes supported by a heterogeneous catalyst agent, SnP2O7 or Sm@t-MSN. SnP2O7, derived from monoammonium phosphate and a solution of SnCl2, or Sm(NO3)3·6H2O, anchored on nanosized silica gel, served as the catalyst. Remarkably, this synthesized catalyst was successfully recycled five times with negligible loss in catalytic efficiency. Aldehydes having both EDG and EWG as substituents exhibited excellent yields (85–97%) and heterocyclic aldehydes also gave excellent yield but aliphatic aldehydes yielded lower percentages (68–73%) (Scheme 11, Method 1).
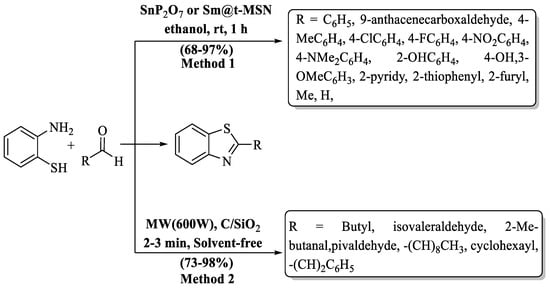
Scheme 11.
Synthesis of 2-substituted BT [76,77].
Sakiyama and co-workers [77] conducted a groundbreaking experiment, synthesizing 2-alkyl BTs with the aid of MW irradiation. Solvents were entirely eliminated, and charcoal and silica gel played pivotal roles. The authors utilized several aliphatic aldehydes with 2-ABT and obtained an impressive range (73–83%) of product yields. The designated process exhibited minimal sensitivity to the steric properties of alkyl groups in aldehydes, resulting in consistently high yields. However, the yield was significantly influenced by the length of the alkyl group, with aldehydes containing shorter alkyl groups yielding higher amounts compared to those with longer alkyl groups (Scheme 11, Method 2).
- (h)
- Metal oxide and sulfate-mediated synthesis of 2-substituted BTs
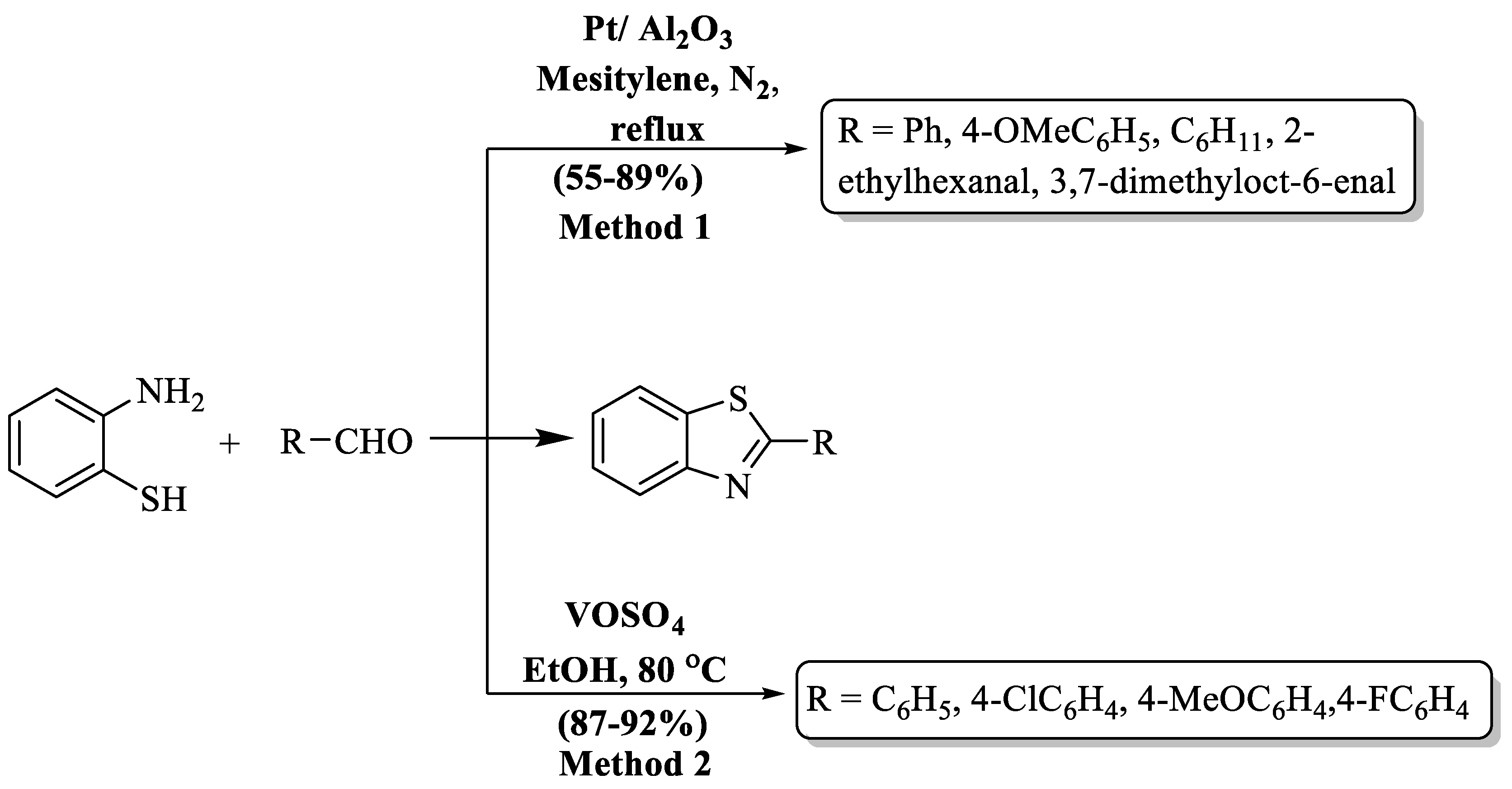
Chaudhari and colleagues [78] introduced an elegant procedure to prepare 2-substituted BTs, utilizing Pt supported over a metal oxide as a versatile catalyst (Scheme 12, Method 1). The authors demonstrated the synthesis in acceptor-free and additive-free conditions. Moderate yields (55–89%) of the desired products were obtained in 3 h. Acyclic, cyclic aliphatic, and substituted aromatic aldehydes were used but yield was not influenced by the nature of the substituent on the aldehyde. The complexity of catalyst recovery and low yields after a single use emerged as limitations in the method and only five derivatives were synthesized. Benzaldehyde achieved the highest yield (89%), whereas 4-methoxybenzaldehyde yielded the lowest (55%).
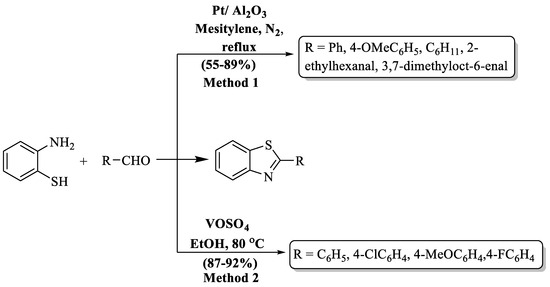
Scheme 12.
Synthesis of 2-substituted BTs via metal-based catalyst [78,79].
Digwal and his research team [79] unveiled a cutting-edge methodology for crafting 2-substituted BTs, capitalizing on VOSO4 as a catalyst in ethanol. This catalyst, known for its accessibility, eco-friendliness, and reusability, yielded the targeted BT compounds with remarkable efficiency (87–92%) in just 40–50 min at RT (Scheme 12, Method 2). The outcome of the process was irrespective to the nature of substituents. The protocol stands out for its low catalyst requirement and mild reaction parameters. A constraint in the method is evident as the authors synthesized only four derivatives of 2-aryl BT.
- (i)
- Synthesis of 2-substituted BTs by cyclodextrins and fruit juice
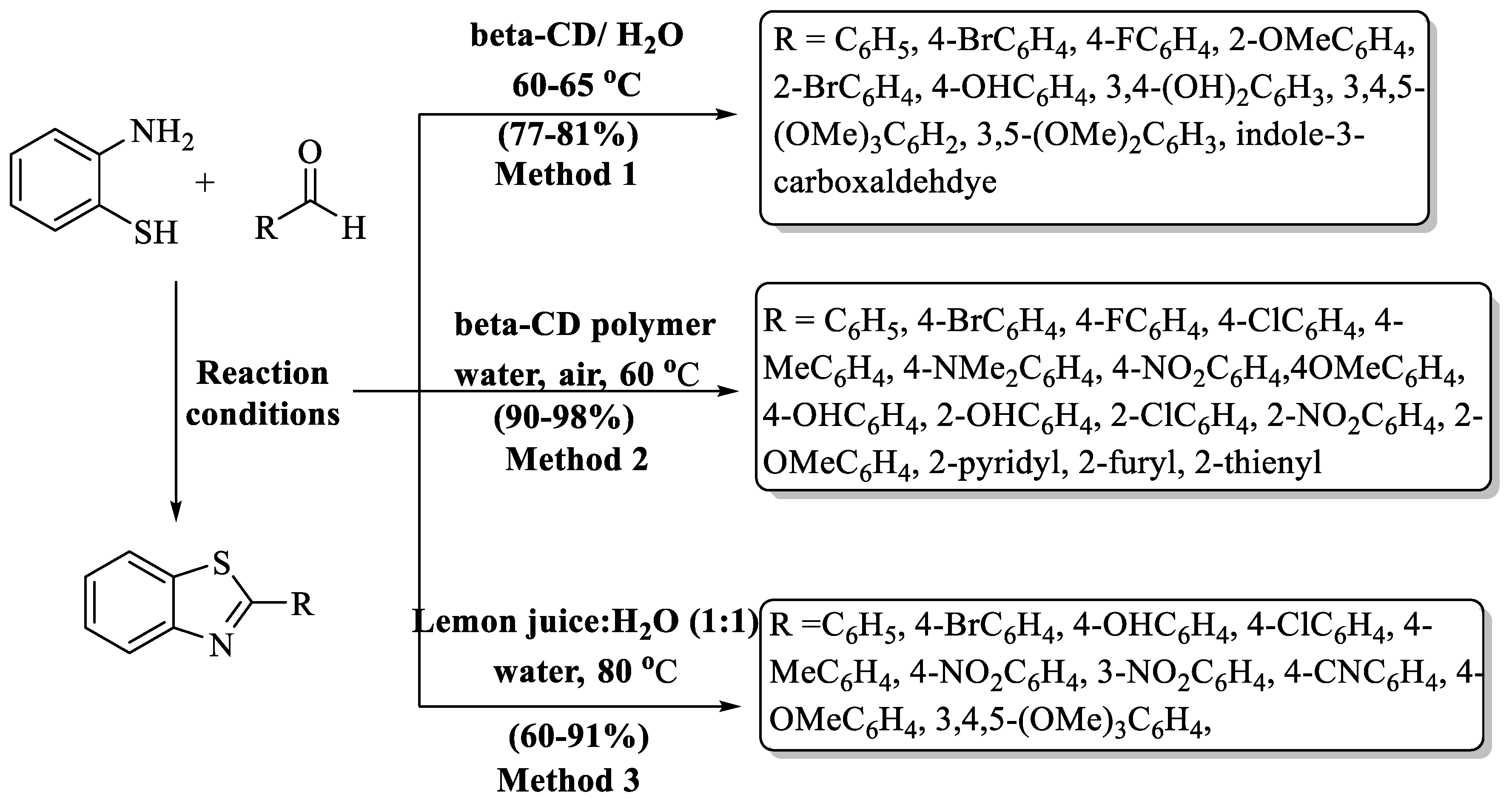
Katla and co-workers [80] designed an eco-conscious method for the synthesis of 2-substituted BTs employing β-cyclodextrin (CD) as a natural catalyst. Cyclodextrins are cyclic oligosaccharides that are composed of five or more α, β, γ glucopyranose through glycosidic linkage. Due to its special structure, i.e., hydrophobic inside and hydrophilic outside, it possesses a very good host–guest relationship and formed 10 derivatives in 4–10 h. It favors the reaction by supramolecular catalysis. The authors also examined α,γ-cyclodextrin but poor yields were obtained. The authors employed a wide range of aldehydes with diverse substituents (EDG/EWG/heteroaryl/di or trisubstituted) and found high yields (77–81%) without influence of the essence of the substituent. Major limitations of this method included the extended reaction duration and the necessity of employing column chromatography for the product isolation (Scheme 13, Method 1).
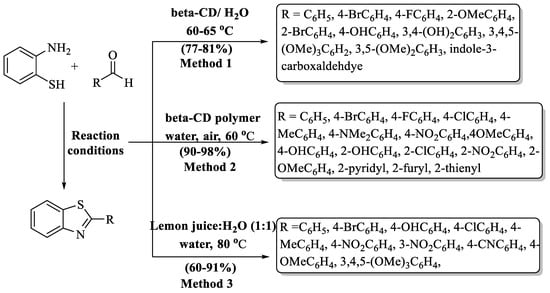
Scheme 13.
Preparation of 2-substituted BTs using cyclodextrins and fruit juice [80,81,82].
Remaily and co-workers [81] innovated an easy-to-follow, eco-conscious, and economically efficient method to synthesize 2-substituted BTs in water using β-cyclodextrin cross-linked with epichlorohydrin (Scheme 13, Method 2). They analyzed the reaction by varying temperature, stirring speed, and catalyst loading, and it was found that the optimum temperature was 60 °C and that 1 gm of catalyst (for 1 mmol of o-ABT) was suitable for the transformation with appreciable yields (90–98%) in 2–4 h. The catalyst was reused after six successive runs. The yield was higher in the process for aldehydes containing electron-deficient or electron-efficient substituents; however, it was lower (below 92) when dealing with hetero aldehydes (2-furyl, 2-thienyl, 2-pyridyl).
Patil and colleagues [82] discovered an eco-smart approach to prepare 2-substituted BTs using lemon juice and other juices like orange and pineapple juice as a natural catalyst (Scheme 13, Method 3). Citric acid and ascorbic acid are present in lemon juice and for this reason it has been used for acid-catalyzed reactions. It was observed that at around 80 °C in the presence of lemon juice (2 mL) and water as media, the reaction occurred smoothly. The desired products were obtained at a yield of 60–91% in 40–120 min and, generally, the yield was produced regardless of the substituents. But 3,4,5-trimethoxy benzaldehyde converted into product over the longest reaction timeframe (120 min with 84%) and 4-cyanobenzaldehyde gave lowest yield (60% in 50 min).
- (j)
- Synthesis of 2-substituted BTs using polymer support
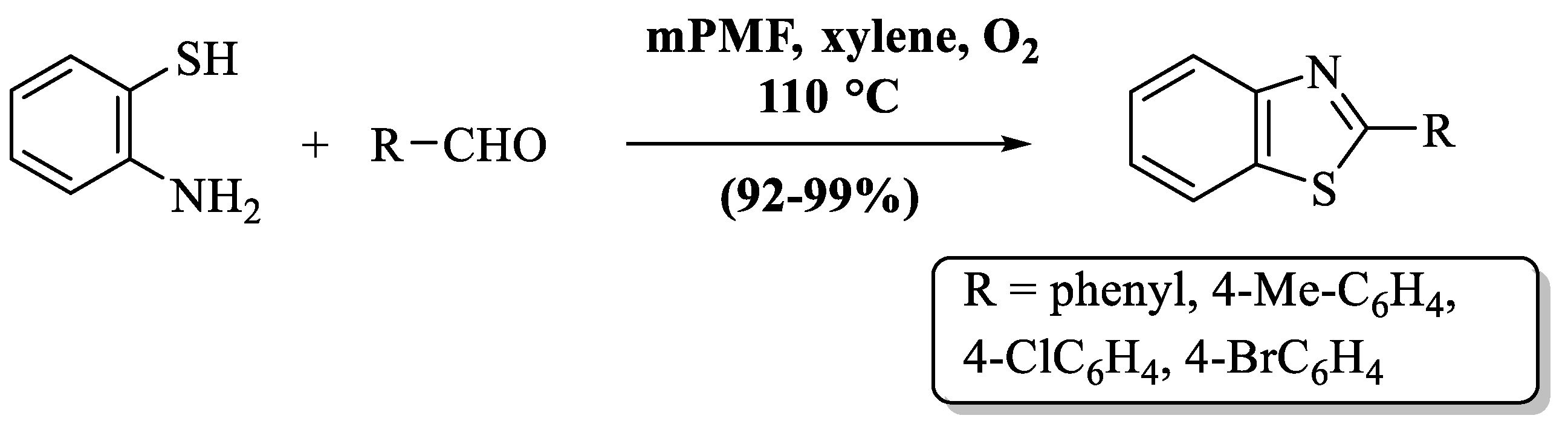
Yang and coauthors [83] developed a sustainable technique for the production of 2-substituted BTs using mesoporous poly (melamine–formaldehyde) as an eco-friendly, heterogeneous organocatalyst (Scheme 14). The authors also discovered that 10 mg of mPMF, xylene as a solvent, and a temperature of (110 °C) were necessary conditions for the reaction to occur with excellent yields. The authors only prepared 4 derivatives of BTs by utilizing ED- or EW-substituted aldehydes, but EDG has a higher yield. The use of xylene as a solvent is also a drawback of this method because of its volatile nature.
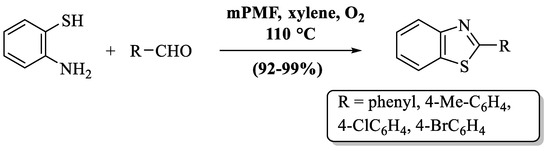
Scheme 14.
Polymer-assisted synthesis of 2-substituted BTs [83].
- (k)
- Microwave-induced synthesis of 2-substituted BTs
Microwave irradiation accelerates chemical synthesis by efficiently heating target molecules with permanent dipole moments, minimizing reaction times compared to conventional methods. This selective and rapid heating enhances control over reactions, enabling the synthesis of complex compounds. Moreover, microwave synthesis often reduces energy consumption and waste production, making it a valuable and sustainable approach in modern chemistry.
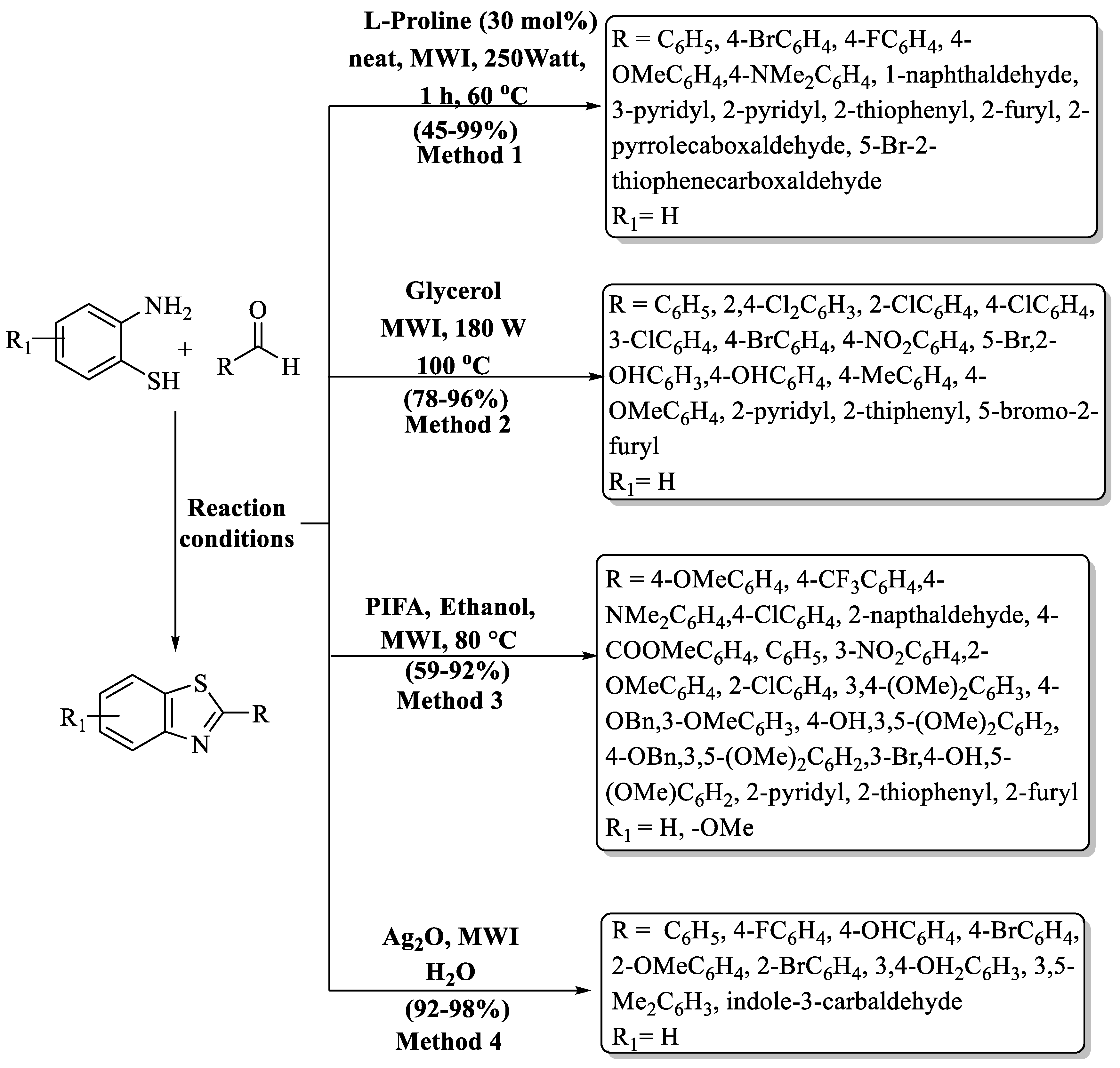
Lee and coworkers [84] developed an innovative, microwave-induced, solvent-free method for crafting a range of bioactive, functionalized 2-substituted BTs, with L-proline serving as the catalyst in solvent-free conditions (Scheme 15, Method 1). Initially, thiophenylcarbaldehye was treated with 2-ABT at 180 °C using L-proline by a conventional method and then the method was put forward towards a greener pathway by performing the model reaction under MW (microwave) irradiation using 30 mol% of catalyst. The process exhibited a 45–99% yield of the desired derivatives. Aldehydes gave excellent yield with both EDG and EWG, except for 4-N,N-dimethyl benzaldehyde (45%). Heteroaromatic aldehydes exhibited moderate-to-good yields by following the procedure; however, pyridyl aldehyde gave better yield (99%) in the absence of L-proline. The authors also used the column chromatography technique to isolate derivatives.
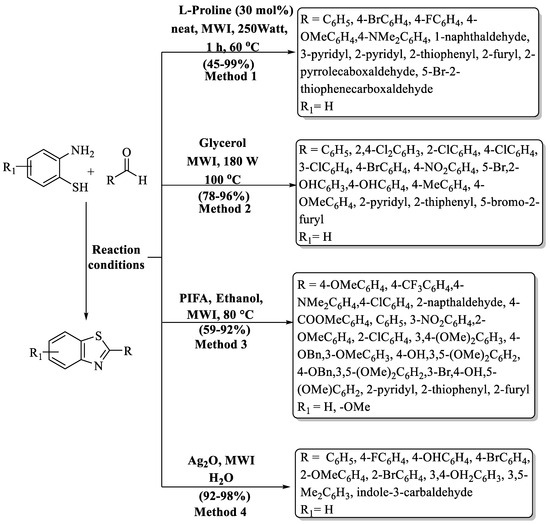
Scheme 15.
Microwave-assisted preparation of 2-substituted BTs [84,85,86,87].
An ecologically benign pathway to prepare 2-substituted BTs by CEM-focused MW irradiation using glycerol as an eco-friendly reaction media was innovated by Zhang and his team [85] (Scheme 15, Method 2). Firstly, the chemical interaction of 2-ABT with aldehydes employing glycerol under CEM-focused MW was investigated and it was noticed that 180 W at 100 °C for 4 min were optimized reaction parameters. The authors obtained a 78–96% yield of products in just 4–8 min. The method exhibited limitations in the yield variation among different aldehydes. While ED- and EW-substituted aldehydes showed higher yields (≥90%), the presence of a hydroxy group resulted in an 86% yield, whereas heteroaromatic aldehydes yielded below 82%, presenting a disparity in the outcomes.
A simple, efficient, environmentally benign, and MW-assisted one-pot condensation using oxidant PIFA (phenyliodoniumbis(trifluoroacetate)) for the production of 2-substituted BTs was evolved by Praveen and co-workers [86] (Scheme 15, Method 3). The synthesized compounds were obtained at a yield of 59–92% in 15 min at 80 °C, and the products were separated by using column chromatography. Heteroaromatic aldehydes gave lower yields as compared to ED and EW substituents, and according to the authors, this happened due to the cleavage of heterocycles under MW conditions.
Sakram and coauthors [87] designed a hassle-free and effective protocol to obtain 2-substituted BTs using Ag2O as a catalyst under MW irradiation (Scheme 15, Method 4). With rapid kinetics (4–8 min), the desired compounds were achieved with impressive yields of 92–98%. The designated protocol gave excellent yields irrespective of the substituent (EDG/EWG/heteroaryl/disubstituted). The method’s limitation lies in the preparation of only nine derivatives, with isolation achieved through column chromatography. While utilizing Ag2O, 2-ABT, and aldehyde in a 5:1:1 ratio for the synthesis of 2-substituted BTs with high power (700 W) stands out, it may pose challenges concerning scalability application.
- (l)
- Ultrasound-assisted synthesis of 2-substituted BTs
Ultrasound irradiation is also known as sonochemistry, based on the acoustic cavitation phenomenon, in the range of 20 KHz to 100 MHz. Ultrasound irradiation initiates the formation of bubbles in the reaction mixture, leading to implosion and the release of considerable heat. This increased temperature is leveraged to conduct chemical transformations.
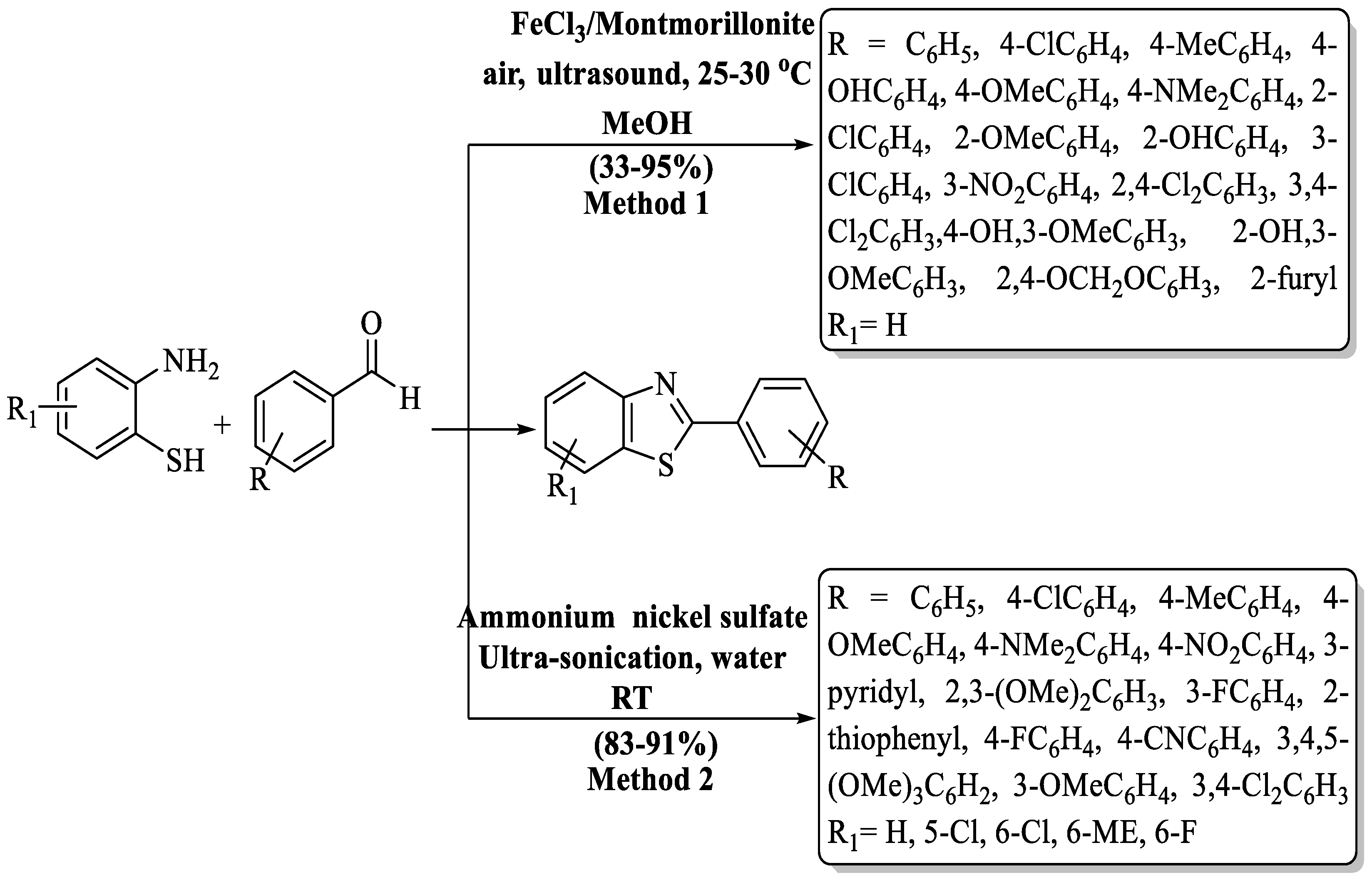
Chen and coworkers [88] designed a green, ultrasound-assisted route for the synthesis of 2-substituted BTs through the reaction of different aldehydes with 2-ABT in a catalytic amount of FeCl3/Montmorillonite K-10. The derivatives were prepared in a 0.7 to 5 h timeframe with 33–95% yield. The catalyst was reused for up to 3 cycles and the yield decreased quickly (69% from 85%). Aliphatic aldehydes were not able to produce products by this method and heteroaromatic aldehydes gave moderate yield. In aryl aldehyde, the process exhibited low-to-high yields but nitro-substituted aldehyde was not suitable for this method. The use of column chromatography was also a drawback of the method. Additionally, the method faced a constraint with limited catalyst reusability, leading to a rapid decline in yield (from 85% to 69%) after just three cycles. (Scheme 16, Method 1).
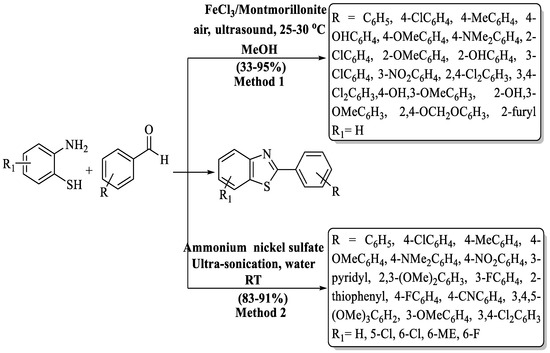
Scheme 16.
Ultrasound-assisted synthesis of 2-substituted BT derivatives [88,89].
Pardeshi and his group [89] designed an ultrasound-assisted production of 2-substituted BTs via 2-ABT with aromatic aldehydes catalyzed by ammonium nickel sulfate (Scheme 16, Method 2). The desired compounds were obtained at a yield of 83–91% in 85–115 min and the yields were not influenced by the essence of the substituents. The drawbacks of the method include the use of column chromatography for obtaining pure derivatives. Moreover, the absence of information on the catalyst recovery and reuse raises concerns about the method’s overall sustainability and cost-effectiveness.
- (m)
- Mechanochemical synthesis of 2-substituted BTs
Mechanochemical synthesis is a sustainable pathway which involves using mechanical energy supplied by ball milling and grinding with a mortar and pestle. It is a simple experimental setup and an environmentally benign method.
- (1)
- Ball milling strategy
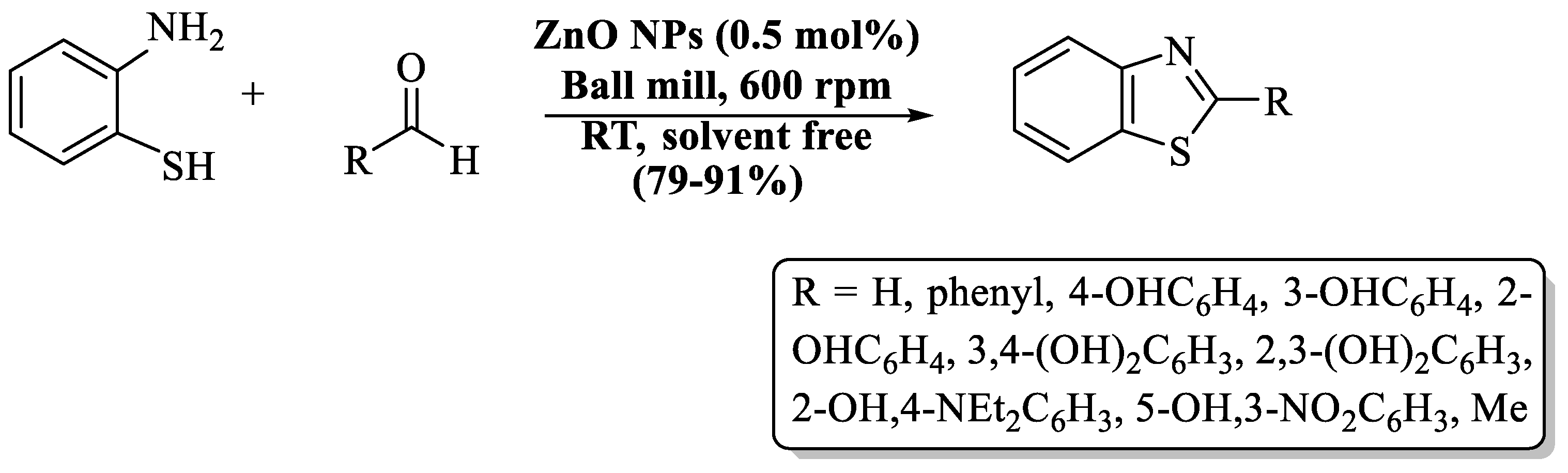
Sharma and colleagues [90] introduced an expedient and cost-friendly method for the synthesis of 2-substituted BT analogs using ZnO NPs by ball milling strategy (Scheme 17). Initially, ZnO NPs were prepared by the sol-gel process and reused for five cycles. 2-substituted BTs were obtained in solvent-free conditions with 79–91% yield in 30 min at RT. The authors utilized various aldehydes, such as aliphatic, electron-rich-substituted, electron-poor-substituted, and disubstituted, but yield was irrespective to their nature. The authors also performed gram-scale synthesis by using salicylaldehyde and obtained a yield of 80%. The utilization of a metal-based catalyst was the negative point of the method.
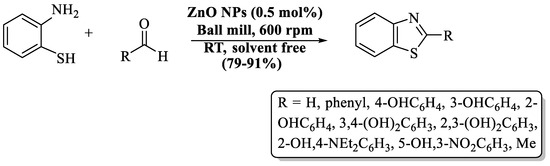
Scheme 17.
Preparation of 2-substituted BT analogs via ZnO NPs [90].
- (2)
- Grindstone technique
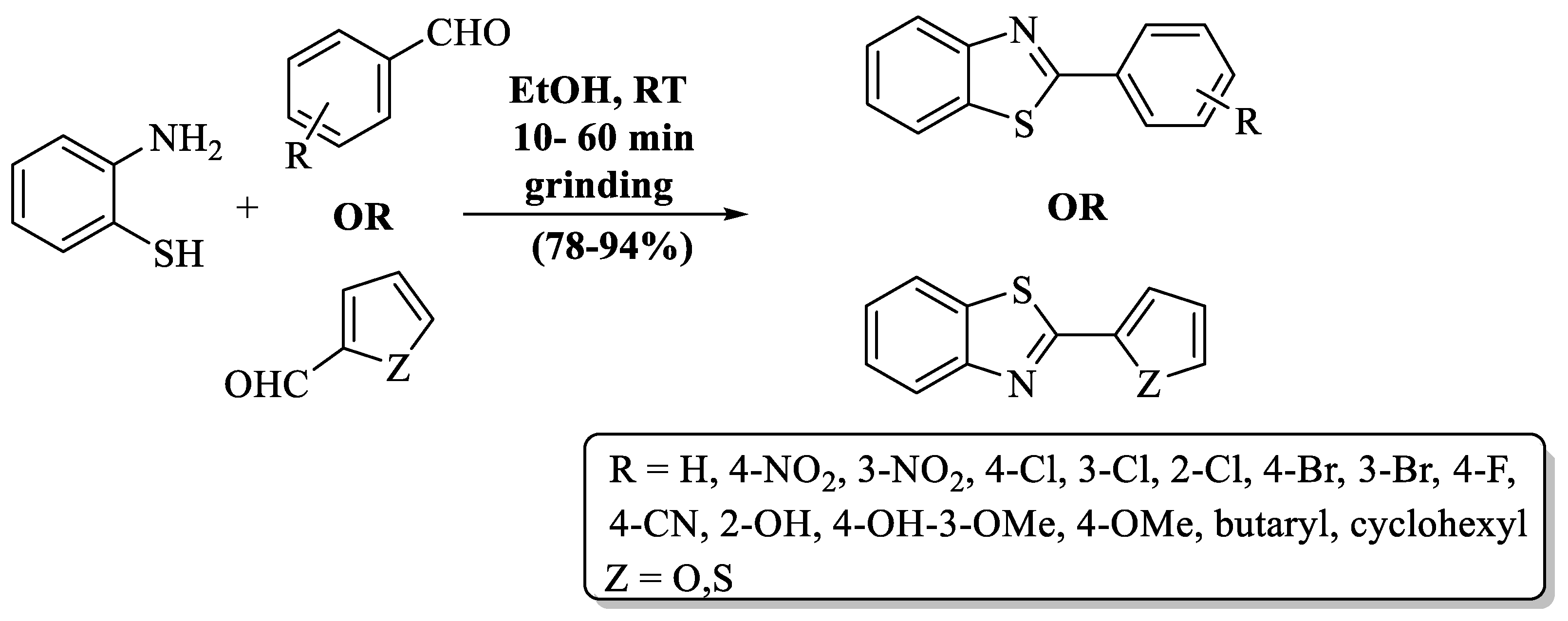
A basic, high-yield, and flexible mechanochemical procedure to prepare 2-substituted BTs was developed by Banerjee and his colleagues [91] (Scheme 18). The pathway involved ethanol-assisted grinding. Catalyst-free conditions, non-toxic solvent, excellent yield (78–94%), lower reaction time (10–60 min), and an absence of need for further workup are the advanced features of this protocol. The yield of the designated pathway occurred despite the nature of substituent on aldehyde. It was suitable for EDG, EWG, and heterocycles, but EDG-substituted aldehydes consumed more time in conversion as compared to others.
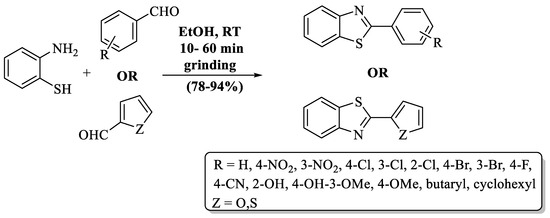
Scheme 18.
Grindstone-based synthesis of 2-substituted BTs [91].
- (n)
- On-surface synthesis of 2-substituted BTs
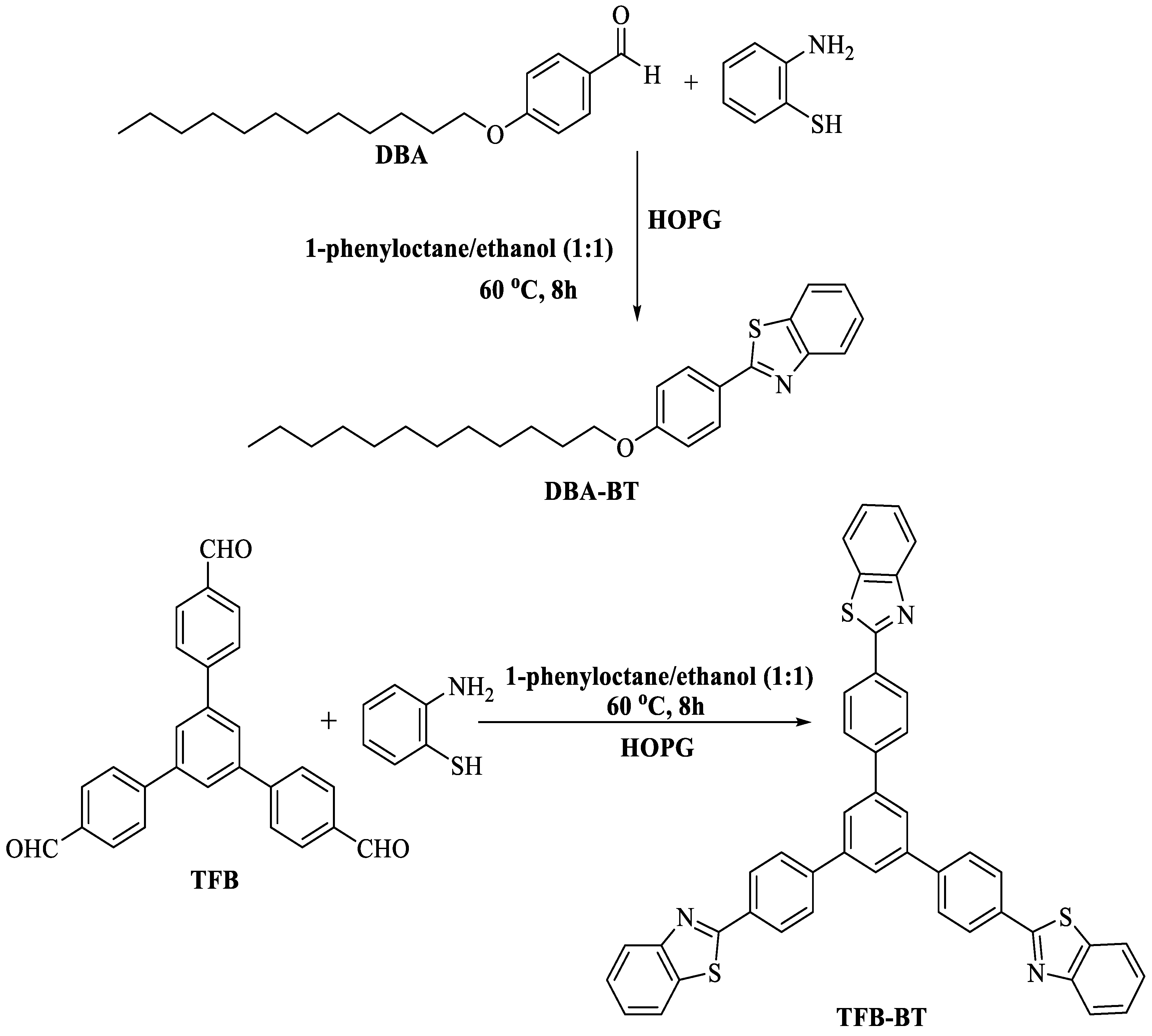
Shi and fellow workers [92] fabricated a groundbreaking and accessible technique for the production of 2-substituted BTs at 60 °C by employing a highly oriented pyrolytic graphite (HOPG) surface via on-surface condensation of 2-ABT with different aldehydes viz. tadpole-like DBA (4-dodecyloxybenzaldehyde) and star-shaped TFB 1,3,5-tri(4′-formylphenyl) benzene in a mixture of (phenyl octane/ethanol (1:1)) at the liquid–solid interface. The method utilized a long reaction timeframe (8 h) (Scheme 19).
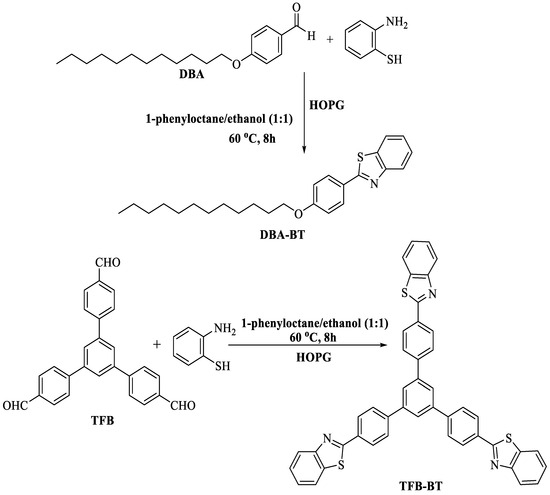
Scheme 19.
On-surface synthesis of 2-substituted BTs [92].
The outcomes of the reactions involving substituted aldehydes and 2-ABT for synthesizing 2-substituted BTs were influenced by the nature of the substituents in a diverse manner. In general, EDGs may have resulted in higher yields in certain instances, while EWGs exhibited this trend elsewhere. Heterocycles, steric hindrance, and reaction conditions also played variable roles, leading to instances where moderate yields or distinct preferences for specific substituent types were observed. This complexity underscores the nuanced nature of the reaction outcomes.
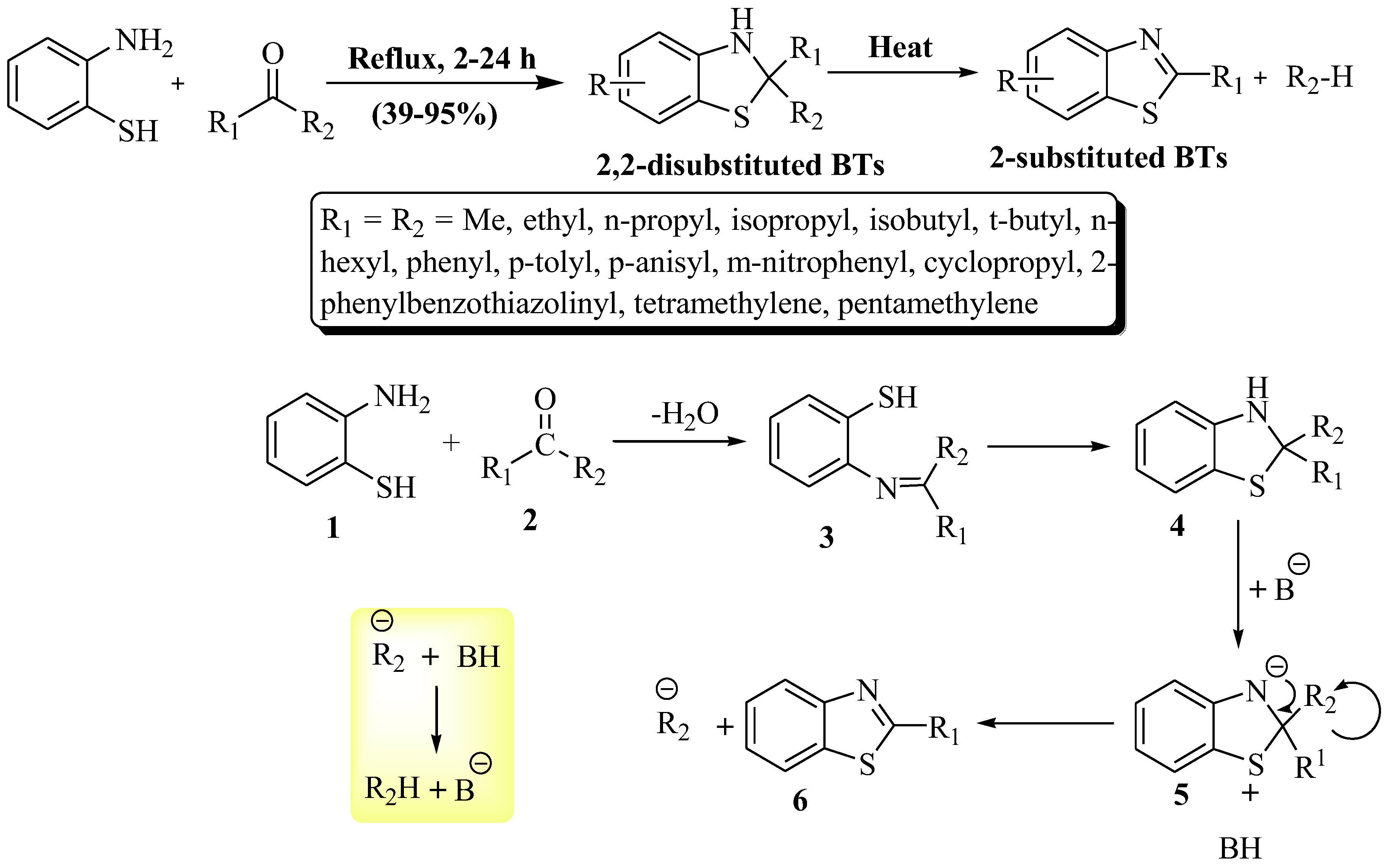
2.2. Reaction of 2-ABT and Ketones
Elderfield et al. [93] synthesized 2-substituted BTs using 2-ABT with excess ketone as a solvent at a yield of 39–95% at reflux temperature within 2 to 24 h reaction time (Scheme 20). The resulting BTs exhibited an oily nature. The synthesis unveils advantages—exploring nitrogen-sulfur dynamics, enabling electronic and steric control, and probing the uncharted territory of 2,2-disubstituted BTs. The method exhibited a broad substrate scope, delivering yields ranging from low to excellent without using a catalyst. Despite its versatility, a drawback lies in the extended reaction time, lasting up to 24 h. The possible reaction mechanism of the reaction is illustrated in Scheme 20, in which both the reactants (1 and 2) converted into imine intermediate (3) by cyclo condensation and, further, a cyclized intermediate (4) was obtained. The cyclized intermediate (4) lost a proton and gave an anionic intermediate (5) in the presence of a base which was converted in the desired product (6).
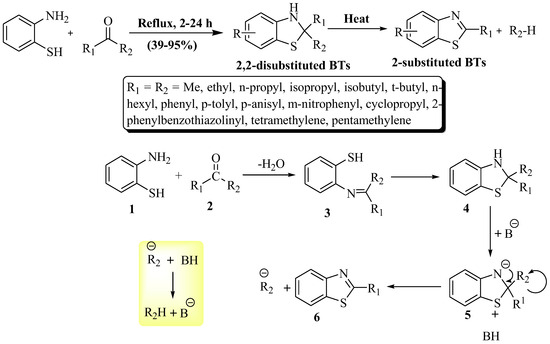
Scheme 20.
Synthesis of 2-substituted BTs with possible reaction mechanism by using 2-ABT and ketones [93].
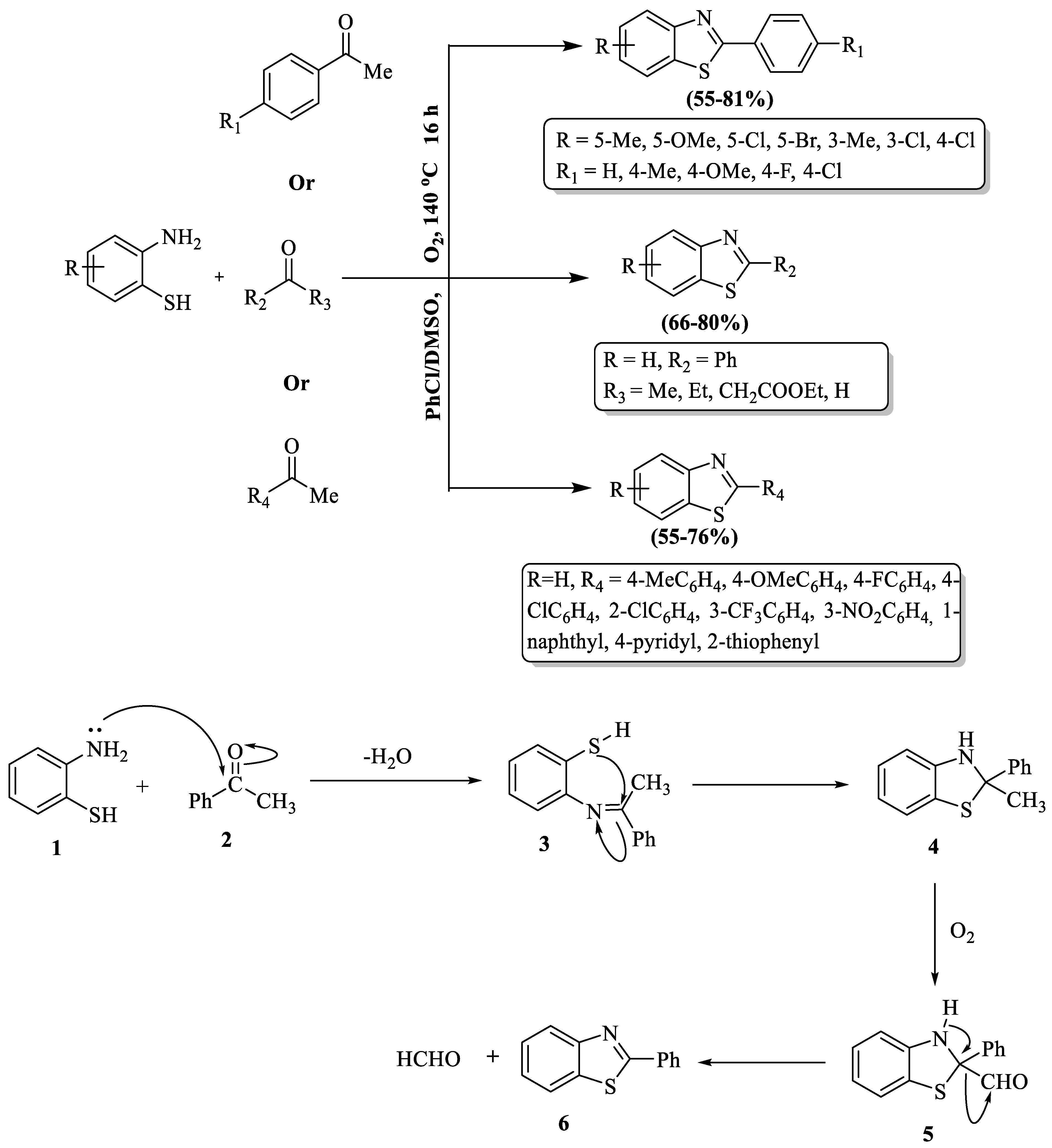
2-substituted BTs were developed by Liao and coauthors [94] using molecular oxygen. The reaction protocol achieved optimal results with a chlorobenzene/DMSO (2:1) solvent mixture and a temperature of 140 °C. A wide substrate scope was studied for the reaction and moderate-to-good yields (55–81%) were obtained for the products. The method’s notable drawbacks involved the requirement for high temperature, an extended reaction time (16 h), and diminished yields for certain compounds. Its unsuitability for aliphatic aldehydes and lower yields with heteroaromatic aldehydes like 2-acetylthiophene and 4-acetylpyridine compared to other aromatic ketones were the limitations. The position of substituents on the phenyl ring of ketones and 2-ABT further influenced the yield, with 2-amino-3-chlorobenzenethiol resulting in only trace amounts of the desired product. The mechanism of this reaction involved the cyclocondensation of 2-ABT) (1) with ketones (2) that formed the imine intermediate (3), which then cyclized into (4). The methyl group in (4) became oxidized, resulting in the aldehyde compound (5). The final product (6) emerged after eliminating a proton and the CHO group from (5) (Scheme 21).
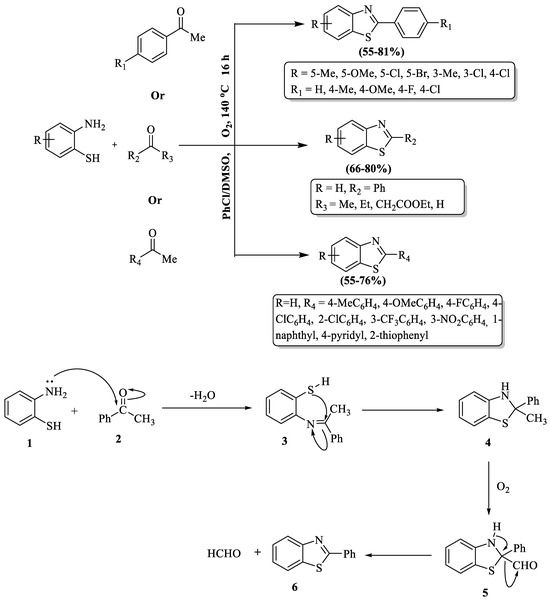
Scheme 21.
Molecular oxygen-assisted synthesis of 2-substituted BTs with possible reaction mechanism [94].
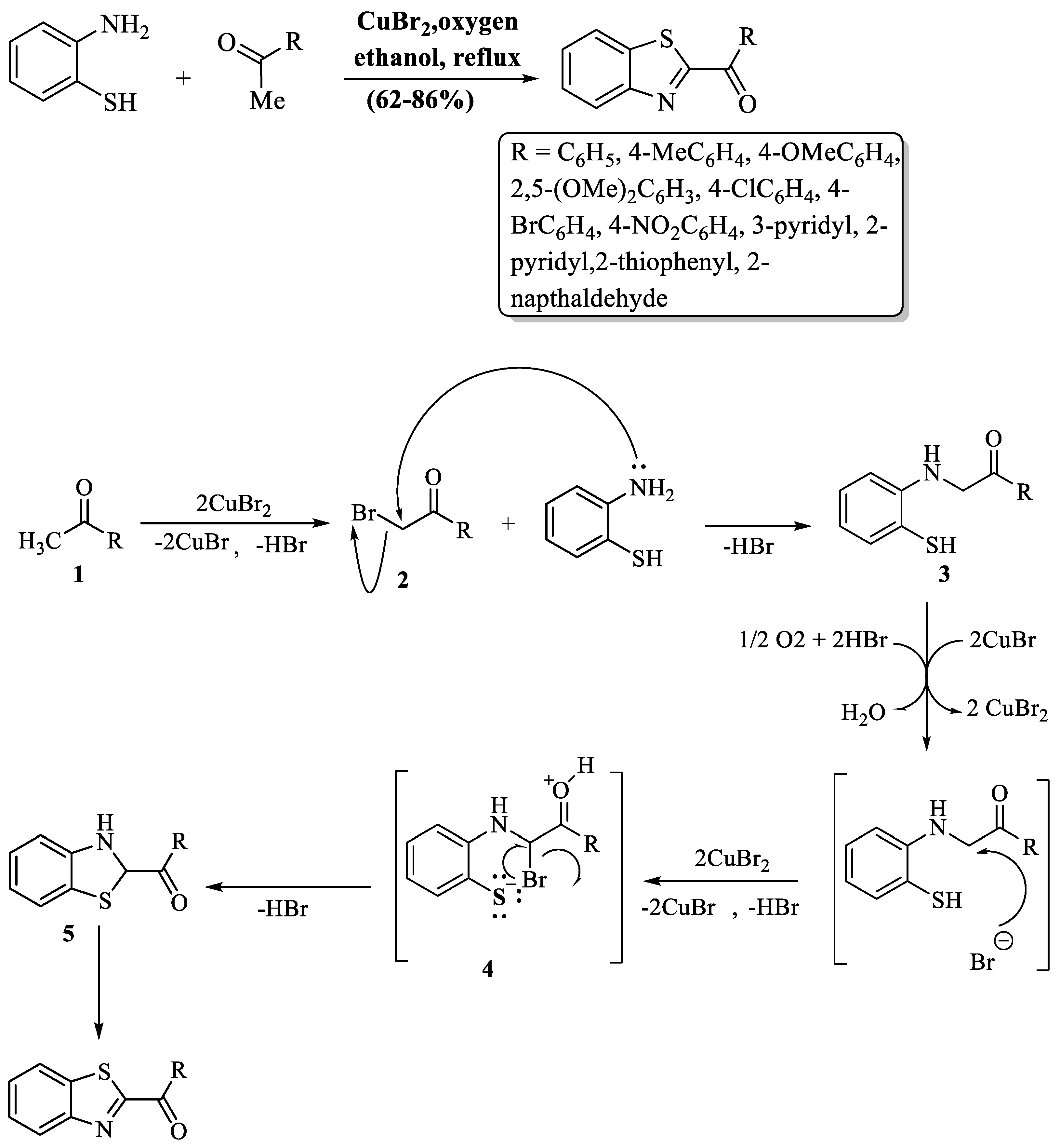
2-substituted BTs were synthesized by Mali et al. [95] using ketones and 2-ABT. CuBr2 was added as a catalytic agent and ethanol as a reaction medium at reflux temperature and products were obtained in an 8 h time period. The method faced significant limitations, including prolonged reaction time, reliance on column chromatography, and unsuitability for aliphatic ketones. While aromatic ketones with various substituents generally yield well (≥70%), exceptions include 4-nitro (62%) and 2,5-dimethoxy groups (62%). The mechanism of this reaction involved the bromination of ketones (1) to yield α-bromoketones (2). Later, 2-ABT gave α-aminoketones (3) via nucleophilic substitution with α-bromoketones (2). The next phase involved the bromination of α-aminoketones (3), generating an intermediate compound (4). This intermediate (4) underwent an intramolecular nucleophilic attack facilitated by thiol, leading to ring closure and the formation of another intermediate (5). Finally, oxidative dehydrogenation of the intermediate (5) concluded the synthesis, producing the desired end product (2-substituted BT) with a 62–86% yield (Scheme 22).
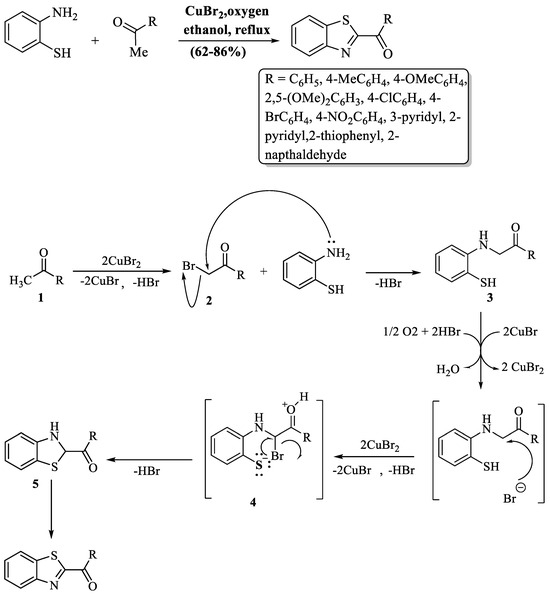
Scheme 22.
Synthesis of 2-substituted BTs with possible reaction mechanism using 2-ABT and ketones [95].
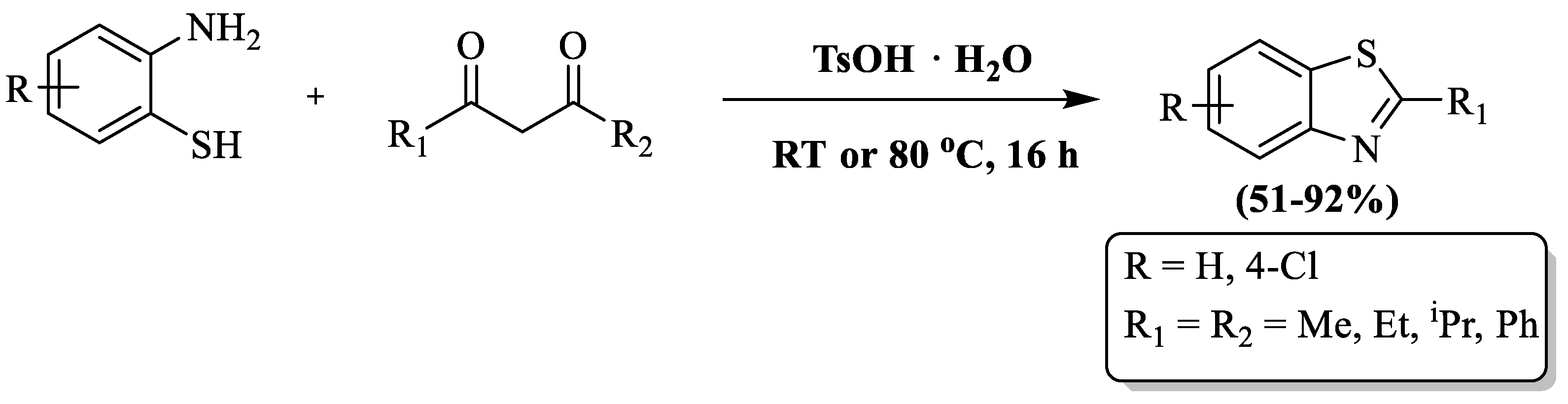
Mayo and coauthors [96] synthesized 2-substituted BTs using β-diketones, 2-ABT, and a p-toluene sulfonic acid catalyst (PTSA) under an oxidant- and metal-free environment in acetonitrile or solvent-free conditions at RT or 80 °C. Easily available raw material, simple experimental setup, mild reaction condition, moderate-to-high yield (51–92%), etc., were the benefits of the method. The method’s performance was influenced by solvent systems, resulting in selective product formation in solvent-free conditions, reliance on acetonitrile for certain reactions, and variable outcomes for certain reactions. For instance, 2,6-dimethylheptane-3,5-dione with 4-chloro-2-aminothiophenol showed no product formation in a solvent-free system but achieved a 63% yield in CH3CN. Notably, 1,3-di(tert-butyl)propane-1,3-dione failed to yield a product in both systems, even at elevated temperatures, while 1,3-diphenylpropane-1,3-dione exhibited a low yield in acetonitrile but a high yield in toluene at 130 °C (Scheme 23).
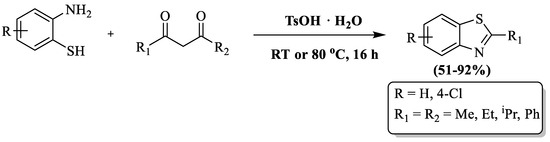
Scheme 23.
Preparation of 2-substituted BT by utilizing 2-ABT and β-diketones using p-toluene sulfonic acid (PTSA) [96].
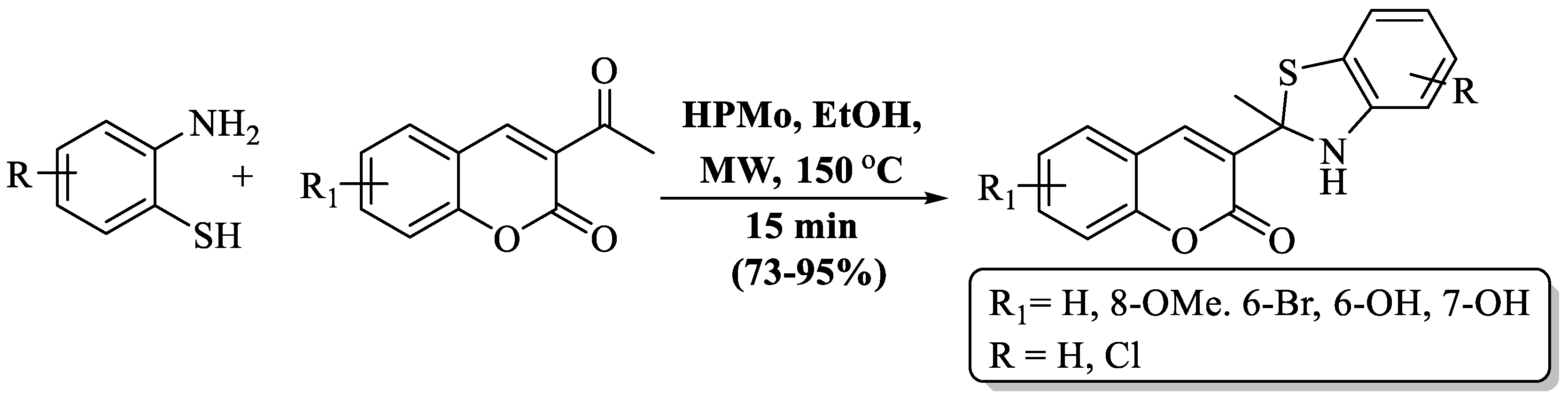
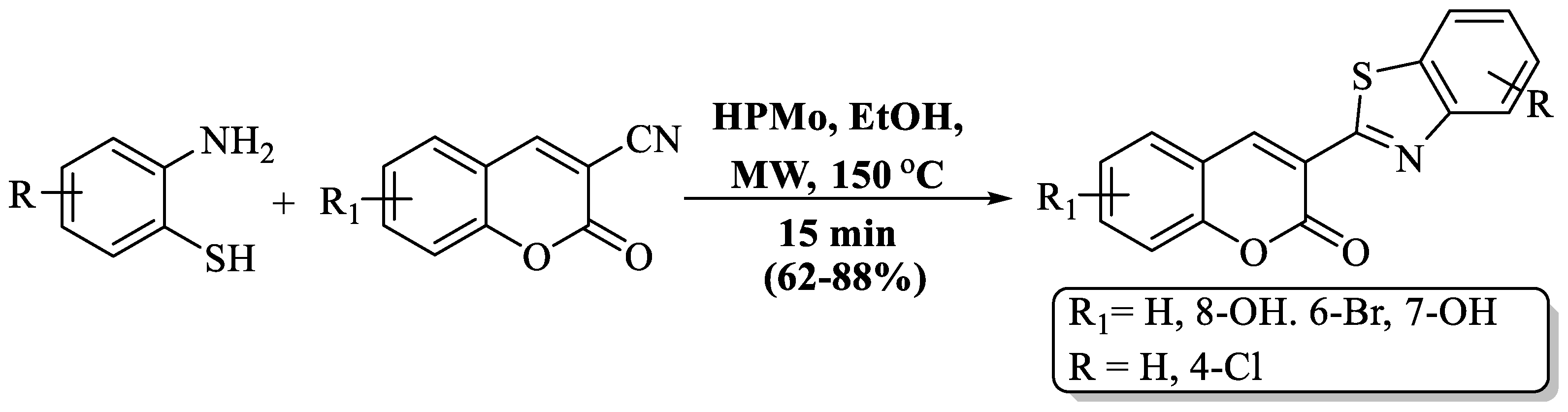
A fast and simple MW-assisted protocol for the preparation of BT derivatives with attached coumarin was developed by Khoobi et al. [97] using 3-COCH3 or 3-CN coumarins and 2-ABT in the existence of heteropolyacid, 12-molybdophosphoric acid (HPMo). A short reaction time, inexpensive catalyst, and moderate-to-high yields (73–95%) are the advantages of this method. The method yields were affected by the presence of a Cl substituent on 2-ABT, with chloro-substituted 2-ABT showing lower yields than unsubstituted 2-ABT. The use of column chromatography and the preparation of only 6 derivatives were the major drawbacks of the method, but the authors successfully prepared a gram-scale product, with a 92% yield of 2-acetyl coumarin with 2-ABT (Scheme 24).
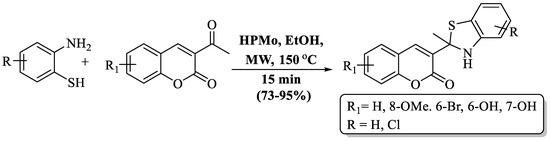
Scheme 24.
Synthesis of 2-substituted BT using 2-ABT and 3-COCH3 or 3-CN coumarin [97].
2.3. Reaction of 2-ABT and Acids
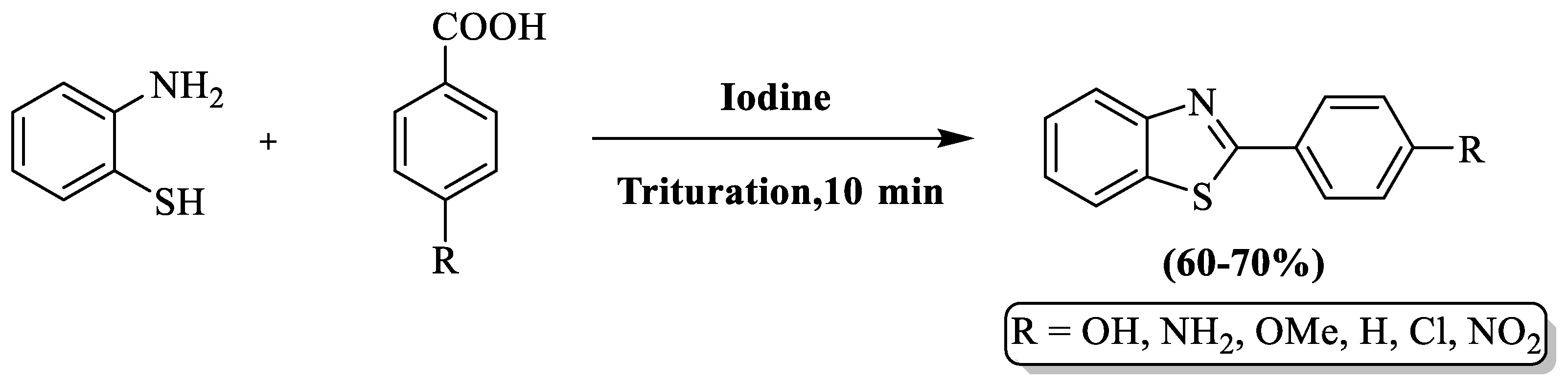
Gupta and colleagues [98] developed a solvent-free approach for 2-substituted BTs using 2-ABT, benzoic acid derivatives, and molecular iodine as catalysts, obtaining products in a 10 min reaction period. The method’s drawbacks included the omission of specific yield details and limited substrate scope, encompassing only 6 derivatives (Scheme 25).
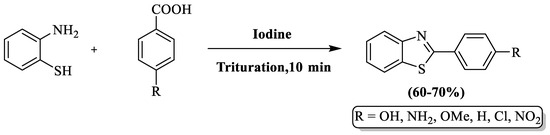
Scheme 25.
Molecular iodine-guided synthesis of 2-substituted BTs [98].
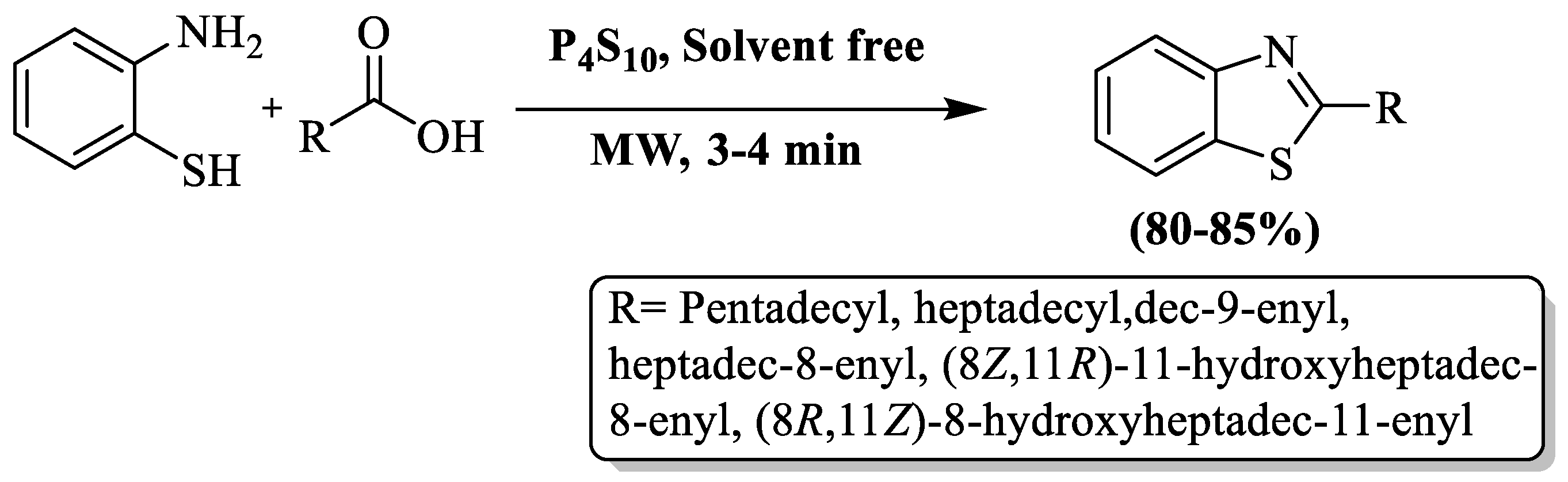
Rauf and coauthors [99] synthesized BT derivatives at a yield of 80–85% using different fatty acids under microwave irradiations and a solvent-free environment with P4S10 as a catalyst. The products were obtained in 3–4 min (Scheme 26). The authors synthesized 2-alkenyl and 2-alkyl BTs and their yield was not influenced by the fatty acid chain length. The method has limitations such as reliance on column chromatography and a narrow substrate scope, comprising only 6 derivatives.
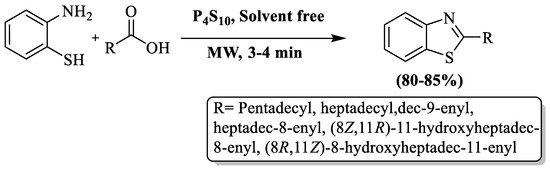
Scheme 26.
Synthesis of 2-substituted BT using 2-ABT and fatty acids [99].
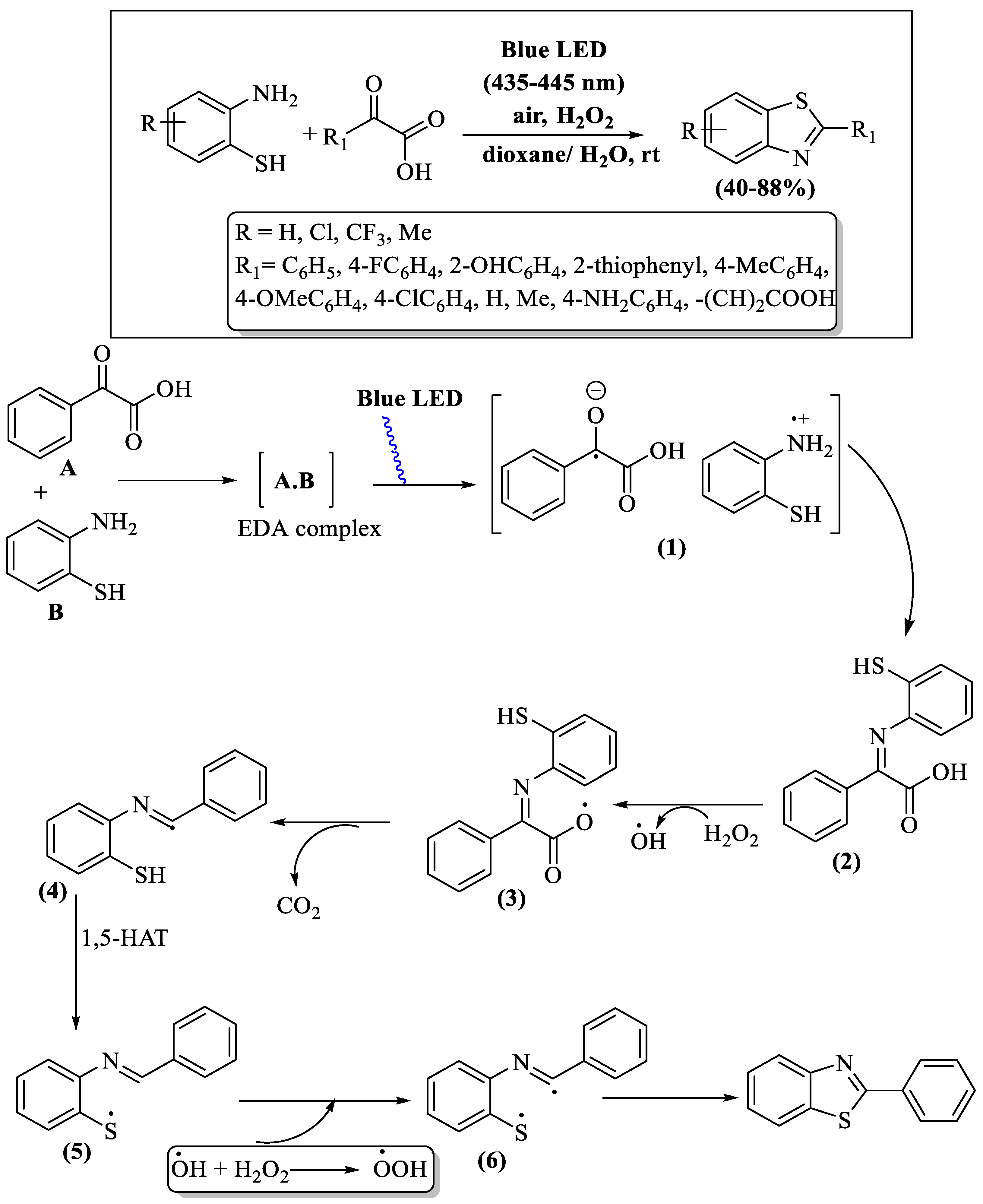
Under blue UV irradiation at ambient temperature, a photo-oxidative cross-coupling process was reported by Monga and co-workers [100], uniting α-oxocarboxylic acids and 2-ABTs with H2O2 as the oxidant, culminating in the production of 2-substituted BTs. This intricate reaction mechanism involved the initiation of a donor–acceptor complex between the reactants, followed by sequential decarboxylation and intramolecular cyclization steps; various intermediates [(1) to (6)] were obtained, as elucidated in Scheme 27. The resulting yields spanned from moderate-to-high (44–88%) in an 8 h reaction time period. Notable drawbacks of this method were a prolonged reaction timeframe and the need for column chromatography. The method’s yield variations were evident based on substitutions on 2-ABT and α-keto acids, as 4-amino-substituted acid failed to yield product, α-ketoglutaric acid resulted in trace amounts, and alkyl-substituted acids showed lower yields compared to aryl-substituted acids.
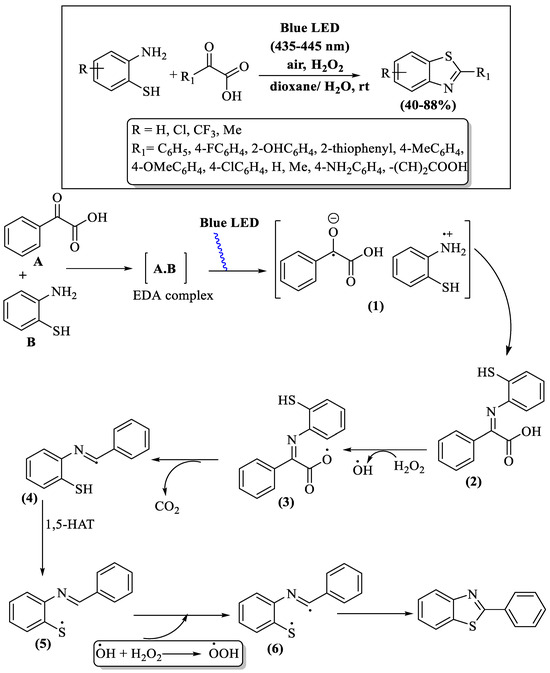
Scheme 27.
Preparation and proposed mechanism of 2-substituted BTs utilizing 2-ABT and α-oxocarboxylic acids [100].
2.4. Condensation of 2-ABT with Acyl Chloride
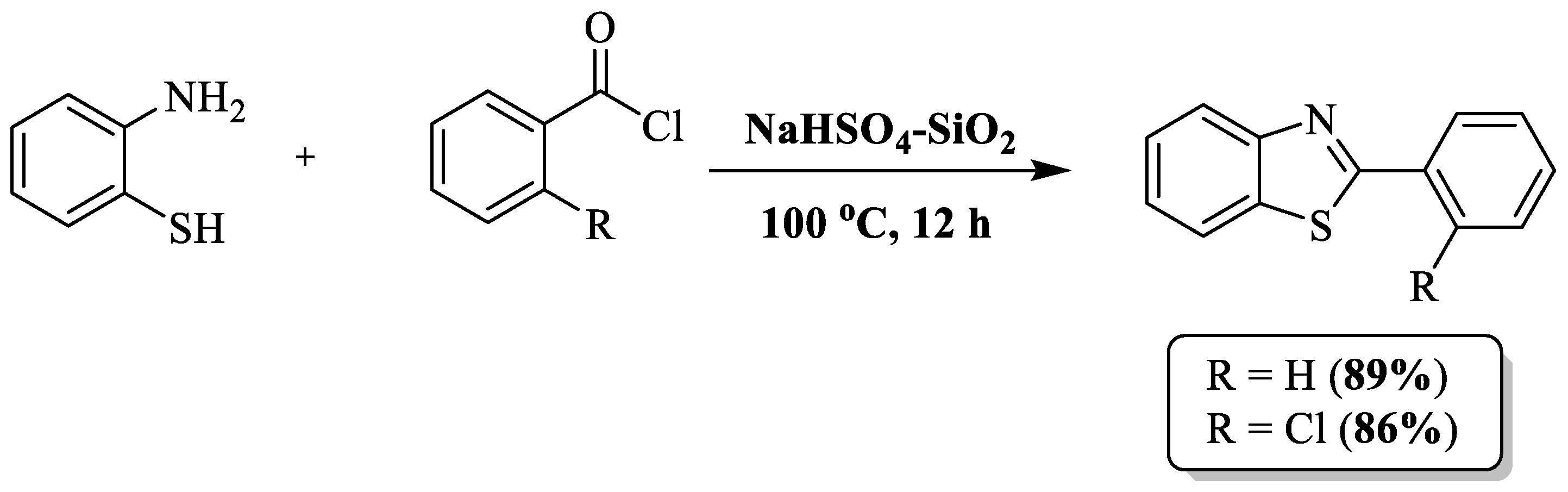
Kumar and coauthors [101] developed a solventless method using 2-ABT, acyl chloride, and a catalyst of silica-supported sodium bisulfate (NaHSO4-SiO2) for the synthesis of 2-substituted BTs. Employing readily available and affordable building blocks, namely silica gel and NaHSO4, the catalyst was designed with simplicity, eco-friendliness, and extended reusability in mind (Scheme 28). The authors only prepared two BTs by utilizing 2 acyl chloride and 2-chloro acyl chloride with 89 and 86% yield, respectively. The drawbacks of the method included an extended reaction time, high-temperature requirements, limited substrate scope, and dependence on column chromatography.
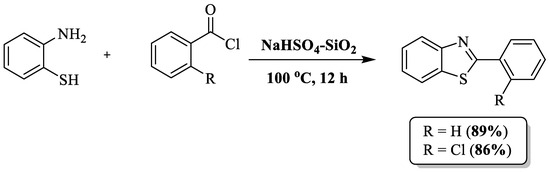
Scheme 28.
2-ABT and acyl chloride as the reagents for 2-substituted BT synthesis [101].
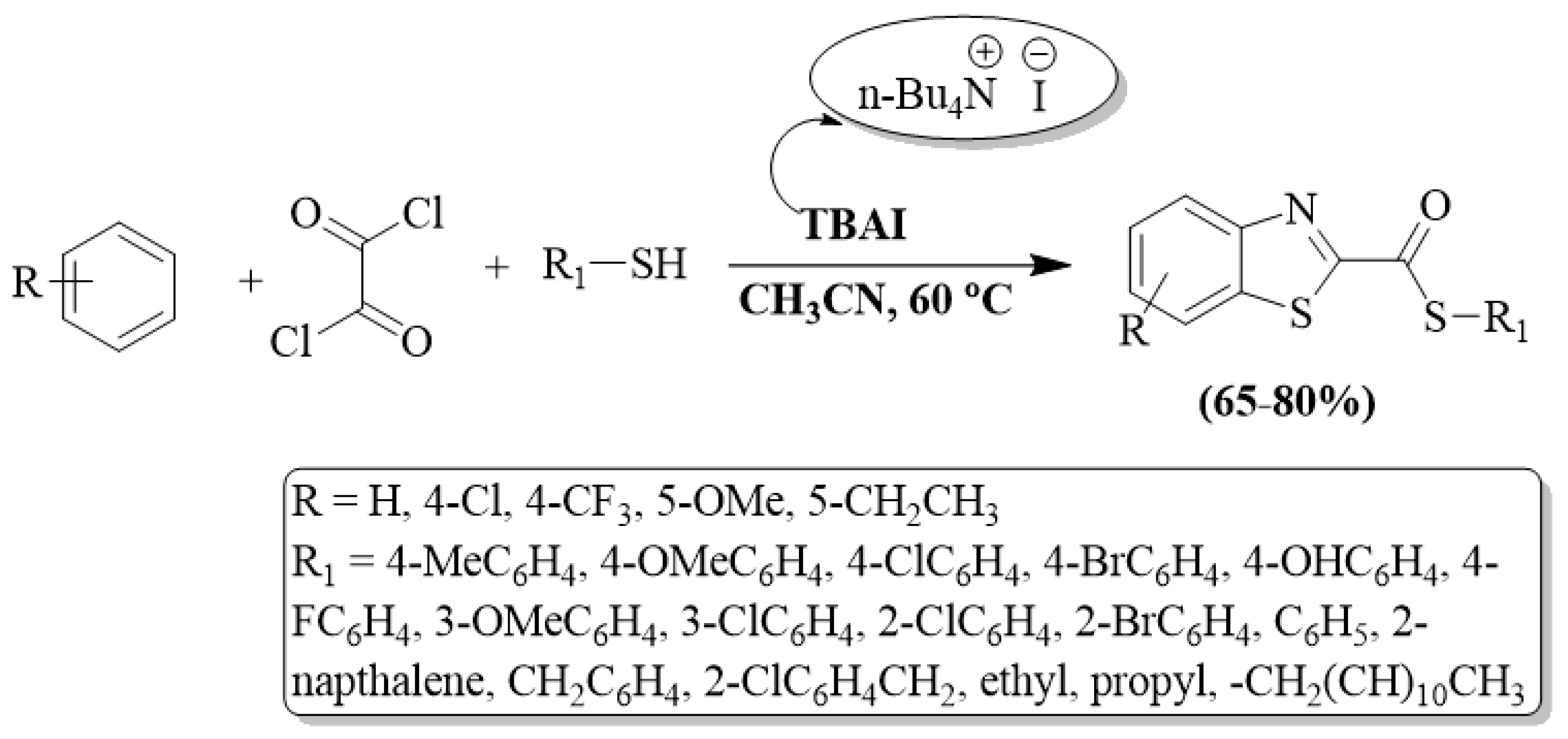
A series of BTs were developed by Dar et al. [102] using thiols, 2-ABT, and oxalyl chloride as reactants in the presence of TBAI (n-tetrabutyl ammonium iodide) catalyst and acetonitrile solvent. A high substrate scope and high yields (65–80%) are the plus points of the protocol. The authors also utilized alkyl/-EDG-substituted aryl thiols/EWG-substituted aryl thiols, and EDG-substituted thiols exhibited slightly higher yields. The method was ineffective for 4-nitro thiophenol under the optimized reaction conditions. The method faced limitations, including the use of column chromatography for product purification, an extended reaction time, and a complex reaction procedure (Scheme 29).
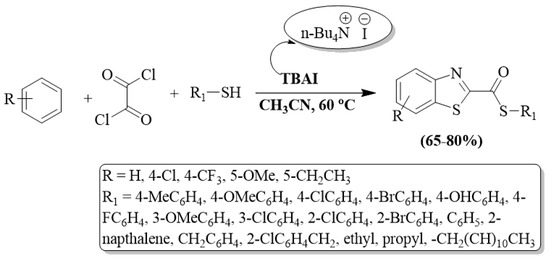
Scheme 29.
Synthesizing 2-substituted BTs by employing 2-ABT and oxalyl chloride [102].
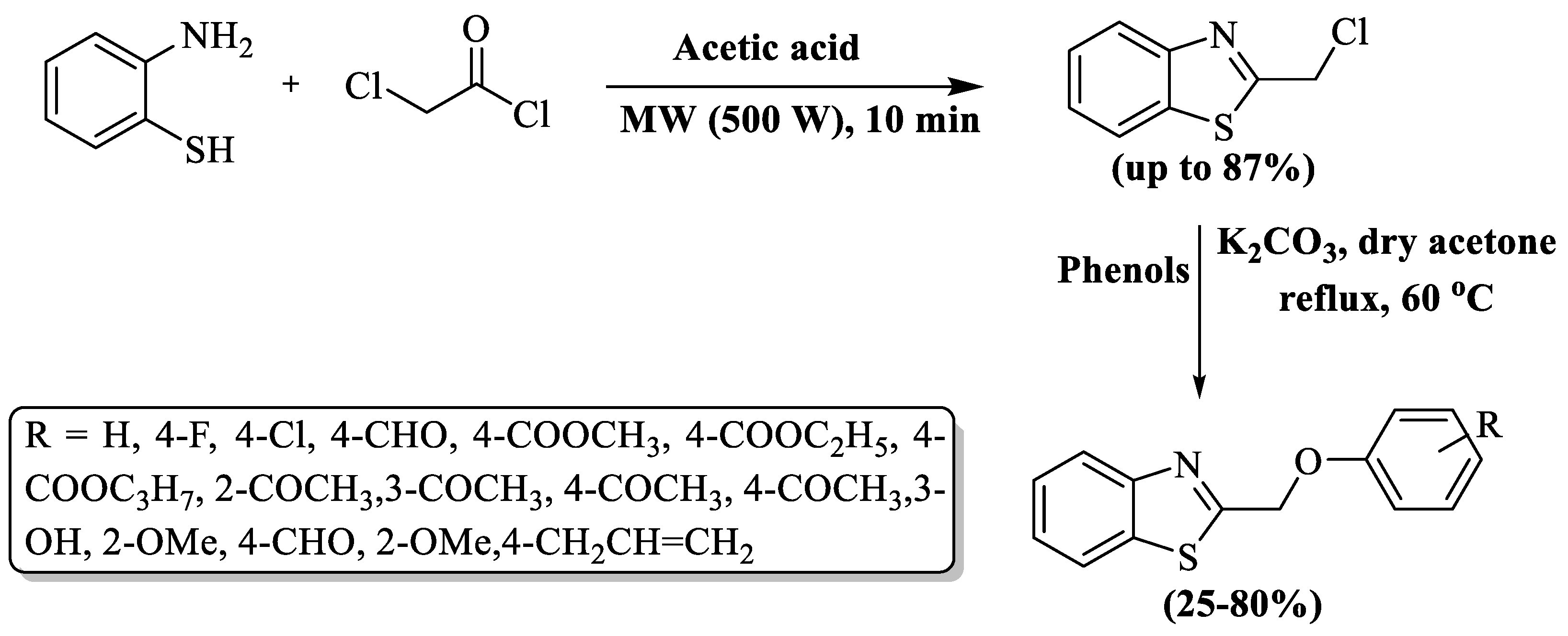
Luo and co-authors [103] developed the synthesis of 2-chloromethyl BTs using 2-ABT and chloro-acetylchloride in AcOH with the aid of microwave irradiations (MWI) for a rapid 10 min reaction. The resulting 2-chloromethyl BTs (up to 87%) strategically obtained in the first step were then employed in subsequent reactions with substituted phenols and K2CO3 in dry acetone. The subsequent reaction mixture, upon refluxing with stirring for 4 h, underwent various workup procedures to yield BT derivatives in moderate-to-high overall yields (25–80%). The authors prepared three novel derivatives of BTs. The method encountered notable limitations, including an extended reaction duration (over 4 h), reliance on column chromatography for product isolation, and the generation of poor yields (<50%) for half of the prepared derivatives. Only 4-chlorophenol and 1-(2-hydroxyphenyl)ethan-1-one derived compounds gave good yields (80%) and methyl/ethyl/propyl- 4-hydroxybenzoate gave lower yields compared to others (Scheme 30).
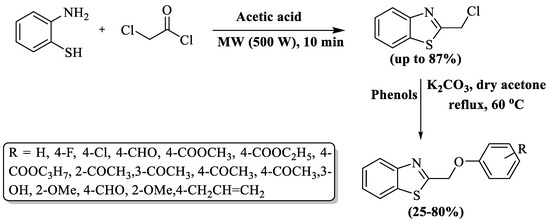
Scheme 30.
MWI-promoted synthesis of 2-substituted BTs [103].
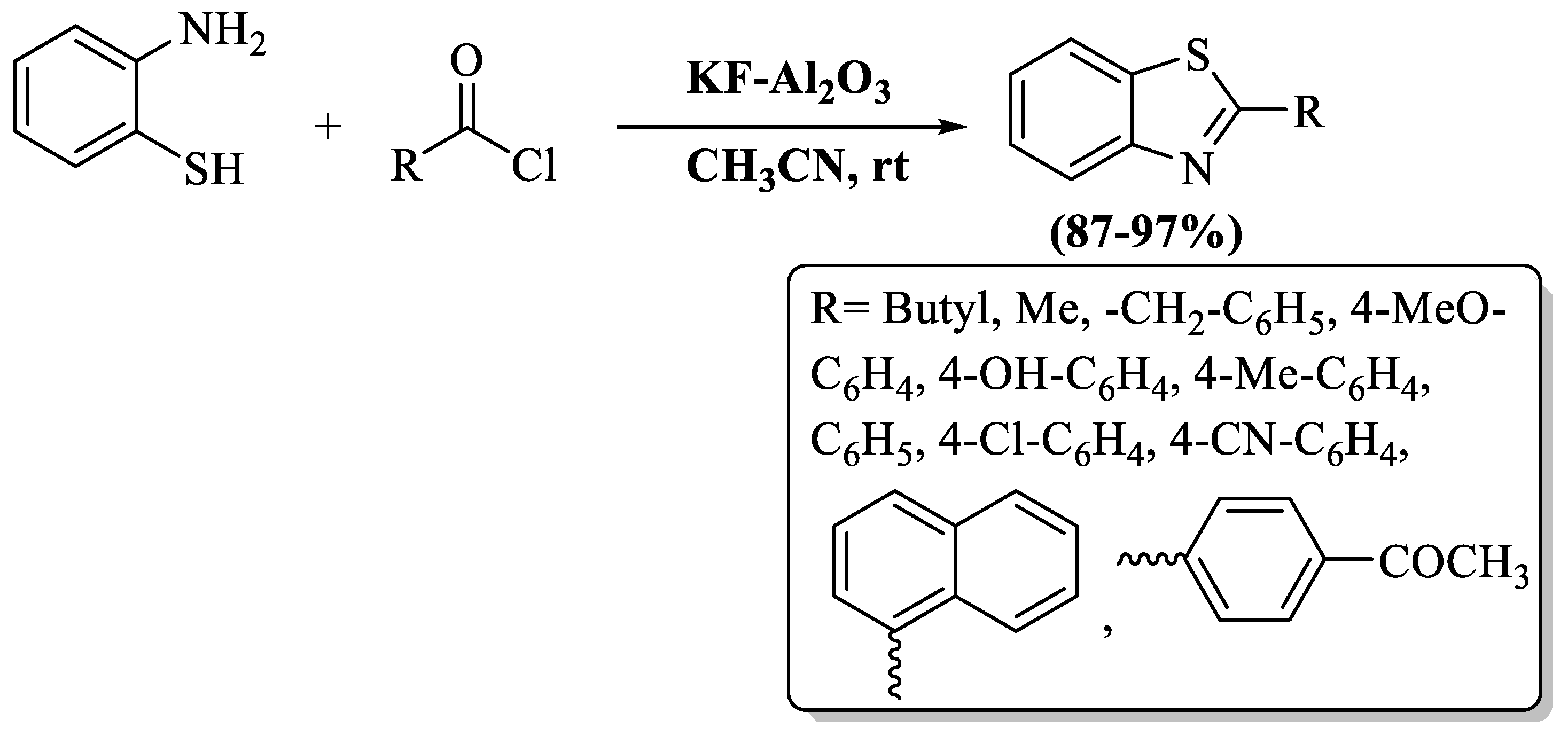
Bahadorikhalili and Sardarian et al. [104] used the basic heterogeneous catalyst KF-Al2O3 for the one-pot synthetic approach of 2-substituted BTs using 2-ABT and acid chlorides/ anhydrides in a 30-to-60 min timeframe. The procedure worked well under mild reaction conditions, and the catalyst was recycled 10 times. The strengths of this protocol include the attainment of superb yields (87–97%), the absence of any unwanted by-products, reactions were executed at ambient temperature, and a hassle-free post-reaction cleanup. The authors examined both alkyl- and aryl-substituted acyl chlorides and both were found to act irrespective to yields, but EDG substitution on aryl acyl chlorides gave higher yields in short reaction time (30 min) (Scheme 31).
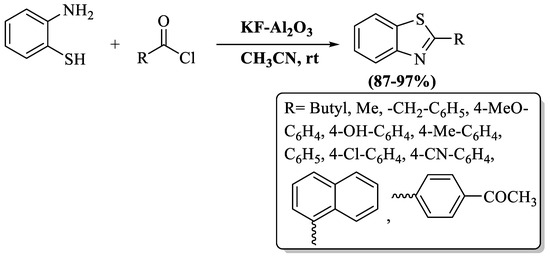
Scheme 31.
Preparation of 2-substituted BT employing 2-ABT and acid chlorides/anhydrides [104].
2.5. Condensation of 2-ABT with Amines
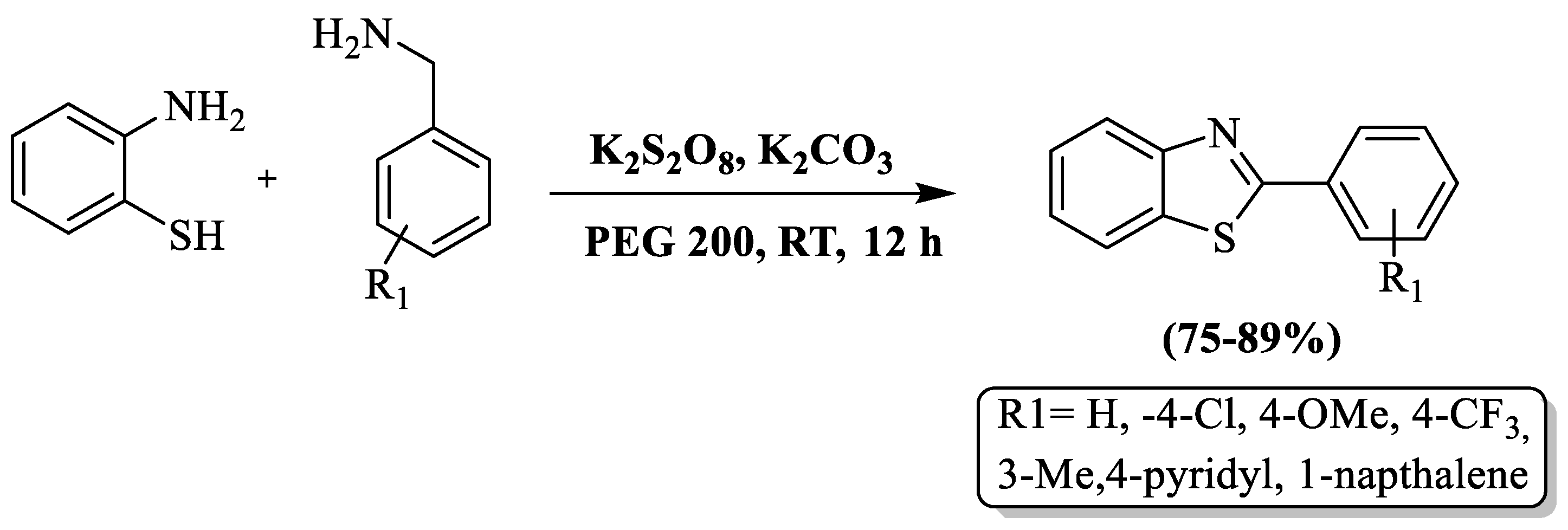
Synthesis of 2-substituted BTs by using 2-ABT and substituted aryl primary amines in the presence of K2S2O8, K2CO3, and PEG 200 (solvent) at RT was developed by Hudwekar et al. [105]. The authors prepared 7 examples with moderate-to-high yields (75–89%) in 12 h of reaction time and the yield was irrespective to the nature of the substituent on amines. The limited aspects of the method included an extended reaction duration, a constrained substrate scope, and reliance on silica gel column chromatography for compound purification. (Scheme 32).
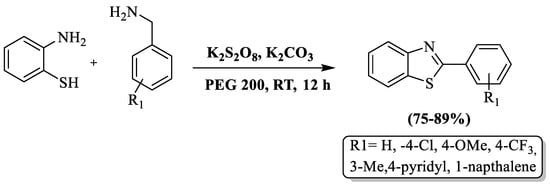
Scheme 32.
Benzylamine and 2-ABT as the agents for preparing 2-substituted BTs [105].
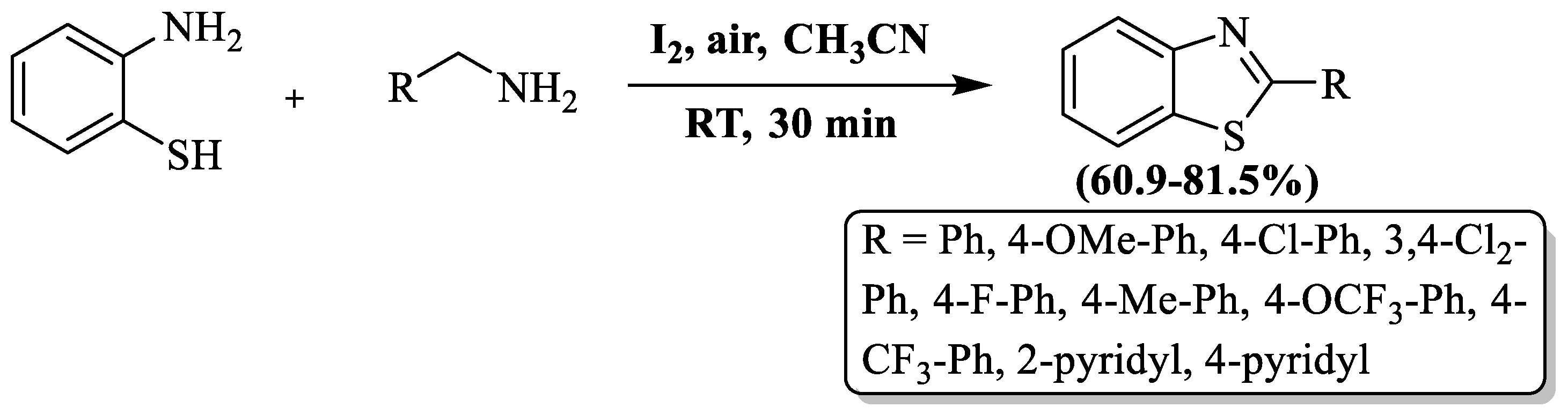
Naresh et al. [106] achieved the synthesis of 2-substituted BTs by combining 2-ABT with substituted amines in the presence of iodine and acetonitrile at room temperature and gave a 60.9–81.5% yield in 30 min of reaction time. Iodine acted as a versatile component, functioning both as an oxidizing agent and a catalyst in the synthesis of 2-substituted BTs. During the optimization of reaction conditions, the authors found a byproduct (2-benzyl-3-phenyl-3,4-dihydro-H-benzo[e][1,2,4]thiadiazine) using ethyl acetate as a solvent. The method was not found to be suitable for aliphatic amines. The authors prepared 11 derivatives and these were isolated by column chromatography (Scheme 33).
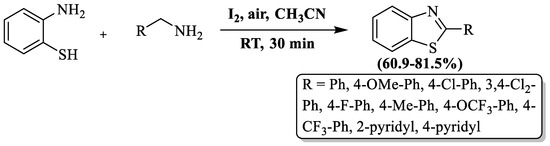
Scheme 33.
A chemical route for the synthesis of 2-substituted BTs with 2-ABT and amines [106].
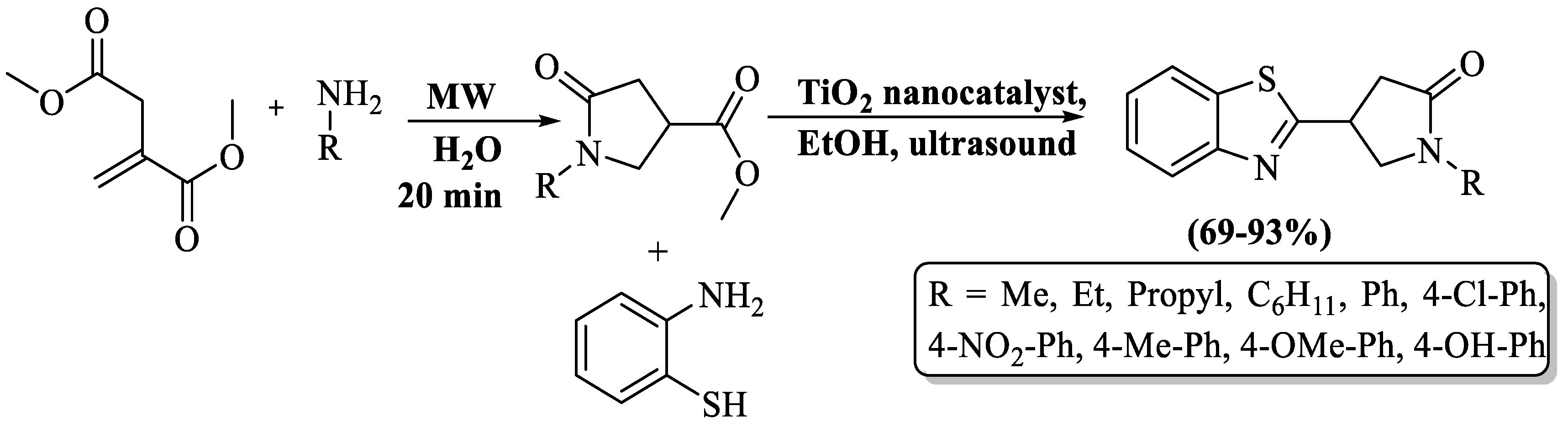
Pyrrolidinone linked to BT moiety was developed by Elsanousi et al. [107] using substituted amines and 2-ABT in the company of TiO2 (as catalytic agent) under ultrasonication. TiO2 was prepared by the sol-gel technique. This method led to the achievement of good-to-excellent yields of product (69–93%) within a remarkably short reaction time. The method involved two steps in synthesis. Initially, substituted amines reacted with dimethyl itaconate to produce substituted methyl 1-benzyl-5-oxopyrrolidine-3-carboxylate, and then this reacted with 2-ABT in the presence of TiO2 NPs to obtain the final desired products via sonication. A short reaction time, multiple reuses of the catalyst, good-to-excellent yield, and suitability for alkyl and aryl substitution were several benefits of the method, but reliance on column chromatography was the notable drawback of the process. (Scheme 34).
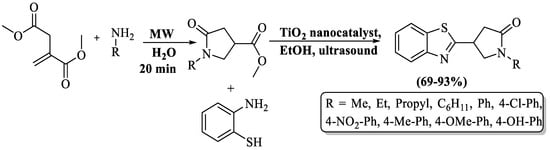
Scheme 34.
Preparation of 2-substituted BT utilizing 2-ABT and amines using TiO2 catalyst under ultrasonication [107].
2.6. Reaction of 2-ABT with Nitriles/Gem Halides/Olefins as Substrates
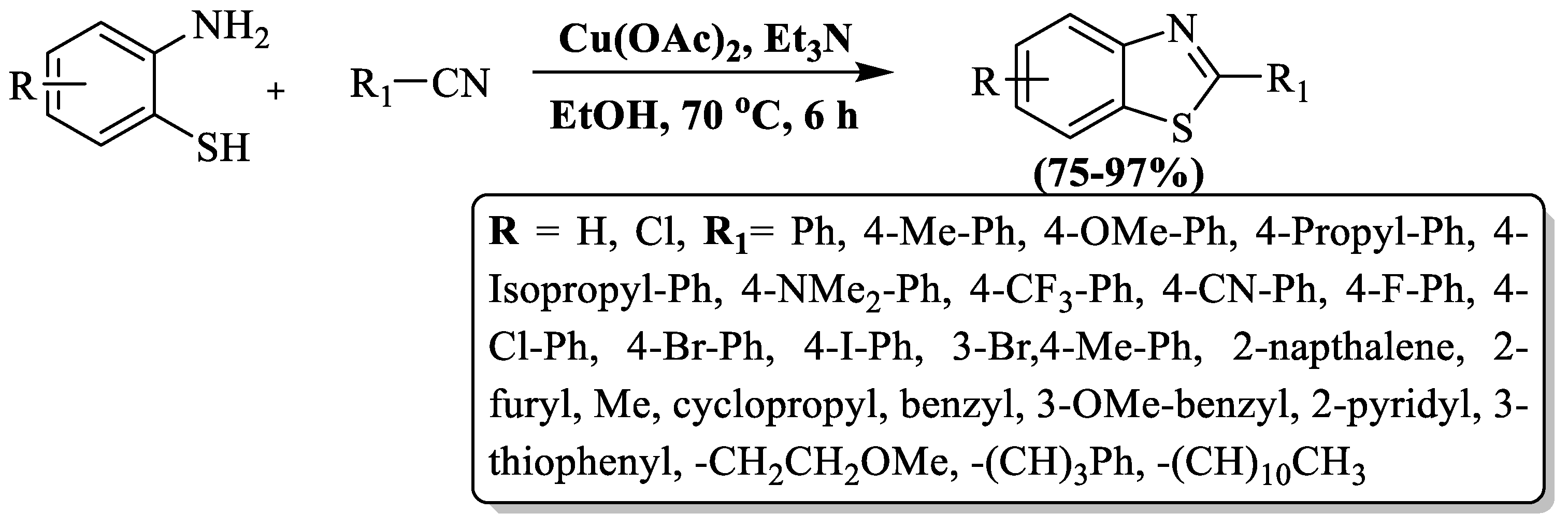
Sun and companions [108] synthesized 2-substituted BTs using 2-ABT with aliphatic and aromatic nitriles in the presence of EtOH and Cu(oAc)2 at 70 °C in 6 h. The reaction brings significant benefits, including impressive yields (75–97%), ambient temperature, green solvent, wild substrate scope, and gram-scale synthesis with high yield. The authors successfully synthesized 15 derivatives using p-substituted benzonitriles and 9 derivatives with aliphatic nitriles, where the nature of the substituent did not significantly affect the yield. However, attempts with o-substituted benzonitriles were unsuccessful under the method’s conditions. The drawbacks of the method included an extended reaction time and incompatibility with ortho-substituted benzonitriles (Scheme 35).
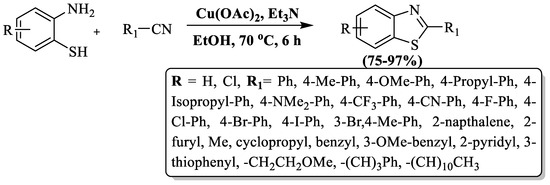
Scheme 35.
Elevating 2-ABT and nitriles in the synthesis of 2-substituted BTs [108].
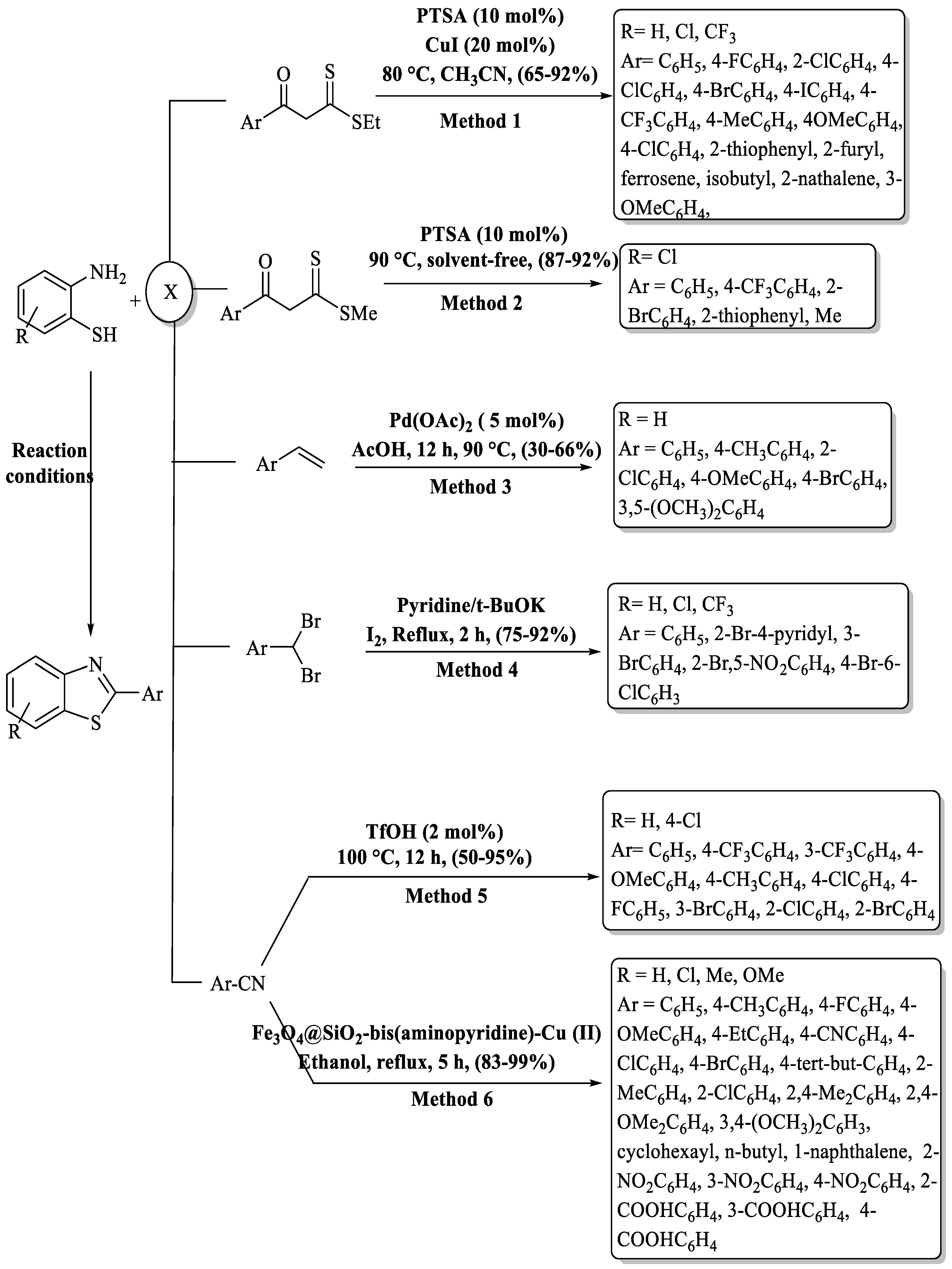
Under the inventive guidance of Ghosh and co-workers [109] a highly efficient and straightforward process was materialized for the synthesis of 2-substituted benzo[d]thiazole derivatives. This method brilliantly employed PTSA and CuI as catalysts, facilitating a one-pot condensation reaction that artfully merged 2-ABTs and β-oxodithioesters in CAN at 90 °C with a 65–92% yield (Scheme 36, Method 1). Remarkably, this methodology is characterized by its user-friendly experimental procedure, mild reaction conditions, and broad substrate compatibility. However, the method encompassed the use of toxic solvent, a long timeframe (16 h), and the use of a metal catalyst. The derivatives derived from alkyl and heterocycles gave lower yields (65–75%) as compared to EDG/EWG-substituted aromatic β-oxodithioesters. 2-(2-chlorophenyl)benzo[d]thiazole was obtained at a lower yield as compared to other halo-substituted derivatives.
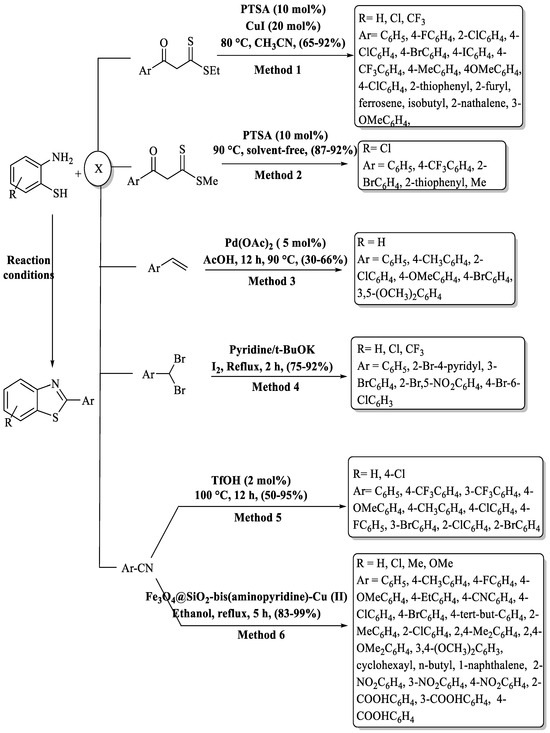
Scheme 36.
Varied pathways to 2-substituted BTs by utilizing 2-ABT as the key building block alongside ketones, nitriles, gem halides, or olefins [109,110,111,112,113,114].
Srivastava and a team of scientists [110] unveiled a groundbreaking PTSA-catalyzed one-pot synthesis method for the synthesis of 2-substituted BTs with 87–92% yield via a [4+1] cascade coupling reaction. Notably, this method was not only eco-friendly, operating in a solvent-free and metal-free manner, but also exhibited substantial DNA topoisomerase-II inhibitory activity. The limited substrate scope (5 compounds), extended reaction time (5–6 h), and only utilization of 2-amino-4-chloro benzenethiol and reliance on column chromatography were the several negative points of the method (Scheme 36, Method 2).
Shaikh and colleagues [111] developed an inventive palladium-catalyzed one-pot method for the synthesis of biologically significant 2-substituted BTs with a 30–66% yield in 12 h at 90 °C. This method utilized styrenes and 2-ABT as its primary substrates (Scheme 36, Method 3). The method has various drawbacks, such as yielding results in the low-to-moderate range, relying on a metal catalyst, preparation of a narrow derivative spectrum, and involving a relatively prolonged reaction time. Halo-substituted styrenes exhibited very poor yield (≤35%).
Siddappa and co-workers [112] introduced a streamlined method for synthesizing 2-substituted BTs with 75–92%. This process involved coupling of o-ABT with gem-dibromomethylarenes under reflux conditions, employing minimal quantities of molecular iodine (I2), tert-butoxide, and pyridine. The reaction was completed in 1.5–2 h and 18 derivatives were synthesized. The method has some pros like gram-scale synthesis, high yields, and adaptability to diverse substrates, but dependence on column chromatography for the isolation of products was the limitation of the method (Scheme 36, Method 4).
Yu and his group [113] devised a Bronsted acid-catalyzed method for the preparation of 2-substituted BTs from 2-ABT and nitriles at 100 °C in 12 h time. This method is characterized by its solvent-free and metal-free synthesis, resulting in moderate-to-high yields (50–95%) of the targeted compounds. The method demonstrated compatibility with various substituted nitriles, offering the benefits of a straightforward experimental setup and an affordable, economically favorable catalyst. High temperature, long reaction completion time, and moderate yields for some of the derivatives were the several negative aspects of the method. The reaction remained unaffected by substitutions on 2-ABT, yet alterations on the benzene ring of benzonitriles impacted the results. Faster reactions were observed with EWG on the benzene ring, while EDGs led to slower reactions, like 4-methyl benzonitrile, which gave the lowest yield (Scheme 36, Method 5).
Mahmood and co-authors [114] introduced a method for the synthesis of 32 compounds of 2-substituted BTs. This innovative approach utilized a [Fe3O4@SiO2-bis(aminopyridine)-Cu(II)] nanocomposite catalyst and involves the reaction between 2-ABT and nitriles, resulting in high yields (83% to 99%) in ethanol at reflux conditions. The method stands out for the use of green solvent, the ease of catalyst separation, the ability to recycle the catalyst up to six times, and the large scope of substrates with excellent yielding. The method has certain drawbacks, including a prolonged reaction time of 5 h, reliance on a metal catalyst, and the use of silica gel column chromatography for the purification of compounds. (Scheme 36, Method 6).
Khoobi et al. [97] introduced an efficient MW-assisted protocol for synthesizing BT derivatives with appended coumarin. The method utilized 3-CN coumarin and 2-ABT in the presence of the cost-effective and environmentally friendly catalyst, HPMo, using ethanol at 150 °C within 15 min. The use of column chromatography and the synthesis of only 5 compounds were notable drawbacks of the procedure. The presence of a Cl substituent on 2-ABT impacted the method’s yields, leading to lower yields with chloro-substituted 2-ABT compared to unsubstituted 2-ABT (Scheme 37).
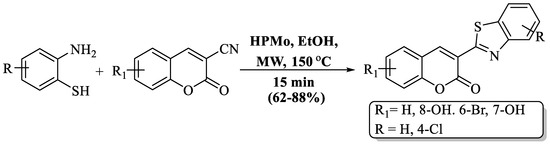
Scheme 37.
Synthesis of 2-substituted BT using 2-ABT and coumarin [97].
2.7. Condensation of 2-ABT with Benzyl Alcohol
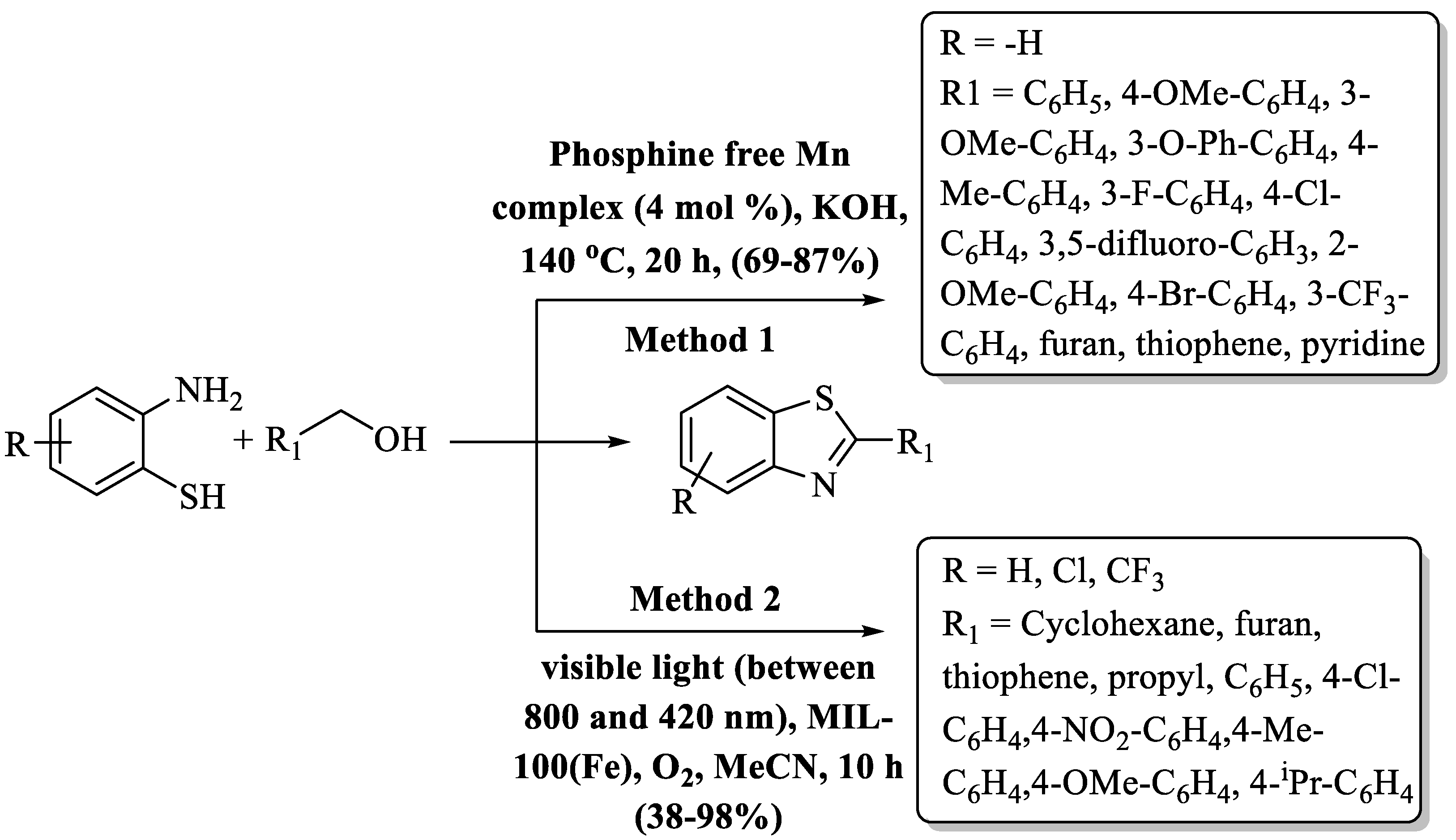
Das and co-workers [115] charted a greener path in the world of chemistry for the preparation of 2-substitiuted BT moieties, guided by the ingenuity of a phosphine-free Mn(I) complex. This transformative method unites 2-ABT and primary alcohols by dehydrogenative coupling at 140 °C and gave a 69–87% yield in a time period of 20 h (Scheme 38, Method 1). The hallmark of this innovation lies in its extraordinary versatility, showcasing an impressive degree of functional group tolerance and an inherent synergy with heteroaromatic aldehydes. The long duration of reaction completion and the condition of high heating were cons of the method. The authors used a library of alcohols like monosubstituted/disubstituted/heteroaromatic benzyl alcohols, and heteroaromatic and 3,5-difluoro-substituted benzyl alcohol gave lower yields.
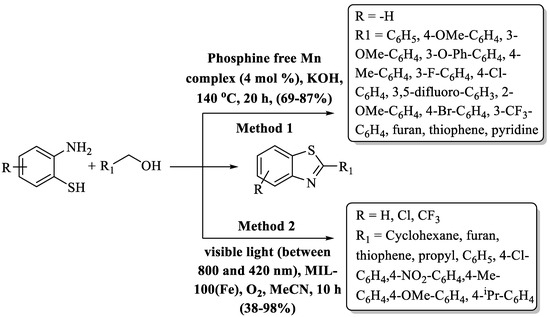
Scheme 38.
Preparation of 2-substituted BT by reaction of 2-ABT with alcohols [115,116].
Wang and their research team [116] unveiled a striking symphony in the preparation of 2-substituted BTs in a 10 h timeframe. The reaction involved oxidative condensing of 2-ABT with various alcohols (alkyl/heterocyclic/aryl), facilitated by visible light (800 nm ≥ λ ≥ 420 nm) and the MIL-100(Fe) photocatalyst in acetonitrile. The catalyst was recovered and reused for 3 cycles. The authors utilized aliphatic, heteroaromatic, and other aryl alcohols and found variations in yield. Aliphatic aldehydes gave poor yields (38–42%) and EWG (Cl, NO2)-substituted benzyl alcohol gave lower yields (46–56%) as compared to EDG-substituted alcohol and heteroaryl alcohol. The reaction showcased moderate-to-high yields (38–96%), with the limitation being a relatively extended reaction duration, use of toxic solvent, and poor yields of some derivatives. In the reaction mechanism, aldehydes were produced as an intermediate due to the oxidation of alcohol to aldehydes which then reacted with o-aminothiophenols to create imine/benzothiazolines. These compounds further oxidized to yield the final 2-substituted BTs, driven by O2 radicals (Scheme 38, Method 2).
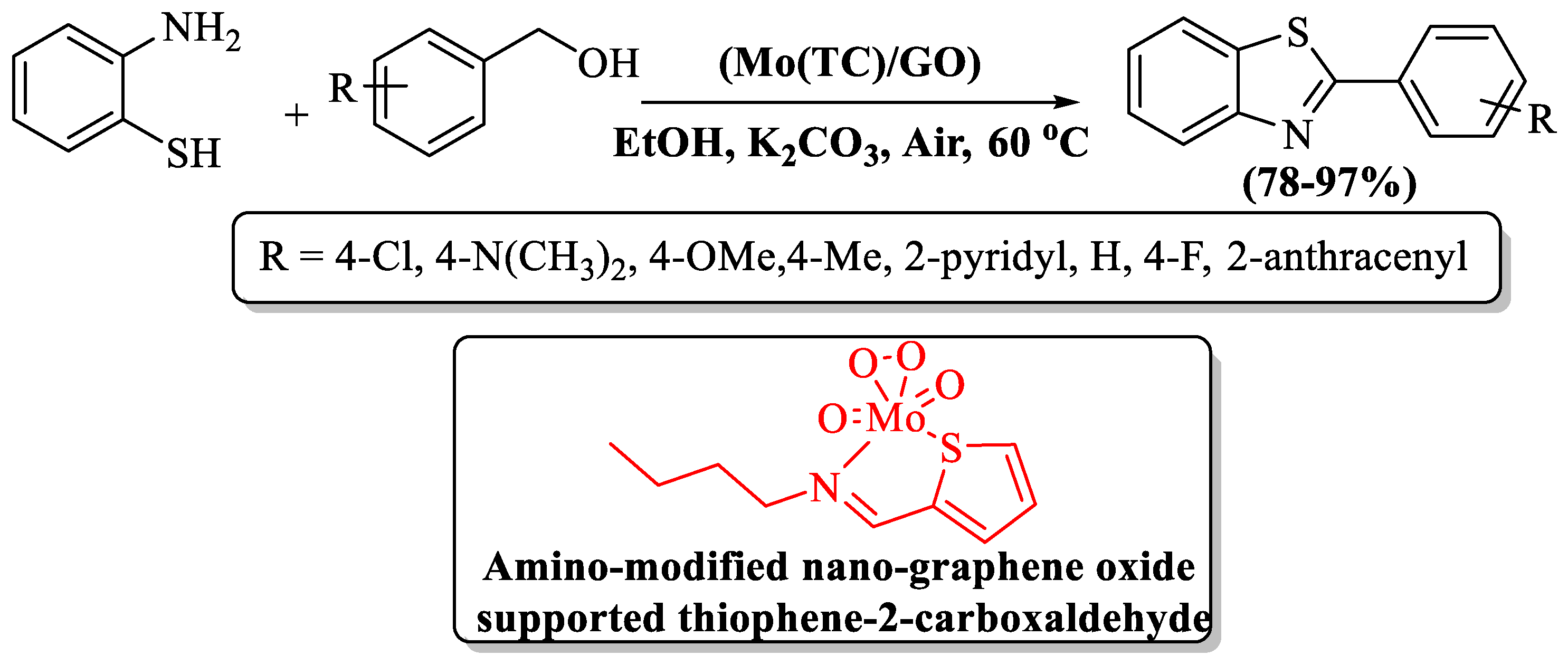
Karami et al. [117] unveiled a pioneering catalyst—the amino-modified nano-graphene oxide-supported thiophene-2-carbaldehyde Mo-complex—affectionately known as Mo(TC)/GO. The catalyst was employed in the synthesis of 2-substituted BTs, harmonizing benzyl alcohol and 2-ABT in ethanol and K2CO3 at 60 °C. The authors prepared 8 derivatives at a yield of 78–97% within 80–175 min reaction time. The catalyst itself, an exemplar of sustainability, gracefully served through repeated use (five runs). Use of column chromatography, a metal-based catalyst, and limited substrate scope were the several limitations of the method, but the high yield with EDG, EWG, and heteroaryl alcohol in ambient reaction conditions were the pros of the method (Scheme 39).
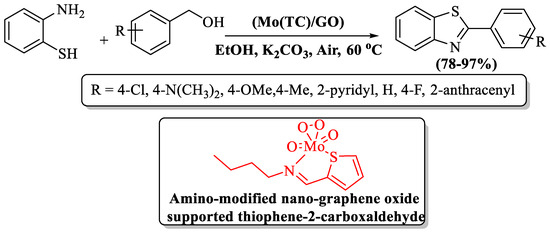
Scheme 39.
Reaction of 2-ABT and Benzyl Alcohol to obtain 2-substitited BTs [117].
2.8. Reaction of 2-ABT and N-Methyl Thioamide
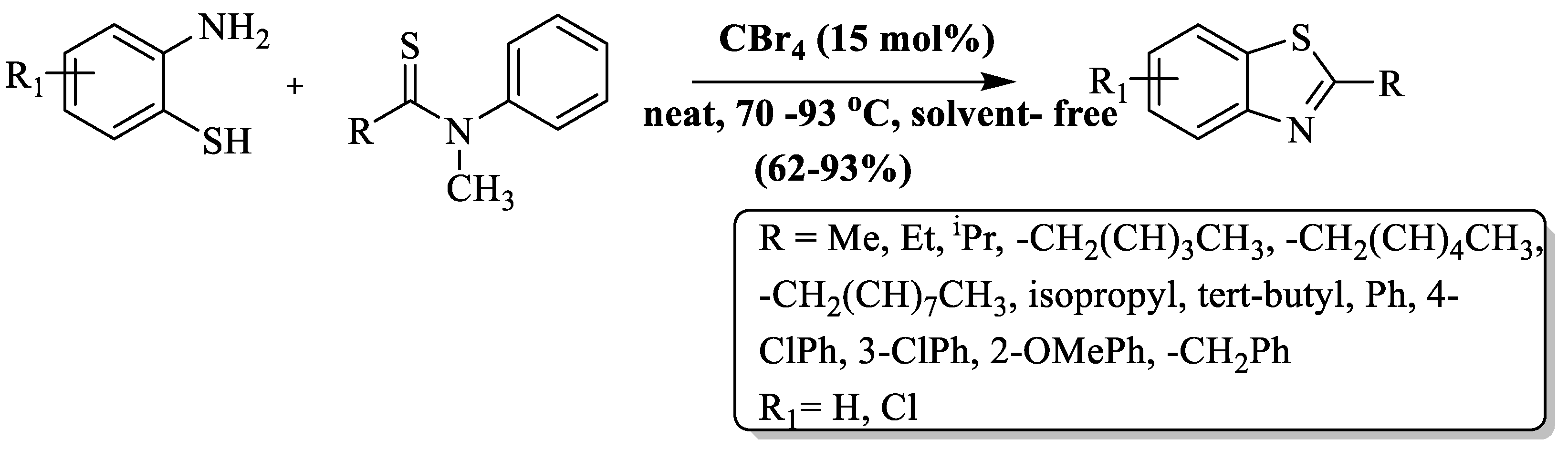
2-ABT and N-methylthioamides were condensed to synthesize 2-substituted BTs at 70–80 °C in 24–36 h by Kazi et al. [118]. Solvent- and metal-free conditions were employed and CBr4 (15 mol%) was used as a promoter. Moderate-to-excellent yields (62–93%), broad substrate scope, solvent-free, metal-free, etc., are the key attributes of the method (Scheme 40). The incorporation of substrates featuring α-branched aliphatic or aromatic thioamides in the synthesis of BT derivatives faced reduced reactivity attributed to steric hindrance. Increasing steric hindrance, for instance, tert-butyl group substitutions, resulted in diminished yields, and the presence of o-positioned groups in aromatic thioamides also led to lower yields; furthermore, aromatic thioamides required a slightly higher temperature (80 °C) to produce products. The influence of substituents on the reaction outcome, prolonged reaction time, and the need for column chromatography were the significant drawbacks of this method.
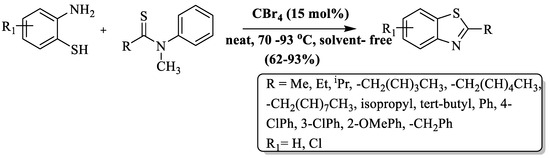
Scheme 40.
Preparation of 2-substituted BTs using N-methylthioamide and 2-ABT [118].
2.9. Reaction of 2-ABT and Dimethylformamide (DMF)Derivatives
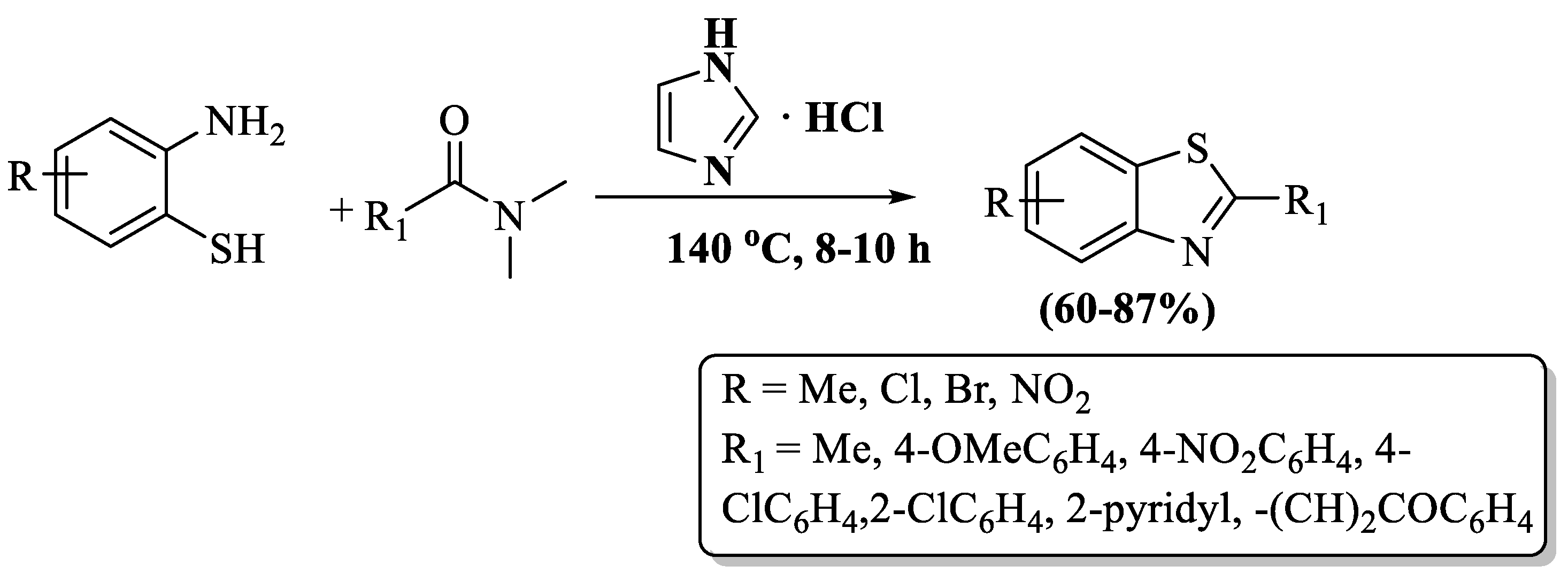
Tian and coworkers [119] unveiled an easy route to prepare 2-substituted BT employing 2-ABTs and DMFs at significant yields (60–87%). The reaction was performed in the presence of imidazolium chloride at 140 °C within 8–10 h. Some of the primary challenges of the reaction are the prolonged time, column chromatography use, and elevated temperatures (Scheme 41).
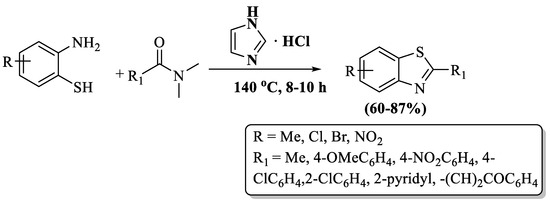
Scheme 41.
The DMF route to 2-substituted BT synthesis with 2-ABT [119].
2.10. Reaction of 2-ABT and Isocyanate/Isothiocyanate
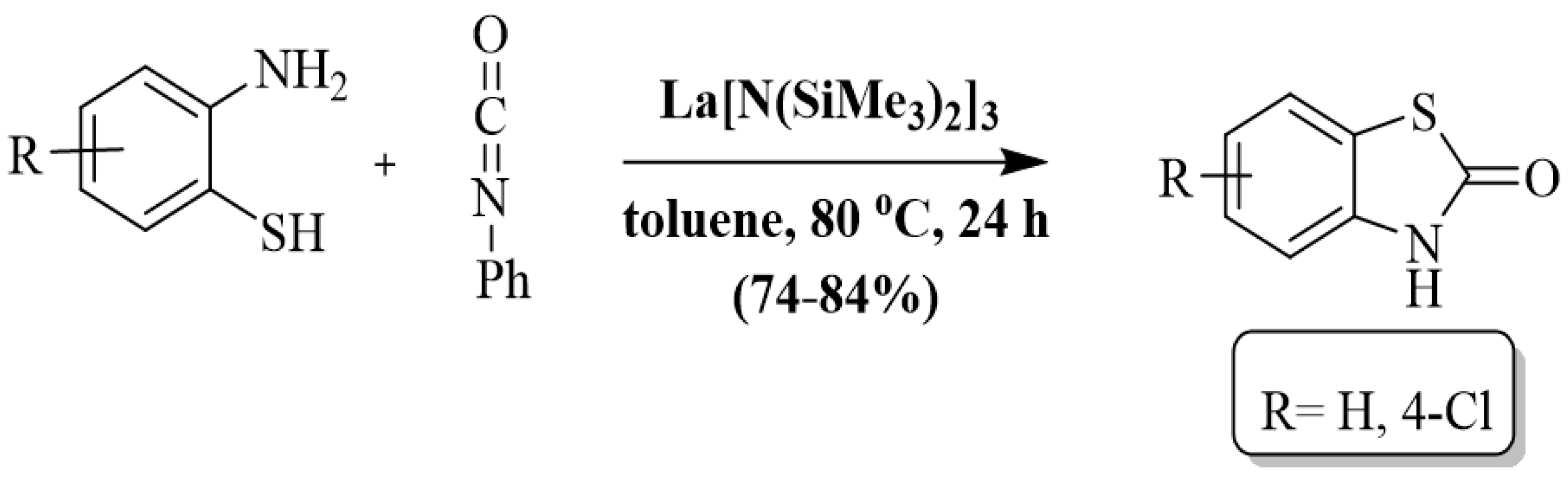
Jing et al. [120] synthesized benzothiazolones using a lanthanum tris(bis(trimethylsilyl)amide) La[N(SiMe3)2]3 catalyst via cyclocarbonylation of 2-ABT with isocyanate in toluene. The reactions were completed in 24 h at 80 °C. The authors only prepared two benzothiazolone derivatives by using 2-ABT and 2-amino-4-chlorobenzenethiol and found 84% and 74% yields, respectively. Along with substrate limitation, this method also has some other drawbacks including long reaction duration, use of toxic solvent, and reliance on column chromatography (Scheme 42).
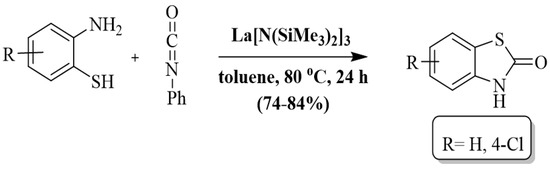
Scheme 42.
Preparation of 2-substituted BT by utilizing 2-ABT and isocyanates [120].
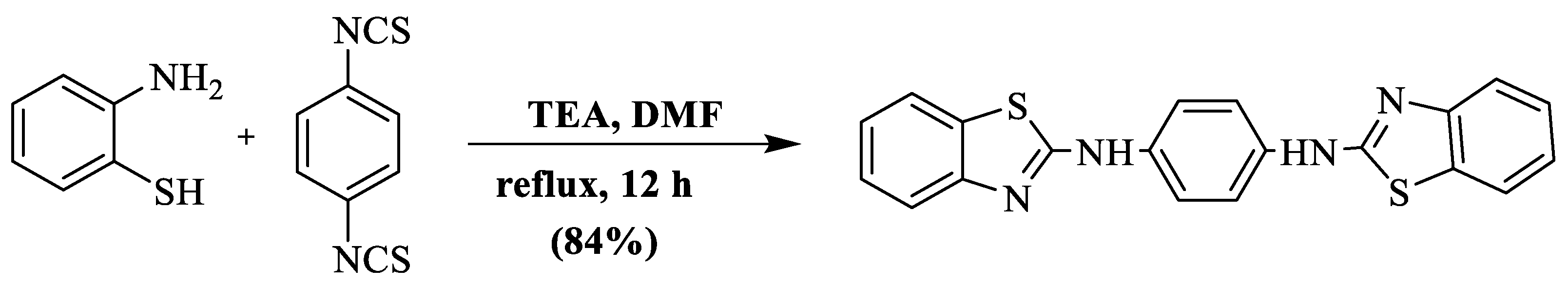
A mixture of 2-ABT and 1,4-phenylene-di-isothiocyanate, in the presence of DMF and triethylamine, gave rise to N,N′-bis(benzothiazole-2-yl)-benzene-1,4-diamine at an 84% yield in 12 h [121]. The use of toxic solvent, high heating, and a long reaction timeframe were the negative aspects of this method (Scheme 43).
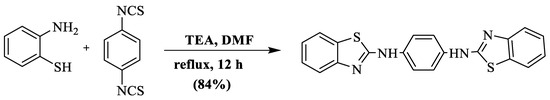
Scheme 43.
Synthesis of 2-substituted BT using 2-ABT and isothiocyanate [121].
2.11. Reaction of 2-ABTs and Carbon Disulfide
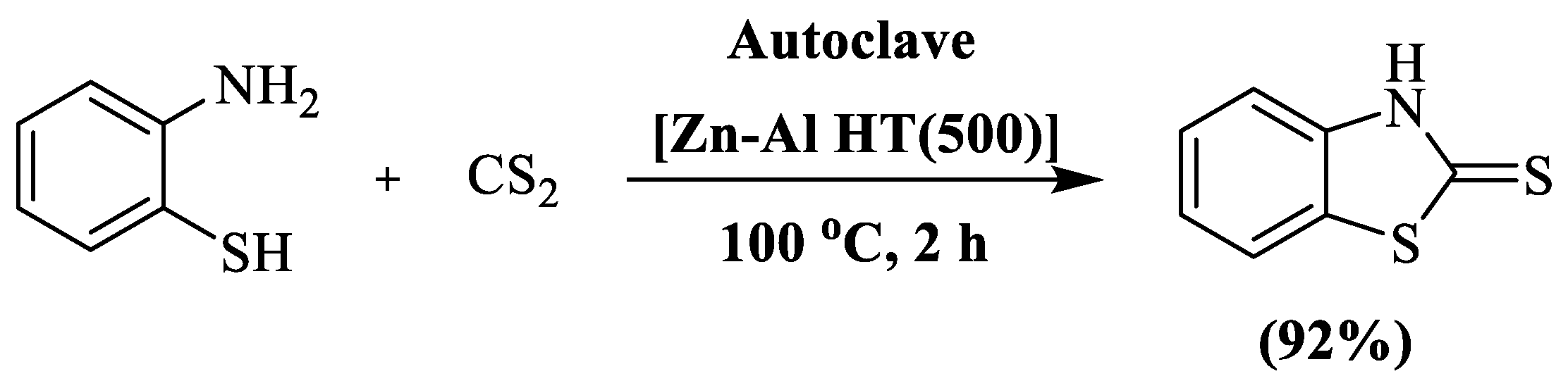
A highly efficient ZnO/Al2O3 composite [Zn-Al HT (500)] catalyzed synthesis ofbenzothiazolel-2-thiones (92%) was developed by Ballebeni et al. [122], involving condensation between 2-aminothiophenol and carbon disulfide at 100 °C in 2 h. The reaction was carried out under reduced pressure in an autoclave. The authors prepared a single compound and utilized silica gel column chromatography to isolate the final pure product (Scheme 44).
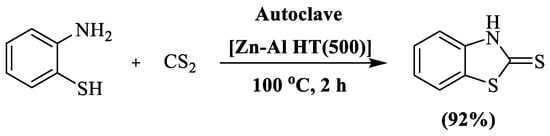
Scheme 44.
Synthesis of 2-substituted BT using 2-ABT and carbon disulfide [122].
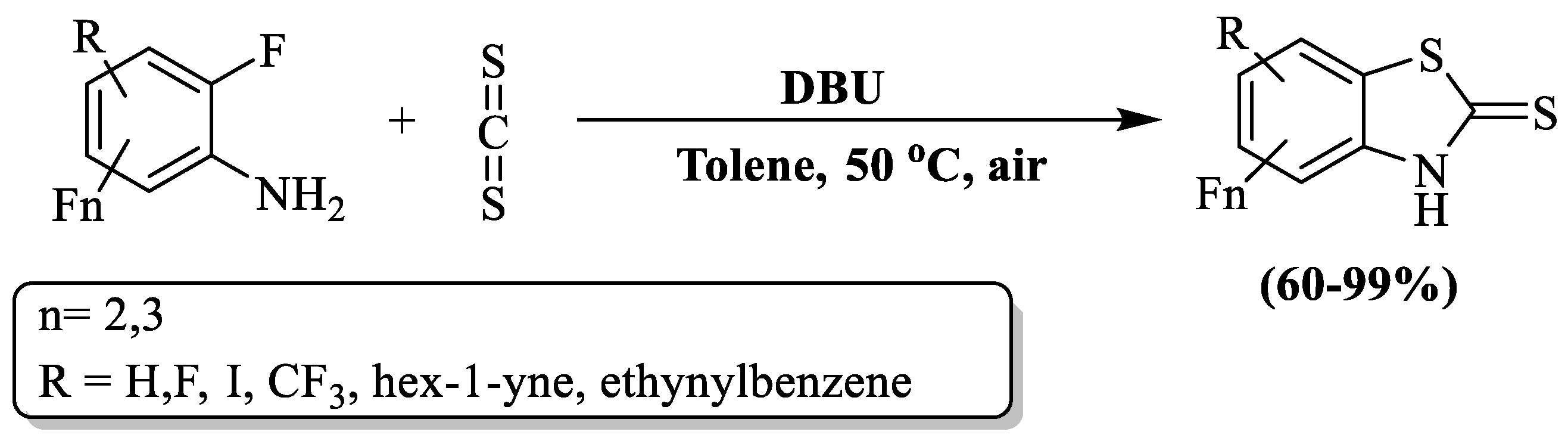
Politanskaya and a research team [123] fabricated an efficient and facile approach to prepare polyfluorinated 2-substituted BTs from aniline and CS2. The reaction was catalyzed by DBU (1,8-diazabicyclo[5.4.0]undec –7–ne at 50 °C in toluene (Scheme 45)). The products were obtained with yields ranging from 60% to 99% within 1 to 25 h of reaction time. In this procedure, the C-atom of CS2 underwent a nucleophilic attack initiated by the N-atom within the amine group of the arene. Subsequently, a targeted intramolecular substitution of the F-atom occurred in the o-position with respect to the amino group. The authors examined 8 substrates to obtain product and found more than 86% yield (within 1–10 h) in optimized conditions, except 2,3,5,6-tetrafluoro-4-iodoaniline (no reaction) and 2,4,5-trifluoro aniline (60% yield in 24 h), but 2,4,5-trifluoro aniline gave a 75% yield at 80 °C in 6 h. The variability in outcomes, long reaction time, constricted substrate scope, use of toxic solvent, and utilization of glass plate chromatography for the isolation of compounds were the limitations.
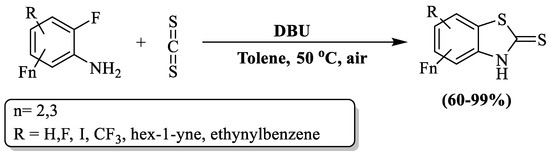
Scheme 45.
Synthesis of polyfluorinated 2-substituted BT using polyfluorinated anilines and carbon disulfide [123].
2.12. Condensation of 2-ABTs with Carbon Dioxide
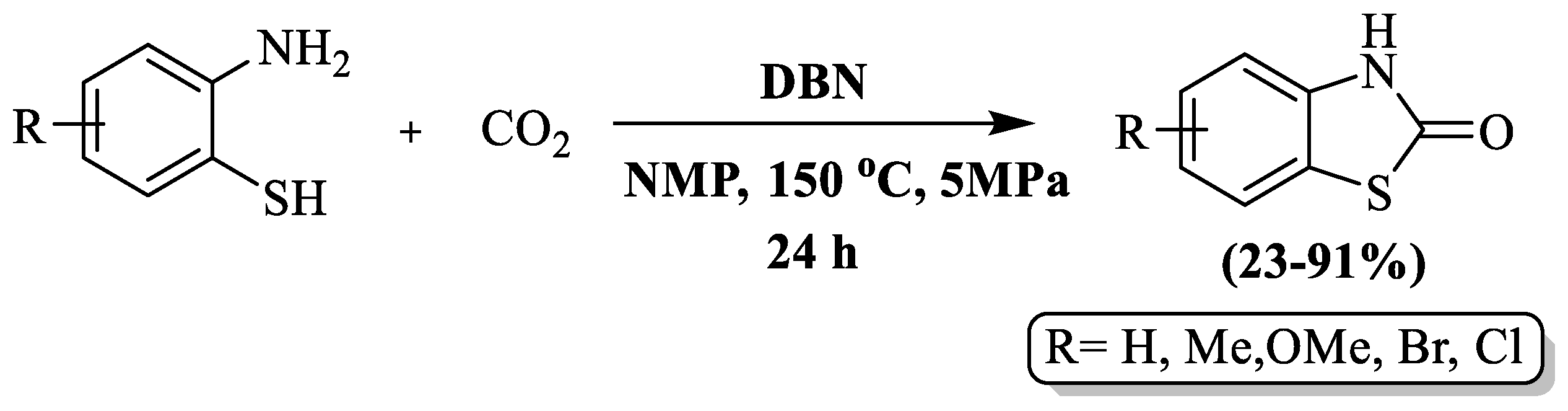
Benzothiazolones were synthesized via cyclocarbonylation of 2-aminothiophenols with CO2 by Gao et al. [124] employing 1,5-diazabicyclo [4.3.0] non-5-ene (DBN) as a metal-free catalytic agent at a yield of 23–91% in the presence of 1-methyl-2-pyrrolidinone (NMP) as solvent. The reaction was carried out at 150 °C within 24 h. The use of EDG-substituted 2-ABT led to high yields, with methyl-substituted 2-ABT achieving a 70% yield. However, EWG substitutions, such as chloro/bromo 2-ABT, resulted in very poor yields after an extended reaction duration (40 h). The reaction did not proceed with nitro-substituted 2-ABT. The limitations of this method include a long reaction duration, poor yields for 2-ABT with EWG substitution, limited substrate scope, high-temperature requirements, reliance on column chromatography, and the use of a complex setup (autoclave with a magnetic stirrer) (Scheme 46).
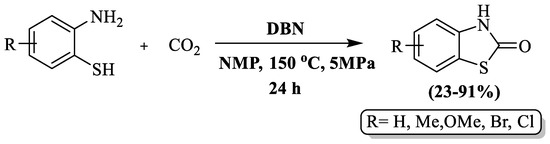
Scheme 46.
Synthesis of 2-substituted BT using 2-ABT and carbon dioxide [124].
2.13. Reaction of 2-ABTs and Thiocarbomyl Chloride/Tetramethyl Thiuram Disulfide/Sodium Dimethyl Dithiocarbamate
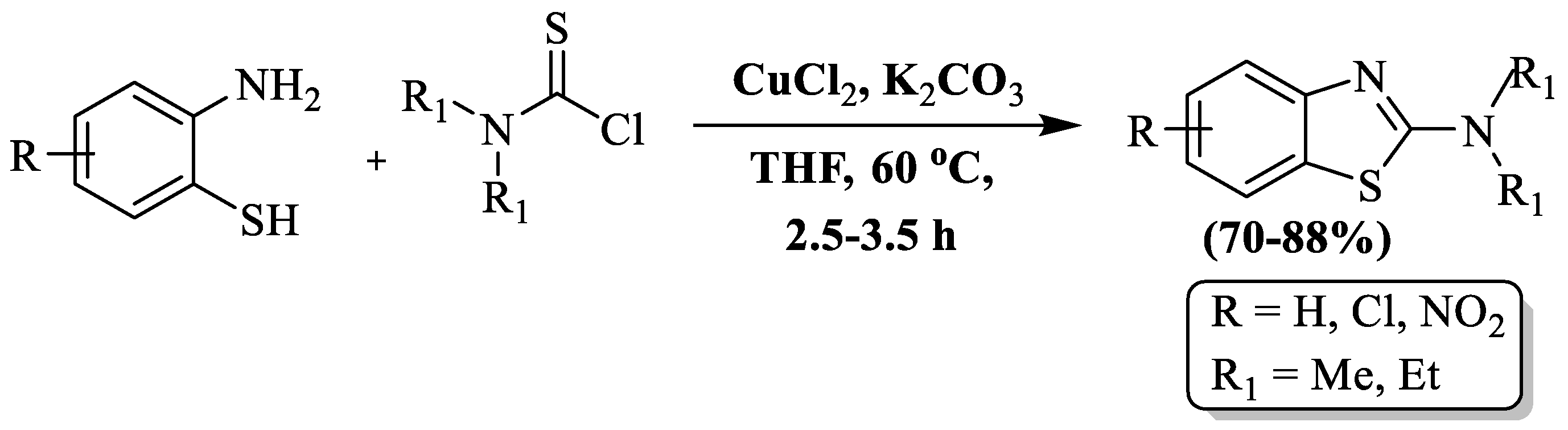
Xu and co-authors [125] synthesized substituted 2-amino BTs using 2-ABT and thiocarbamoyl chloride in the residence of CuCl2 catalyst, K2CO3, and THF as solvent at 60 °C. The reaction unveiled its treasures with high yields (70–88%). The method utilized both dimethyl thiocarbamoyl and diethyl thiocarbamoyl, but diethyl thiocarbamoyl demonstrated superior results. However, the approach has drawbacks, including the use of toxic solvent, a lack of details on the reaction mechanism, synthesis limited to only 6 BT derivatives, utilization of a metal catalyst, and an absence of information on catalyst recovery/recycling (Scheme 47).
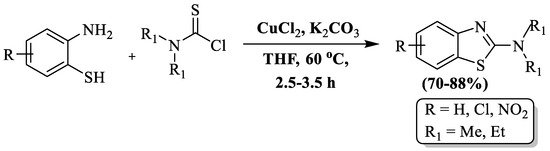
Scheme 47.
Preparation of 2-substituted BT using 2-ABT and thiocarbamoyl chloride [125].
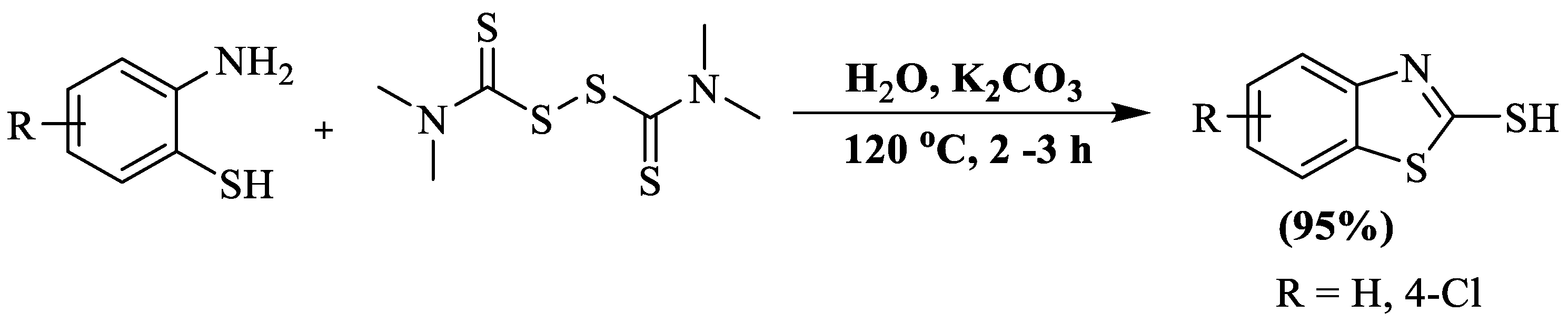
Liu et al. [126] synthesized BT-2-thiols involving cyclization of 2-ABTs with tetramethylthiuram disulfide (TMTD) in an aqueous medium using K2CO3 (base) at 120 °C. TMTD was used as a thiocarbonylation trigger in the reaction. The protocol involved a simple method under metal/catalyst-free conditions and resulted in a 95% yield within 2–3 h. The method exhibited high yields, utilized a catalyst-free system, and employed water as a solvent, but also had challenges like high-temperature requirements, limited synthesis of only two BT-2-thiol derivatives, and the necessity of flash column chromatography for product isolation (Scheme 48).
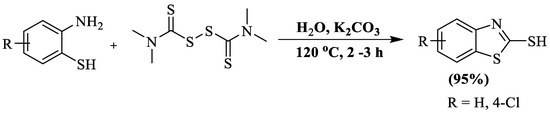
Scheme 48.
Synthesis of 2-substituted BT using 2-ABT and TMTD [126].
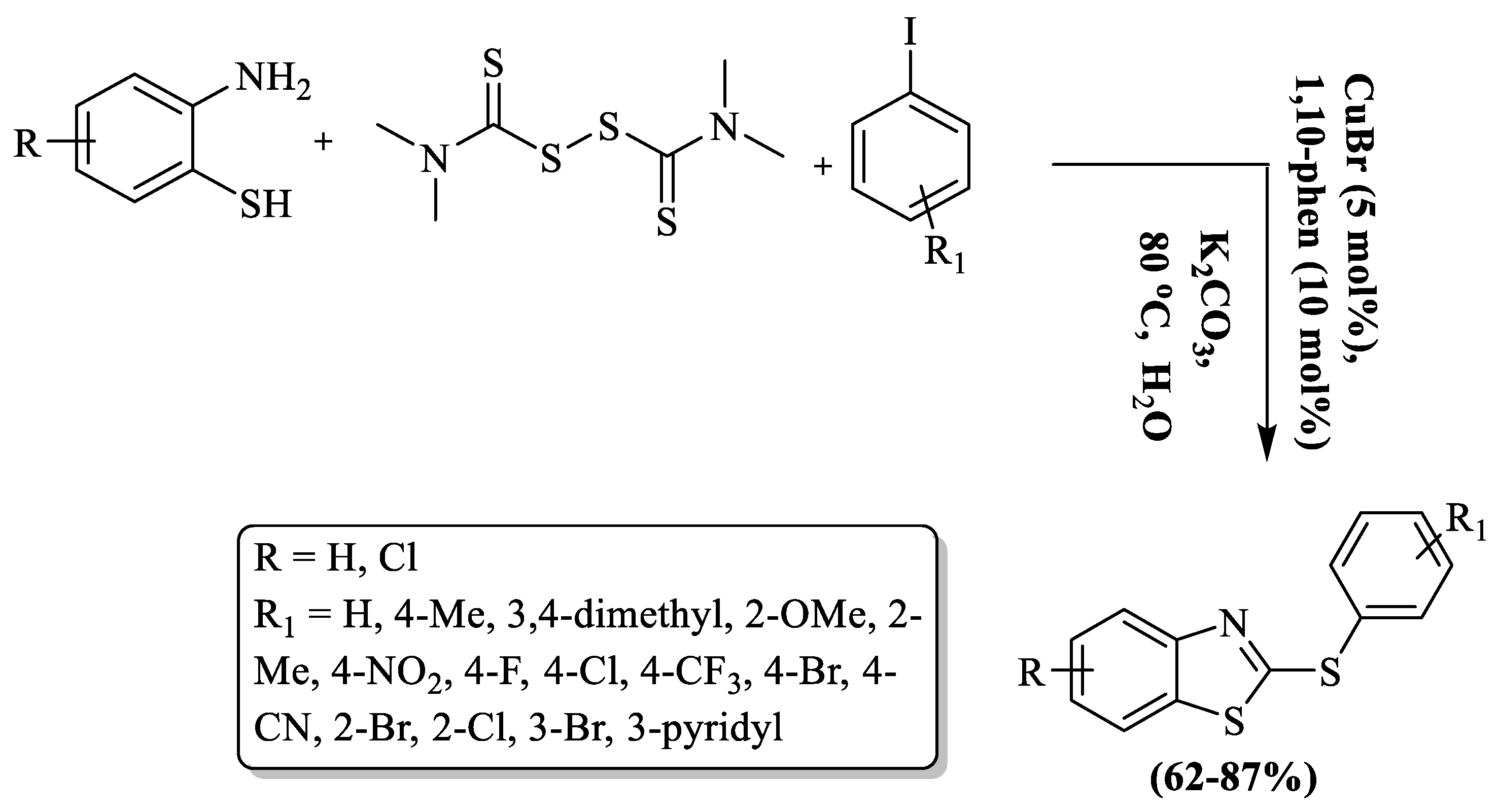
Furthermore, their group (Liu and co-workers) also synthesized 2-arylthio BTs involving S-arylation of 2-ABT using TMTD in an aqueous medium and CuBr catalyst at 80 °C [127]. The mechanism involved in situ generation of 2-thiobenzazoles that underwent C–S bond formation with iodobenzene and produced 2-arylthio BTs. In the process, 2-ABT and TMTD were stirred for 3 h and then CuBr and iodobenzene were added and the reaction continued for 15 h. The protocol involved the use of water solvent, moderate-to-high yields (62–87%), wide substrate scope, and a moderate reaction temperature. The method faced challenges with the use of flash column chromatography and an extended reaction duration. While the authors successfully synthesized 17 derivatives by employing various substituted iodobenzenes, lower yields were observed for strong EWG substituents like CN and NO2. Additionally, the protocol resulted in decreased yields for 2-amino-4-chlorobenzenethiol, and steric hindrance in certain cases also impacted the overall outcome (Scheme 49).
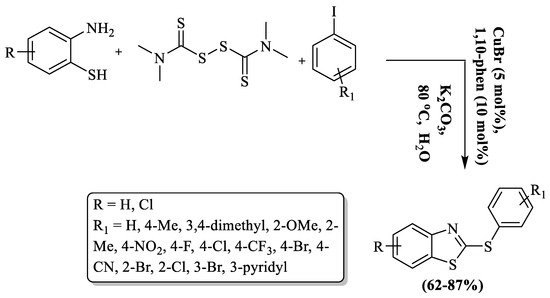
Scheme 49.
Synthesis of 2-substituted BT using 2-ABT and TMTD with iodobenzene [127].
In the year 2018, Liu et al. [128] further developed a synthesis of 2-thiobenzothiazoles involving double condensation of 2-ABT with sodium dimethyl dithiocarbamate in the presence of AlCl3 as a catalyst and DMF as a solvent at 120 °C. The authors used 2-ABT and 2-amino-4-chlorobenzenethiol and obtained 94% and 95% yields, respectively, within 10–30 min. However, the utilization of a toxic solvent, elevated reaction temperature, and dependency on column chromatography were the significant drawbacks of the protocol (Scheme 50).
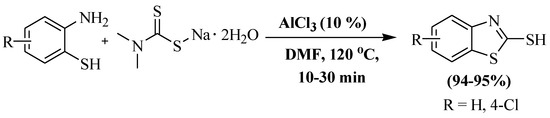
Scheme 50.
2-substituted BT synthesized by utilizing 2-ABT and sodium dimethyldithiocarbamate [128].
3. Discussion
In the extensive exploration of the synthetic strategies for 2-substituted BTs using 2-ABT as a pivotal precursor with aldehydes, ketones, acids, amines, acyl chlorides, etc., we have meticulously examined diverse methodologies, including visible-light-assisted, acid-catalyzed, base-catalyzed, and resin/silica-supported pathways. One noteworthy aspect is the eco-friendly nature of visible-light-assisted synthesis, particularly in employing CdS nanospheres, 3,6-disubstituted-s-tetrazine, and CdSe nanoparticles dispersed in montmorillonite, which showcased a noteworthy advancement with moderate-to-high yields. Similarly, acid-catalyzed strategies involving nitric acid on silica gel, urea nitrate, succinimide-N-sulfonic acid, and H2O2/HCl presented efficient, solvent-free, and green routes for 2-substituted BT synthesis. While these methods exhibit distinct advantages, such as eco-friendliness, high yields, and diverse catalyst systems, it is crucial to acknowledge their certain limitations. For instance, some approaches rely on toxic solvents, conventional heating, and the use of metal catalysts, posing challenges from an environmental perspective. Certain methods, like resin-supported microwave irradiation, may require specialized equipment, potentially limiting their broad application. Scalability challenges, especially with green synthesis approaches involving nanoparticles and ionic liquids, could pose obstacles to large-scale production. Additionally, reaction times and elevated temperatures are the drawbacks in certain methodologies, demanding further exploration for improved efficiency. Despite these limitations, this review serves as a valuable resource for researchers in synthetic organic chemistry. We believe that this discussion will guide future research endeavors, inspiring the development of more sustainable and efficient pathways for synthesizing these essential heterocyclic compounds.
4. Conclusions and Future Perspectives
This paper delivers the recent progress and development in the synthetic approaches of 2-substituted BT analogs under various reaction parameters. In this review, 2-ABT was employed as one of the precursors with aldehydes/ketones/cyanides/esters/acids/acyl halides/olefins/alcohol/amines/nitriles/isocyanate/carbon dioxide/carbon disulfide, etc., to devise more efficient and eco-benign pathways to prepare 2-substituted BTs. Moreover, diverse reaction conditions and techniques, viz. grindstone, ball milling, MW-assisted, sonication, were employed in the company of different catalytic agents, like nanoparticles, acid, metal, base, solid acid catalyst, and other eco-friendly catalysts, using diverse solvents like H2O, DMSO, CH3OH, EtOH, PEG, etc.
With each passing year, science and technology have made enormous development; however, there are plenty of challenges in pharmaceutical sciences that require a vital investigation. A range of methodologies have been formulated to synthesize 2-substituted BTs. Each one of the protocols has its own pros and cons that must be resolved.
In contemplating future perspectives, this manuscript lays the foundation for ongoing exploration and innovation in the synthesis of 2-substituted BTs. The challenges identified, including reaction optimization, scalability, and method-specific limitations, present exciting opportunities for forthcoming research endeavors. These challenges are opportunities for future studies. The scientific community could work on creating new catalysts, using eco-friendly liquids, and developing methods that are safe for the environment. Exploring what these compounds can do in medicine and various industries is also a promising direction. Embracing new technologies like smart machines and clever computer programs can make the whole process even better. To sum up, the present work not only gathers information about the syntheses of these compounds but also acts as a starting point for more sustainable and effective ways in the future.
Author Contributions
Conceptualization, A.S. and S.A.; validation, S.T., A.S. and S.A.; data curation, S.T., A.S. and S.A.; writing—original draft preparation, S.T., A.S. and S.A.; writing—review and editing, S.T.; visualization, S.A.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Department of Chemistry for providing the necessary facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balaban, A.T.; Oniciu, D.C.; Katritzky, A.R. Aromaticity as a cornerstone of heterocyclic chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef]
- Arora, P.; Arora, V.; Lamba, H.; Wadhwa, D. Importance of heterocyclic chemistry: A review. Int. J. Pharm. Sci. Res. 2012, 3, 2947. [Google Scholar]
- Soni, S.; Sahiba, N.; Teli, S.; Teli, P.; Agarwal, L.K.; Agarwal, S. Advances in the synthetic strategies of benzoxazoles using 2-aminophenol as a precursor: An up-to-date review. RSC Adv. 2023, 13, 24093–24111. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Soldatenkov, A.T.; Katritzky, A.R. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Amin, A.; Qadir, T.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on The Medicinal and Industrial Applications of N-Containing Heterocycles. Open Med. Chem. J. 2022, 16, e187410452209010. [Google Scholar] [CrossRef]
- Teli, S.; Teli, P.; Soni, S.; Sahiba, N.; Agarwal, S. Synthetic aspects of 1, 4-and 1, 5-benzodiazepines using o-phenylenediamine: A study of past quinquennial. RSC Adv. 2023, 13, 3694–3714. [Google Scholar] [CrossRef]
- Channapur, M.B. Synthetic and Biological Studies on Selected Heterocyclic Systems; Goa University: Goa, India, 2019. [Google Scholar]
- Singh, M. Design, Synthesis and Biological Evaluation of Some Novel Benzothiazole Derivatives; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mustafa, B.S.I. Synthesis and Evaluation of the Antimicrobial Activity of Newly Synthesized Benzothiazole Derivatives; University of Petra: Amman, Jordan, 2015. [Google Scholar]
- Ambhaikar, N.B. Thiazoles and Benzothiazoles. Heterocyclic Chemistry in Drug Discovery; Li, J.K., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 283–322. [Google Scholar]
- Botha, M. Synthesis, Characterization and Biological Evaluation of Benzo [d] Isothiazole Ring Systems, Fused to Nitrogen Heterocycles. Master’s Thesis, University of Hertfordshire, Hertfordshire, UK, 2014. [Google Scholar]
- Bhat, M.; Belagali, S.L. Structural activity relationship and importance of benzothiazole derivatives in medicinal chemistry: A comprehensive review. Mini-Rev. Org. Chem. 2020, 17, 323–350. [Google Scholar] [CrossRef]
- Liao, C.; Kim, U.-J.; Kannan, K. A review of environmental occurrence, fate, exposure, and toxicity of benzothiazoles. Environ. Sci. Technol. 2018, 52, 5007–5026. [Google Scholar] [CrossRef] [PubMed]
- Day, J.J.; Yang, Z.; Chen, W.; Pacheco, A.; Xian, M. Benzothiazole sulfinate: A water-soluble and slow-releasing sulfur dioxide donor. ACS Chem. Biol. 2016, 11, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-W.; Liu, J.-Q.; Chen, Y.-A.; Chao, C.-M.; Liu, K.-M.; Chen, C.-L.; Lin, T.-C.; Hung, C.-H.; Chou, Y.-L.; Lin, T.-C. Harnessing excited-state intramolecular proton-transfer reaction via a series of amino-type hydrogen-bonding molecules. J. Phys. Chem. Lett. 2015, 6, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzym. Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef] [PubMed]
- Stenger-Smith, J.; Chakraborty, I.; Sameera, W.; Mascharak, P.K. Antimicrobial silver (I) complexes derived from aryl-benzothiazoles as turn-on sensors: Syntheses, properties and density functional studies. Inorg. Chim. Acta 2018, 471, 326–335. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.K.; Gangwar, M.; Nath, G.; Singh, S.K. Design, synthesis and mode of action of novel 2-(4-aminophenyl) benzothiazole derivatives bearing semicarbazone and thiosemicarbazone moiety as potent antimicrobial agents. Med. Chem. Res. 2016, 25, 263–282. [Google Scholar] [CrossRef]
- Fedorchenko, T.; Lipunova, G.; Shchepochkin, A.; Valova, M.; Tsmokalyuk, A.; Slepukhin, P.; Chupakhin, O. Synthesis and Spectral, Electrochemical, and Antioxidant Properties of 2-(5-Aryl-6-R-3-phenyl-5, 6-dihydro-4 H-1, 2, 4, 5-tetrazin-1-yl)-1, 3-benzothiazoles. Russ. J. Org. Chem. 2020, 56, 38–48. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Agarwal, S.; Gandhi, D. Benzothiazole: A Versatile Synthetic Auxillary for Antiepileptic Drugs. Chem. Biol. Interface 2018, 8, 135. [Google Scholar]
- Abdellatif, K.R.; Belal, A.; El-Saadi, M.T.; Amin, N.H.; Said, E.G.; Hemeda, L.R. Design, synthesis, molecular docking and antiproliferative activity of some novel benzothiazole derivatives targeting EGFR/HER2 and TS. Bioorg. Chem. 2020, 101, 103976. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Salado, I.G.; Sanz-San Cristobal, M.; Gil, C.; Perez-Castillo, A.; Martínez, A.; Perez, D.I. Biological and pharmacological characterization of benzothiazole-based CK-1δ inhibitors in models of Parkinson’s disease. ACS Omega 2017, 2, 5215–5220. [Google Scholar] [CrossRef] [PubMed]
- Van Zandt, M.C.; Jones, M.L.; Gunn, D.E.; Geraci, L.S.; Jones, J.H.; Sawicki, D.R.; Sredy, J.; Jacot, J.L.; DiCioccio, A.T.; Petrova, T. Discovery of 3-[(4, 5, 7-trifluorobenzothiazol-2-yl) methyl] indole-N-acetic acid (lidorestat) and congeners as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. J. Med. Chem. 2005, 48, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Inoue, H.; Kim, H.-S.; Suzuki, M.; Shishido, T.; Wataya, Y.; Ihara, M. Rhodacyanine dyes as antimalarials. 1. Preliminary evaluation of their activity and toxicity. J. Med. Chem. 2002, 45, 995–998. [Google Scholar] [CrossRef]
- Ghonim, A.E.; Ligresti, A.; Rabbito, A.; Mahmoud, A.M.; Di Marzo, V.; Osman, N.A.; Abadi, A.H. Structure-activity relationships of thiazole and benzothiazole derivatives as selective cannabinoid CB2 agonists with in vivo anti-inflammatory properties. Eur. J. Med. Chem. 2019, 180, 154–170. [Google Scholar] [CrossRef]
- Bilel, M.; Mehdi, B.; Elhadi, S.M.; Lyamine, M.; Ali, B.; Hocine, M.; Aissa, C.; Sofiane, B.; Abdelmalek, B. Synthesis, X-ray structure and theoretical study of benzazole thioether and its zinc complex as corrosion inhibitors for steel in acidic medium. Res. Chem. Intermed. 2016, 42, 7447–7470. [Google Scholar] [CrossRef]
- Soriano, E.; Holder, C.; Levitz, A.; Henary, M. Benz [c, d] indolium-containing monomethine cyanine dyes: Synthesis and photophysical properties. Molecules 2015, 21, 23. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Wang, W.-C.; Hsu, C.-P.; Lu, W.-Y.; Chuang, W.-J.; Chiang, M.Y.; Lai, Y.-C.; Chen, H.-Y. The ring-opening polymerization of ε-caprolactone and l-lactide using aluminum complexes bearing benzothiazole ligands as catalysts. Polym. Chem. 2016, 7, 4367–4377. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Zhai, Y.; Chen, C.; Wang, Q.; Han, J.; Zhang, Z.; Liu, F.; Mu, W. Sublethal concentration of benzothiazole adversely affect development, reproduction and longevity of Bradysiaodoriphaga (Diptera: Sciaridae). Phytoparasitica 2016, 44, 115–124. [Google Scholar] [CrossRef]
- Luongo, G.; Avagyan, R.; Hongyu, R.; Östman, C. The washout effect during laundry on benzothiazole, benzotriazole, quinoline, and their derivatives in clothing textiles. Environ. Sci. Pollut. Res. 2016, 23, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Navizet, I.; Liu, Y.-J.; Ferré, N.; Xiao, H.-Y.; Fang, W.-H.; Lindh, R. Color-tuning mechanism of firefly investigated by multi-configurational perturbation method. J. Am. Chem. Soc. 2010, 132, 706–712. [Google Scholar] [CrossRef]
- Bhagdev, K.; Sarkar, S. In Benzothiazole moiety and its derivatives as antiviral agents. Med. Sci. Forum 2021, 7, 9. [Google Scholar]
- Jena, J. Significance of benzothiazole moiety in the field of cancer. Int. J. Pharm. Pharm. Sci 2014, 6, 16–22. [Google Scholar]
- Amir, M.; Javed, S.A.; Zaheen Hassan, M. Synthesis and antimicrobial activity of pyrazolinones and pyrazoles having benzothiazole moiety. Med. Chem. Res. 2012, 21, 1261–1270. [Google Scholar] [CrossRef]
- Gordon, D.M. Ethoxzolamide: A new carbonic anhydrase inhibitor. Am. J. Ophthalmol. 1958, 46, 41–44. [Google Scholar] [CrossRef]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012, 4. [Google Scholar] [CrossRef]
- Chrienova, Z.; Rysanek, D.; Novak, J.; Vasicova, P.; Oleksak, P.; Andrys, R.; Skarka, A.; Dumanovic, J.; Milovanovic, Z.; Jacevic, V. Frentizole derivatives with mTOR inhibiting and senomorphic properties. Biomed. Pharmacother. 2023, 167, 115600. [Google Scholar] [CrossRef] [PubMed]
- Cavalluzzi, M.M.; Budriesi, R.; De Salvia, M.A.; Quintieri, L.; Piarulli, M.; Milani, G.; Gualdani, R.; Micucci, M.; Corazza, I.; Rosato, A. Lubeluzole: From anti-ischemic drug to preclinical antidiarrheal studies. Pharmacol. Rep. 2021, 73, 172–184. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Naali, F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J. Org. Chem. 2008, 73, 6835–6837. [Google Scholar] [CrossRef]
- Panda, S.S.; Ibrahim, M.A.; Oliferenko, A.A.; Asiri, A.M.; Katritzky, A.R. Catalyst-free facile synthesis of 2-substituted benzothiazoles. Green Chem. 2013, 15, 2709–2712. [Google Scholar] [CrossRef]
- Senapak, W.; Saeeng, R.; Jaratjaroonphong, J.; Sirion, U. Brönsted acid-surfactant-combined ionic liquid catalyzed green synthesis of 2-alkyl and 2-arylbenzothiazoles in water: Reusable catalyst and metal-free conditions. Mol. Catal. 2018, 458, 97–105. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, W.; Liu, Y.; Guan, J.; Yan, J.; Yuan, J.-J.; Tao, D.-J.; Song, Z. Oxidative NHC catalysis for base-free synthesis of benzoxazinones and benzoazoles by thermal activated NHCs precursor ionic liquid catalyst using air as oxidant. Mol. Catal. 2020, 492, 111013. [Google Scholar] [CrossRef]
- Ghafuri, H.; Esmaili, E.; Talebi, M. Fe3O4@ SiO2/collagen: An efficient magnetic nanocatalyst for the synthesis of benzimidazole and benzothiazole derivatives. Comptes Rendus Chim. 2016, 19, 942–950. [Google Scholar] [CrossRef]
- Kommula, D.; Madugula, S.R.M. Synthesis of benzimidazoles/benzothiazoles by using recyclable, magnetically separable nano-Fe2O3 in aqueous medium. J. Iran. Chem. Soc. 2017, 14, 1665–1671. [Google Scholar] [CrossRef]
- Goswami, M.; Dutta, M.M.; Phukan, P. Sulfonic-acid-functionalized activated carbon made from tea leaves as green catalyst for synthesis of 2-substituted benzimidazole and benzothiazole. Res. Chem. Intermed. 2018, 44, 1597–1615. [Google Scholar] [CrossRef]
- Gandhi, D.; Agarwal, S. Urea nitrate catalyzed synthesis of 2-arylbenzothiazoles using the grindstone technique. Heterocycl. Commun. 2018, 24, 307–310. [Google Scholar] [CrossRef]
- Kaur, G.; Moudgil, R.; Shamim, M.; Gupta, V.K.; Banerjee, B. Camphor sulfonic acid catalyzed a simple, facile, and general method for the synthesis of 2-arylbenzothiazoles, 2-arylbenzimidazoles, and 3 H-spiro [benzo [d] thiazole-2, 3′-indolin]-2′-ones at room temperature. Synth. Commun. 2021, 51, 1100–1120. [Google Scholar] [CrossRef]
- Ye, L.-m.; Chen, J.; Mao, P.; Mao, Z.-f.; Zhang, X.-j.; Yan, M. Visible-light-promoted synthesis of benzothiazoles from 2-aminothiophenols and aldehydes. Tetrahedron Lett. 2017, 58, 874–876. [Google Scholar] [CrossRef]
- Sirgamalla, R.; Kommakula, A.; Konduru, S.; Ponakanti, R.; Devaram, J.; Boda, S. Cupper-catalyzed an efficient synthesis, characterization of 2-substituted benzoxazoles, 2-substituted benzothiazoles derivatives and their anti-fungal activity. Chem. Data Collect. 2020, 27, 100362. [Google Scholar] [CrossRef]
- Maphupha, M.; Juma, W.P.; de Koning, C.B.; Brady, D. A modern and practical laccase-catalysed route suitable for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. RSC Adv. 2018, 8, 39496–39510. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; He, Y.; Zhang, X.; Wang, J. Ru (III)-catalyzed oxidative reaction in ionic liquid: An efficient and practical route to 2-substituted benzothiazoles and their hybrids with pyrimidine nucleoside. Tetrahedron Lett. 2010, 51, 3493–3496. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, M.; Kant, R.; Gupta, V.K. Formation of a nanorod shaped ionogel and its high catalytic activity for one-pot synthesis of benzothiazoles. New J. Chem. 2015, 39, 5116–5120. [Google Scholar] [CrossRef]
- Sayyahi, S.; Shabani, S.; Ghasemi, S.; Azin, A.; Hasani, S.M. Magnetic ionic liquid [bmim][FeCl4] as an efficient catalyst for the synthesis of 2-aryl benzimidazoles and 2-aryl benzothiazoles derivatives. Orient. J. Chem. 2015, 31, 1773. [Google Scholar] [CrossRef]
- Hang, A.-H.T.; Nguyen, T.-L.H.; Chau, D.-K.N.; Tran, P.H. Phosphonium acidic ionic liquid: An efficient and recyclable homogeneous catalyst for the synthesis of 2-arylbenzoxazoles, 2-arylbenzimidazoles, and 2-arylbenzothiazoles. RSC Adv. 2018, 8, 11834–11842. [Google Scholar]
- Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S. Synthesis and characterization of Cu (II) containing nanosilica triazine dendrimer: A recyclable nanocomposite material for the synthesis of benzimidazoles, benzothiazoles, bis-benzimidazoles and bis-benzothiazoles. J. Mol. Catal. A Chem. 2013, 379, 243–254. [Google Scholar] [CrossRef]
- Shelkar, R.; Sarode, S.; Nagarkar, J. Nano ceria catalyzed synthesis of substituted benzimidazole, benzothiazole, and benzoxazole in aqueous media. Tetrahedron Lett. 2013, 54, 6986–6990. [Google Scholar] [CrossRef]
- Banerjee, S.; Payra, S.; Saha, A.; Sereda, G. ZnO nanoparticles: A green efficient catalyst for the room temperature synthesis of biologically active 2-aryl-1, 3-benzothiazole and 1, 3-benzoxazole derivatives. Tetrahedron Lett. 2014, 55, 5515–5520. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Naali, F. TiO2 nanoparticles catalysed synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles using hydrogen peroxide under ambient light. J. Exp. Nanosci. 2016, 11, 148–160. [Google Scholar] [CrossRef]
- Dighore, N.R.; Anandgaonker, P.; Gaikwad, S.T.; Rajbhoj, A.S. Green synthesis of 2-aryl benzothiazole heterogenous catalyzed by MoO3 nanorods. Green Process. Synth. 2016, 5, 139–143. [Google Scholar] [CrossRef]
- Sharma, J.; Bansal, R.; Soni, P.; Singh, S.; Halve, A. One Pot synthesis of 2-substituted benzothiazoles catalyzed by Bi2O3 nanoparticles. Asian J. Nanosci. Mater. 2018, 1, 135–142. [Google Scholar]
- Pesyan, N.N.; Batmani, H.; Havasi, F. Copper supported on functionalized MCM-41 as a novel and a powerful heterogeneous nanocatalyst for the synthesis of benzothiazoles. Polyhedron 2019, 158, 248–254. [Google Scholar] [CrossRef]
- Hassanloie, N.; NorooziPesyan, N.; Sheykhaghaei, G.; Aalinejad, M.; OjaghiAghbash, K.; Alamgholiloo, H. Preparation of Fe3O4@ SiO2@ Cu-MoO3 core–shell nanostructures: Synergistics effects of copper and molybdenum for catalytic enhancement. J. Porous Mater. 2023, 30, 859–869. [Google Scholar] [CrossRef]
- Cai, Y.; Yuan, H.; Gao, Q.; Wu, L.; Xue, L.; Feng, N.; Sun, Y. Palladium (II) complex supported on magnetic nanoparticles modified with phenanthroline: A highly active reusable nanocatalyst for the synthesis of benzoxazoles, benzothiazoles and cyanation of aryl halides. Catal. Lett. 2023, 153, 460–476. [Google Scholar] [CrossRef]
- Das, S.; Samanta, S.; Maji, S.K.; Samanta, P.K.; Dutta, A.K.; Srivastava, D.N.; Adhikary, B.; Biswas, P. Visible-light-driven synthesis of 2-substituted benzothiazoles using CdS nanosphere as heterogenous recyclable catalyst. Tetrahedron Lett. 2013, 54, 1090–1096. [Google Scholar] [CrossRef]
- Samanta, S.; Das, S.; Biswas, P. Photocatalysis by 3, 6-disubstituted-s-tetrazine: Visible-light driven metal-free green synthesis of 2-substituted benzimidazole and benzothiazole. J. Org. Chem. 2013, 78, 11184–11193. [Google Scholar] [CrossRef]
- Wade, A.R.; Pawar, H.R.; Biware, M.V.; Chikate, R.C. Synergism in semiconducting nanocomposites: Visible light photocatalysis towards the formation of C–S and C–N bonds. Green Chem. 2015, 17, 3879–3888. [Google Scholar] [CrossRef]
- Le, H.A.N.; Nguyen, L.H.; Nguyen, Q.N.B.; Nguyen, H.T.; Nguyen, K.Q.; Tran, P.H. Straightforward synthesis of benzoxazoles and benzothiazoles via photocatalytic radical cyclization of 2-substituted anilines with aldehydes. Catal. Commun. 2020, 145, 106120. [Google Scholar] [CrossRef]
- Kumar, P.; Bhatia, R.; Kumar, D.; Kamboj, R.C.; Kumar, S.; Kamal, R.; Kumar, R. An economic, simple and convenient synthesis of 2-aryl/heteroaryl/styryl/alkylbenzothiazoles using SiO2–HNO3. Res. Chem. Intermed. 2015, 41, 4283–4292. [Google Scholar] [CrossRef]
- Kumar, P.; Bhatia, R.; Khanna, R.; Dalal, A.; Kumar, D.; Surain, P.; Kamboj, R.C. Synthesis of some benzothiazoles by developing a new protocol using urea nitrate as a catalyst and their antimicrobial activities. J. Sulfur Chem. 2017, 38, 585–596. [Google Scholar] [CrossRef]
- Shaikh, K.A.; Chaudhar, U.N.; Ningdale, V.B. A facile and rapid access towards the synthesis of 2-aryl benzothiazoles using succinimide-N-sulphonic acid: A Reusable catalyst. Can. Chem. Trans. 2016, 4, 133–142. [Google Scholar]
- Guo, H.Y.; Li, J.C.; Le Shang, Y. A simple and efficient synthesis of 2-substituted benzothiazoles catalyzed by H2O2/HCl. Chin. Chem. Lett. 2009, 20, 1408–1410. [Google Scholar] [CrossRef]
- Sahu, P.K. A green approach to the synthesis of a nano catalyst and the role of basicity, calcination, catalytic activity and aging in the green synthesis of 2-aryl bezimidazoles, benzothiazoles and benzoxazoles. RSC Adv. 2017, 7, 42000–42012. [Google Scholar] [CrossRef]
- Chhabra, M.; Sinha, S.; Banerjee, S.; Paira, P. An efficient green synthesis of 2-arylbenzothiazole analogues as potent antibacterial and anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Waengdongbung, W.; Hahnvajanawong, V.; Theramongkol, P. A Simple and Efficient Route for Synthesis of 2-alkylbenzothiazoles. Orient. J. Chem 2016, 32, 941–945. [Google Scholar] [CrossRef][Green Version]
- Mohammadi, M.; Bardajee, G.R.; Pesyan, N.N. A novel method for the synthesis of benzothiazole heterocycles catalyzed by a copper—DiAmSar complex loaded on SBA-15 in aqueous media. RSC Adv. 2014, 4, 62888–62894. [Google Scholar] [CrossRef]
- Samanta, P.K.; Biswas, R.; Das, T.; Nandi, M.; Adhikary, B.; Richards, R.M.; Biswas, P. Mesoporous silica supported samarium as recyclable heterogeneous catalyst for synthesis of 5-substituted tetrazole and 2-substituted benzothiazole. J. Porous Mater. 2019, 26, 145–155. [Google Scholar] [CrossRef]
- Sakiyama, R.; Aoyama, T.; Akazawa, H.; Kikuchi, N.; Omura, K.; Ohsaki, A.; Yasukawa, K.; Iida, T.; Kodomari, M. Solvent-free synthesis of 2-alkylbenzothiazoles and bile acid derivatives containing benzothiazole ring by using active carbon/silica gel and microwave. J. Oleo Sci. 2018, 67, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, C.; Siddiki, S.H.; Shimizu, K.-i. Acceptorless dehydrogenative synthesis of benzothiazoles and benzimidazoles from alcohols or aldehydes by heterogeneous Pt catalysts under neutral conditions. Tetrahedron Lett. 2015, 56, 4885–4888. [Google Scholar] [CrossRef]
- Digwal, C.S.; Yadav, U.; Sakla, A.P.; Ramya, P.S.; Aaghaz, S.; Kamal, A. VOSO4 catalyzed highly efficient synthesis of benzimidazoles, benzothiazoles, and quinoxalines. Tetrahedron Lett. 2016, 57, 4012–4016. [Google Scholar] [CrossRef]
- Katla, R.; Chowrasia, R.; Manjari, P.S.; Domingues, N.L.C. An efficient aqueous phase synthesis of benzimidazoles/benzothiazoles in the presence of β-cyclodextrin. RSC Adv. 2015, 5, 41716–41720. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Soliman, A.M. Epichlorohydrin cross-linked β-cyclodextrin: An environmental method for the synthesis of 2-arylbenzothiazoles derivatives in water. J. Sulfur Chem. 2016, 37, 70–79. [Google Scholar] [CrossRef]
- Patil, M.A.; Ubale, P.A.; Karhale, S.S.; Helavi, V.B. Lemon juice: An environmentally benign catalyst for synthesis of benzothiazoles and benzoxazole derivatives in aqueous medium. Chem. Sin. 2017, 8, 198–205. [Google Scholar]
- Yang, D.; Liu, P.; Zhang, N.; Wei, W.; Yue, M.; You, J.; Wang, H. Mesoporous poly (melamine–formaldehyde): A green and recyclable heterogeneous organocatalyst for the synthesis of benzoxazoles and benzothiazoles using dioxygen as oxidant. ChemCatChem 2014, 6, 3434–3439. [Google Scholar] [CrossRef]
- Lee, A.S.-Y.; Chung, C.-H.; Chang, Y.-T.; Chen, P.-L. L-proline catalyzed condensation reaction of aldehyde or carboxylic acid with 2-aminothiophenol under solvent-free and microwave irradiation. J. Appl. Sci. Eng. 2012, 15, 311–315. [Google Scholar]
- Zhang, X.-Z.; Zhou, W.-J.; Yang, M.; Wang, J.-X.; Bai, L. Microwave-assisted synthesis of benzothiazole derivatives using glycerol as green solvent. J. Chem. Res. 2012, 36, 489–491. [Google Scholar] [CrossRef]
- Praveen, C.; Nandakumar, A.; Dheenkumar, P.; Muralidharan, D.; Perumal, P. Microwave-assisted one-pot synthesis of benzothiazole and benzoxazole libraries as analgesic agents. J. Chem. Sci. 2012, 124, 609–624. [Google Scholar] [CrossRef]
- Sakram, B.; Rambabu, S.; Ashok, K.; Sonyanaik, B.; Ravi, D. Microwave assisted aqueous phase synthesis of benzothiazoles and benzimidazoles in the presence ofAg2O. Russ. J. Gen. Chem. 2016, 86, 2737–2743. [Google Scholar] [CrossRef]
- Chen, G.-F.; Jia, H.-M.; Zhang, L.-Y.; Chen, B.-H.; Li, J.-T. An efficient synthesis of 2-substituted benzothiazoles in the presence of FeCl3/Montmorillonite K-10 under ultrasound irradiation. Ultrason. Sonochem. 2013, 20, 627–632. [Google Scholar] [CrossRef]
- Pardeshi, S.D.; Sonar, J.P.; Pawar, S.S.; Dekhane, D.; Gupta, S.; Zine, A.M.; Thore, S.N. Sonicated assisted synthesis of benzimidazoles, benzoxazoles and benzothiazoles in aqueous media. J. Chil. Chem. Soc. 2014, 59, 2335–2340. [Google Scholar] [CrossRef][Green Version]
- Sharma, H.; Singh, N.; Jang, D.O. A ball-milling strategy for the synthesis of benzothiazole, benzimidazole and benzoxazole derivatives under solvent-free conditions. Green Chem. 2014, 16, 4922–4930. [Google Scholar] [CrossRef]
- Banerjee, M.; Chatterjee, A.; Kumar, V.; Bhutia, Z.T.; Khandare, D.G.; Majik, M.S.; Roy, B.G. A simple and efficient mechanochemical route for the synthesis of 2-aryl benzothiazoles and substituted benzimidazoles. RSC Adv. 2014, 4, 39606–39611. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Y.; Song, J.; Lu, X.; Geng, Y.; Zhang, J.; Xie, J.; Zeng, Q. On-surface synthesis of self-assembled monolayers of benzothiazole derivatives studied by STM and XPS. Langmuir 2017, 33, 4216–4223. [Google Scholar] [CrossRef]
- Elderfield, R.C.; McClenachan, E.C. Pyrolysis of the products of the reaction of o-aminobenzenethiols with ketones1. J. Am. Chem. Soc. 1960, 82, 1982–1988. [Google Scholar] [CrossRef]
- Liao, Y.; Qi, H.; Chen, S.; Jiang, P.; Zhou, W.; Deng, G.-J. Efficient 2-aryl benzothiazole formation from aryl ketones and 2-aminobenzenethiols under metal-free conditions. Org. Lett. 2012, 14, 6004–6007. [Google Scholar] [CrossRef]
- Mali, J.K.; Mali, D.A.; Telvekar, V.N. Copper-II mediated tandem reaction between aromatic ketones and 2-aminobenzenethiol for the synthesis of 2-aroylbenzothiazoles. Tetrahedron Lett. 2016, 57, 2324–2326. [Google Scholar] [CrossRef]
- Mayo, M.S.; Yu, X.; Zhou, X.; Feng, X.; Yamamoto, Y.; Bao, M. Convenient synthesis of benzothiazoles and benzimidazoles through Brønsted acid catalyzed cyclization of 2-amino thiophenols/anilines with β-diketones. Org. Lett. 2014, 16, 764–767. [Google Scholar] [CrossRef]
- Khoobi, M.; Ramazani, A.; Foroumadi, A.; Hamadi, H.; Hojjati, Z.; Shafiee, A. Efficient microwave-assisted synthesis of 3-benzothiazolo and 3-benzothiazolino coumarin derivatives catalyzed by heteropoly acids. J. Iran. Chem. Soc. 2011, 8, 1036–1042. [Google Scholar] [CrossRef]
- Gupta, S.D.; Singh, H.P.; Moorthy, N. Iodine-catalyzed, one-pot, solid-phase synthesis of benzothiazole derivatives. Synth. Commun. 2007, 37, 4327–4329. [Google Scholar] [CrossRef]
- Rauf, A.; Gangal, S.; Sharma, S. Solvent-free synthesis of 2-alkyl and 2-alkenylbenzothiazoles from fatty acids under microwave irradiation. Indian. J. Chem. 2008, 47B, 601–605. [Google Scholar] [CrossRef]
- Monga, A.; Bagchi, S.; Soni, R.K.; Sharma, A. Synthesis of benzothiazoles via photooxidative decarboxylation of α-keto acids. Adv. Synth. Catal. 2020, 362, 2232–2237. [Google Scholar] [CrossRef]
- Kumar, K.R.; Satyanarayana, P.; Srinivasa Reddy, B. NaHSO4-SiO2-Promoted solvent-free synthesis of benzoxazoles, benzimidazoles, and benzothiazole derivatives. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Dar, A.A.; Shadab, M.; Khan, S.; Ali, N.; Khan, A.T. One-pot synthesis and evaluation of antileishmanial activities of functionalized S-alkyl/aryl benzothiazole-2-carbothioate scaffold. J. Org. Chem. 2016, 81, 3149–3160. [Google Scholar] [CrossRef]
- Luo, B.; Li, D.; Zhang, A.-L.; Gao, J.-M. Synthesis, antifungal activities and molecular docking studies of benzoxazole and benzothiazole derivatives. Molecules 2018, 23, 2457. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Sardarian, A.R. KF-Al2O3 as a base heterogeneous catalyst for the synthesis of 2-substituted benzoxazoles and benzothiazoles under mild reaction conditions at room temperature. Polycycl. Aromat. Compd. 2020, 40, 990–997. [Google Scholar] [CrossRef]
- Hudwekar, A.D.; Verma, P.K.; Kour, J.; Balgotra, S.; Sawant, S.D. Transition metal-free oxidative coupling of primary amines in polyethylene glycol at room temperature: Synthesis of imines, azobenzenes, benzothiazoles, and disulfides. Eur. J. Org. Chem. 2019, 2019, 1242–1250. [Google Scholar] [CrossRef]
- Naresh, G.; Kant, R.; and Narender, T. Molecular iodine promoted divergent synthesis of benzimidazoles, benzothiazoles, and 2-benzyl-3-phenyl-3, 4-dihydro-2 H-benzo [e][1, 2, 4] thiadiazines. J. Org. Chem. 2014, 79, 3821–3829. [Google Scholar] [CrossRef]
- Elsanousi, A.; Riadi, Y.; Ouerghi, O.; Geesi, M.H. Synthesis, Characterization of TiO2-based nanostructure as efficient catalyst for the synthesis of new heterocycles benzothiazole-linked pyrrolidin-2-one: Catalytic performances are particle’s size dependent. Polycycl. Aromat. Compd. 2023, 43, 2404–2417. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, H.; Wu, W.; Zeng, W.; Wu, X. Copper-catalyzed synthesis of substituted benzothiazoles via condensation of 2-aminobenzenethiols with nitriles. Organ. Lett. 2013, 15, 1598–1601. [Google Scholar] [CrossRef]
- Ghosh, A.; Gattu, R.; Khan, A.T. Synthesis of benzothiazoles via condensation reaction of 2-aminothiophenols and β-oxodithioesters using a combination of PTSA and CuI as catalyst. ChemistrySelect 2018, 3, 13773–13776. [Google Scholar] [CrossRef]
- Srivastava, A.; Shukla, G.; Singh, M.S. p-Toluenesulfonic acid-catalyzed metal-free formal [4+ 1] heteroannulation via NH/OH/SH functionalization: One-pot access to 2-aryl/hetaryl/alkyl benzazole derivatives. Tetrahedron 2017, 73, 879–887. [Google Scholar] [CrossRef]
- Shaikh, A.; Ravi, O.; Ragini, S.P.; Sadhana, N.; Bathula, S.R. Benzimidazoles and benzothiazoles from styrenes and N-vinylimidazole via palladium catalysed oxidative CC and CN bond cleavage. Tetrahedron Lett. 2020, 61, 151356. [Google Scholar] [CrossRef]
- Siddappa, C.; Kambappa, V.; Umashankara, M.; Rangappa, K.S. One-pot approach for the synthesis of 2-aryl benzothiazoles via a two-component coupling of gem-dibromomethylarenes and o-aminothiophenols. Tetrahedron Lett. 2011, 52, 5474–5477. [Google Scholar] [CrossRef]
- Yu, X.; Yin, Q.; Zhang, Z.; Huang, T.; Pu, Z.; Bao, M. Synthesis of 2-substituted benzothiazoles via the Brønsted acid catalyzed cyclization of 2-amino thiophenols with nitriles. Tetrahedron Lett. 2019, 60, 1964–1966. [Google Scholar] [CrossRef]
- Hameed Mahmood, Z.; Riadi, Y.; Hammoodi, H.A.; Alkaim, A.F.; Fakri Mustafa, Y. Magnetic nanoparticles supported copper nanocomposite: A highly active nanocatalyst for synthesis of benzothiazoles and polyhydroquinolines. Polycycl. Aromat. Compd. 2023, 43, 3687–3705. [Google Scholar] [CrossRef]
- Das, K.; Mondal, A.; Srimani, D. Phosphine free Mn-complex catalysed dehydrogenative C–C and C–heteroatom bond formation: A sustainable approach to synthesize quinoxaline, pyrazine, benzothiazole and quinoline derivatives. Chem. Commun. 2018, 54, 10582–10585. [Google Scholar] [CrossRef]
- Wang, D.; Albero, J.; García, H.; Li, Z. Visible-light-induced tandem reaction of o-aminothiophenols and alcohols to benzothiazoles over Fe-based MOFs: Influence of the structure elucidated by transient absorption spectroscopy. J. Catal. 2017, 349, 156–162. [Google Scholar] [CrossRef]
- Karami, N.; Zarnegaryan, A. Fabrication of immobilized molybdenum complex on functionalized graphene oxide as a novel catalyst for the synthesis of benzothiazoles. J. Organomet. Chem. 2023, 992, 122696. [Google Scholar] [CrossRef]
- Kazi, I.; Sekar, G. An efficient synthesis of benzothiazole using tetrabromomethane as a halogen bond donor catalyst. Org. Biomol. Chem. 2019, 17, 9743–9756. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Luo, W.; Gan, Z.; Li, D.; Dai, Z.; Wang, H.; Wang, X.; Yuan, J. Eco-friendly syntheses of 2-substituted benzoxazoles and 2-substituted benzothiazoles from 2-aminophenols, 2-aminothiophenols and DMF derivatives in the presence of imidazolium chloride. Molecules 2019, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, R.; Lin, Y.; Zhou, X. Lanthanide-catalyzed cyclocarbonylation and cyclothiocarbonylation: A facile synthesis of benzannulated 1, 3-diheteroatom five-and six-membered heterocycles. Sci. China Chem. 2014, 57, 1117–1125. [Google Scholar] [CrossRef]
- El-Sharief, A.S.; Ammar, Y.; Zahran, M.; Sabet, H.K. 1, 4-Phenylenediisothiocyanate in the synthesis of bis-(thiourea, benzothiazole, quinazoline, 1, 3-benzoxazine and imidazolidineiminothiones) derivatives. Phosphorus Sulfur Silicon 2004, 179, 267–275. [Google Scholar] [CrossRef]
- Ballabeni, M.; Ballini, R.; Bigi, F.; Maggi, R.; Parrini, M.; Predieri, G.; Sartori, G. Synthesis of symmetrical N, N’-disubstituted thioureas and heterocyclic thiones from amines and CS (2) over a ZnO/Al (2) O (3) composite as heterogeneous and reusable catalyst. J. Org. Chem. 1999, 64, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Politanskaya, L.; Duan, Z.; Bagryanskaya, I.; Eltsov, I.; Tretyakov, E.; Xi, C. Highly efficient synthesis of polyfluorinated 2-mercaptobenzothiazole derivatives. J. Fluor. Chem. 2018, 212, 130–136. [Google Scholar] [CrossRef]
- Gao, X.; Deng, Y.; Lu, C.; Zhang, L.; Wang, X.; Yu, B. Organic base-catalyzed C–S bond construction from CO2: A new route for the synthesis of benzothiazolones. Catalysts 2018, 8, 271. [Google Scholar] [CrossRef]
- Xu, W.; Zeng, M.-T.; Liu, S.-S.; Li, Y.-S.; Dong, Z.-B. Copper catalyzed synthesis of benzoxazoles and benzothiazoles via tandem manner. Tetrahedron Lett. 2017, 58, 4289–4292. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Xu, W.; Zeng, M.-T.; Zhu, H.; Chang, C.-Z.; Dong, Z.-B. An environmentally benign and efficient synthesis of substituted benzothiazole-2-thiols, benzoxazole-2-thiols, and benzimidazoline-2-thiones in water. Green Chem. 2017, 19, 5591–5598. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.-B.; Zhu, H.; Dong, Z.-B. Copper (I)-catalyzed tandem one-pot synthesis of 2-arylthiobenzothiazoles and 2-arylthiobenzoxazoles in water. J. Org. Chem. 2018, 83, 11703–11711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.B.; Dong, Z.B. AlCl3-promoted synthesis of 2-mercapto benzoheterocycles by using sodium dimethyldithiocarbamate as thiocarbonyl surrogate. Eur. J. Org. Chem. 2018, 2018, 5406–5411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).