Molecular Modeling and Potential Ca2+ Channel Blocker Activity of Diphenylmethoxypiperidine Derivatives
Abstract
1. Introduction
2. Materials and Methods
3. Results
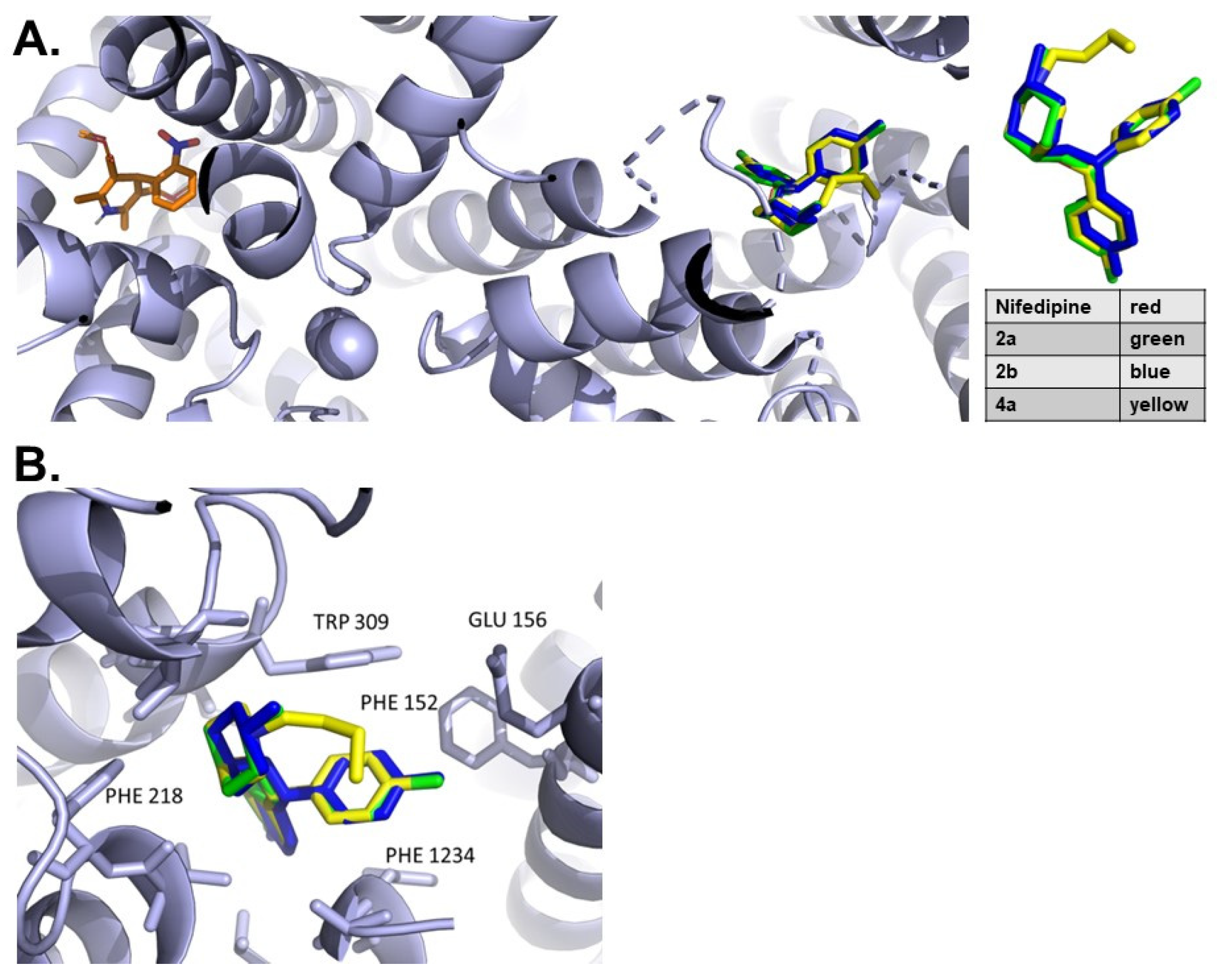
3.1. Molecular Interactions with Cav1.1
3.2. Docking Analysis
3.3. Protein Sequence Alignment of LTCC Alpha Subunits
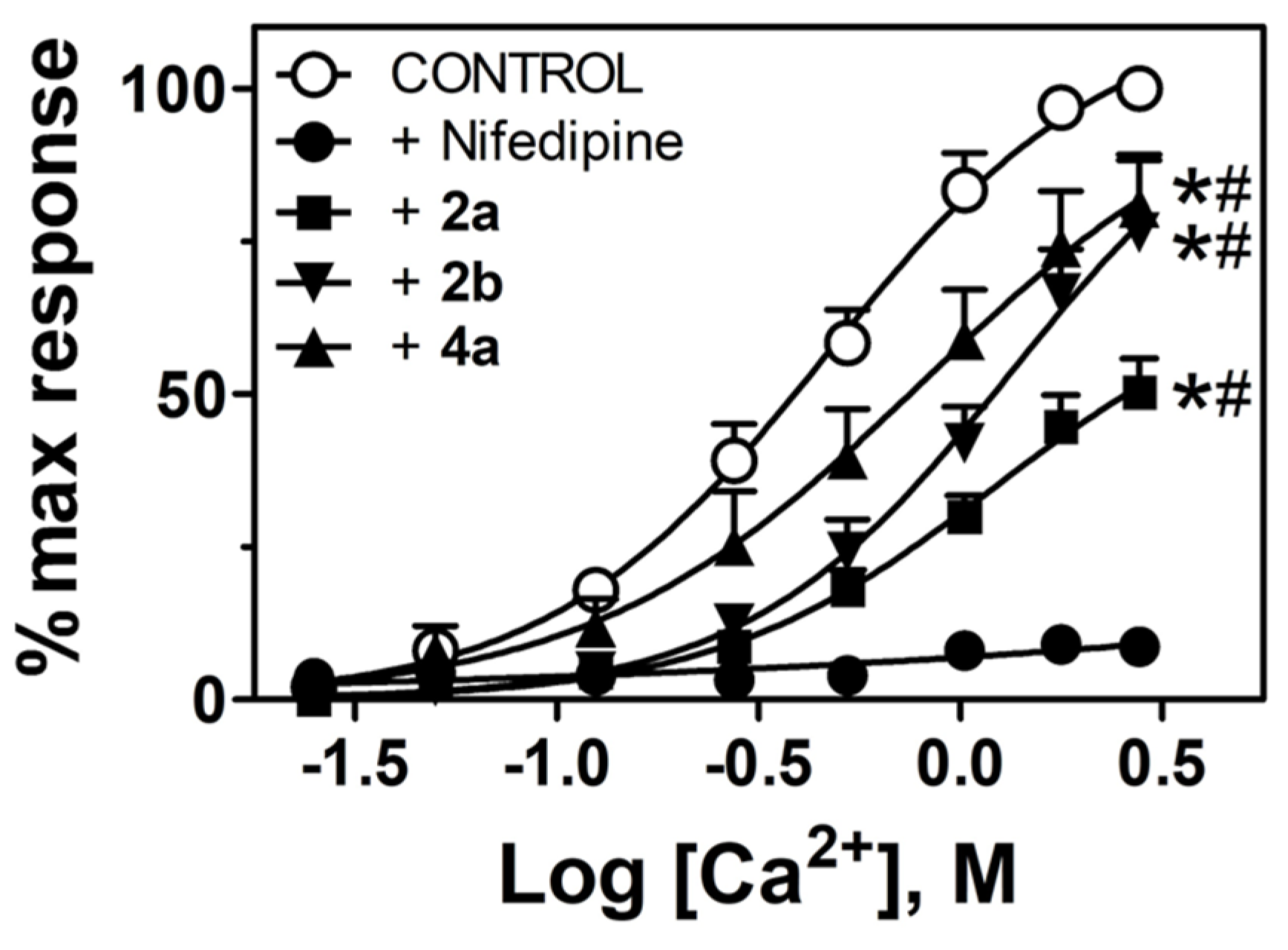
3.4. Diphenylmethoxypiperidine Derivatives Block Ca2+-Dependent Contractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintrón, M.; Navedo, M.F. Calcium channels in vascular smooth muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Richard, S. Vascular effects of calcium channel antagonists: New evidence. Drugs 2005, 65 (Suppl. 2), 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moosmang, S.; Schulla, V.; Welling, A.; Feil, R.; Feil, S.; Wegener, J.W.; Hofmann, F.; Klugbauer, N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003, 22, 6027–6034. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Pulgar, V.M.; Keith Harp, J. Vascular effects of diphenylmethoxypiperidine-derived dopamine uptake inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 2429–2432. [Google Scholar] [CrossRef]
- Tang, L.; Gamal El-Din, T.M.; Payandeh, J.; Martinez, G.Q.; Heard, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 2014, 505, 56–61. [Google Scholar] [CrossRef]
- Tang, L.; Gamal El-Din, T.M.; Lenaeus, M.J.; Zheng, N.; Catterall, W.A. Structural Basis for Diltiazem Block of a Voltage-Gated Ca(2+) Channel. Mol. Pharmacol. 2019, 96, 485–492. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, G.; Wu, J.; Wu, Q.; Gao, S.; Yan, Z.; Lei, J.; Yan, N. Molecular basis for ligand modulation of a mammalian voltage-gated Ca(2+) channel. Cell 2019, 177, 1495–1506.e1412. [Google Scholar] [CrossRef]
- Wu, J.; Yan, Z.; Li, Z.; Yan, C.; Lu, S.; Dong, M.; Yan, N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science 2015, 350, aad2395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.L.; Yeo, M.; Zhang, Q.J.; López-Romero, A.E.; Ding, H.P.; Zhang, X.; Zeng, Q.; Morales-Lázaro, S.L.; Moore, C.; et al. Epithelia-sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317.e316. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Word, J.M.; Lovell, S.C.; Richardson, J.S.; Richardson, D.C. Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 1999, 285, 1735–1747. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol Molecular Viewer (V1.2r3pre). 2002. Available online: http://www.pymol.org (accessed on 3 October 2022).
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2002, 50 (W1), W276–W279. [Google Scholar] [CrossRef]
- Pulgar, V.M.; Yasuda, M.; Gan, L.; Desnick, R.J.; Bonkovsky, H.L. Sex differences in vascular reactivity in mesenteric arteries from a mouse model of acute intermittent porphyria. Mol. Genet. Metab. 2019, 128, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Halpern, W. Mechanical properties of vascular smooth muscle cells in situ. Nature 1976, 260, 617–619. [Google Scholar] [CrossRef]
- Jurkat-Rott, K.; Lehmann-Horn, F. The impact of splice isoforms on voltage-gated calcium channel alpha1 subunits. J. Physiol. 2004, 554 Pt 3, 609–619. [Google Scholar] [CrossRef]
- Ramirez, D.; Caballero, J. Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Striessnig, J.; Ortner, N.J.; Pinggera, A. Pharmacology of L-type calcium channels: Novel drugs for old targets? Curr. Mol. Pharmacol. 2015, 8, 110–122. [Google Scholar] [CrossRef]
- Abd El-Rahman, R.R.; Harraz, O.F.; Brett, S.E.; Anfinogenova, Y.; Mufti, R.E.; Goldman, D.; Welsh, D.G. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: Role in myogenic tone development. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H58–H71. [Google Scholar] [CrossRef] [PubMed]

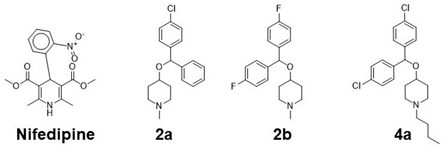 | |||
| Name | SMILES | FW g/mol | Kcal/mol |
| Nifedipine | COC(=O)C1=C(C)NC(=C(C1c1ccccc1[N+](=O)[O-])C(=O)OC)C | 346.3 | −5.8 |
| 2a | CN3CCC(OC(c1ccccc1)c2ccc(Cl)cc2)CC3 | 315.8 | −8.2 |
| 2b | CN3CCC(OC(c1ccc(F)cc1)c2ccc(F)cc2)CC3 | 317.4 | −8.3 |
| 4a | CCCCN3CCC(OC(c1ccc(Cl)cc1)c2ccc(Cl)cc2)CC3 | 392.4 | −8.4 |
| EMAX (%KMAX) | EC50 (mM) | |
|---|---|---|
| Control | 113 ± 2 | 0.48 ± 0.07 |
| 2a | 62 ± 9 * | 1.02 ± 0.11 * |
| 2b | 87 ± 7 * | 1.2 ± 0.07 * |
| 4a | 94 ± 2 * | 0.81 ± 0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulgar, V.M.; Harp, J.; Reeves, T.E. Molecular Modeling and Potential Ca2+ Channel Blocker Activity of Diphenylmethoxypiperidine Derivatives. Chemistry 2023, 5, 713-719. https://doi.org/10.3390/chemistry5020050
Pulgar VM, Harp J, Reeves TE. Molecular Modeling and Potential Ca2+ Channel Blocker Activity of Diphenylmethoxypiperidine Derivatives. Chemistry. 2023; 5(2):713-719. https://doi.org/10.3390/chemistry5020050
Chicago/Turabian StylePulgar, Victor M., Jill Harp, and Tony E. Reeves. 2023. "Molecular Modeling and Potential Ca2+ Channel Blocker Activity of Diphenylmethoxypiperidine Derivatives" Chemistry 5, no. 2: 713-719. https://doi.org/10.3390/chemistry5020050
APA StylePulgar, V. M., Harp, J., & Reeves, T. E. (2023). Molecular Modeling and Potential Ca2+ Channel Blocker Activity of Diphenylmethoxypiperidine Derivatives. Chemistry, 5(2), 713-719. https://doi.org/10.3390/chemistry5020050







