Synthesis and Structural Analysis of a Nitrobenzofurazan Derivative of Dibenzo-18-Crown-6 Ether
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry: Where from? Where to? Chem. Soc. Rev. 2017, 46, 2378–2379. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and kinetic data for macrocycle interaction with cations, anions, and neutral molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.G.; Stavarache, C.; Paun, A.; Popescu, C.C.; Hădade, N.D.; Ionita, P.; Matache, M. A novel profluorescent paramagnetic diaza-crown ether: Synthesis, characterization and alkaline metal-ion complexation. RSC Adv. 2019, 9, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Móczár, I.; Huszthy, P. Optically active crown ether-based fluorescent sensor molecules: A mini-review. Chirality 2018, 2, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I.; Dubonosov, A.D.; Bren, V.A.; Tsukanovb, A.V. Chemosensors with crown ether-based receptors. Arkivoc 2008, iv, 90–102. [Google Scholar] [CrossRef]
- Feigenbaum, W.M.; Michel, R.H.J. Novel polyamides from macrocyclic ethers. Polym. Sci. Part A Polym. Chem. 1971, 9, 817–820. [Google Scholar] [CrossRef]
- Glushko, V.N.; Sadovskaya, N.Y.; Blokhina, L.I.; Zhila, M.Y.; Belus, S.K.; Vashchenkova, E.S.; Shmeleva, I.A. Synthesis of isomeric dinitro and diamino derivatives of polycyclic crown ethers: Dibenzo-18-crown-6 and dibenzo-24-crown-8. Rus. J. Gen. Chem. 2018, 88, 1595–1600. [Google Scholar] [CrossRef]
- Bujor, A.; Matei, I.; Culita, D.C.; Hanganu, A.; Tecuceanu, V.; Ionita, P. Linear and cyclic ethylene-glycols labelled with nitrobenzofurazan motifs. Rev. Roum. Chim. 2022; in press. [Google Scholar]
- Dongyun, W.; Chunhai, Y.; Beibei, Z.; Bolun, Y. Synthesis and characterization of trans-di(nitrobenzo)- and di(aminobenzo)-18-crown-6 derivatives with high selectivity. Synth. Comm. 2018, 48, 329–335. [Google Scholar] [CrossRef]
- Gutowska, N.; Seliger, P.; Romański, J.; Zięba, M.; Adamus, G.; Kowalczuk, M. Mass spectrometry reveals complexing properties of modified pnp-lariat ether containing benzyl derivative of (s)–prolinamine. Molecules 2020, 25, 136. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, S.Y.; Solovieva, D.O.; Zaitsev, I.S. Membrane and films based on novel crown-containing dyes as promising chemosensoring materials. Materials 2010, 3, 5293–5310. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, A.; Bovenkamp, J.W.; Lacroix, B.V.; Bannard, R.A.B.; Buchanan, G.W. Complexes of 18-crown-6 macrocyclic ethers obtained from ethereal solvents. Complexes of potassium and sodium salts with host: Guest ratios of 1:2 and 1:3. Can. J. Chem. 1986, 64, 808–815. [Google Scholar] [CrossRef]
- Lvova, L.; Acciari, E.; Mandoj, F.; Pomarico, G.; Natale, C.D.; Paolesse, R. Crown-porphyrin ligand for optical sensors development. Proceedings 2018, 2, 922. [Google Scholar] [CrossRef]
- Valeur, B. Design principles of fluorescent molecular sensors for cation recognition. Coordin. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Zaleskaya-Hernik, M.; Dobrzycki, Ł.; Karbarz, M.; Romański, J. Fluorescence recognition of anions using a heteroditopic receptor: Homogenous and two-phase sensing. Int. J. Mol. Sci. 2021, 22, 13396. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and fluorescent properties of crown ether functionalized graphene quantum dots for potassium and sodium ions detection. Nanomaterials 2021, 11, 2897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, F.; Dong, S.; Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef] [PubMed]
- Bem, M.; Badea, F.; Draghici, C.; Caproiu, M.T.; Vasilescu, M.; Voicescu, M.; Beteringhe, A.; Caragheorgheopol, A.; Maganu, M.; Constantinescu, T.; et al. Synthesis and fluorescent properties of new derivatives of 4-amino-7-nitrobenzofurazan. Arkivoc 2007, xiii, 87–104. [Google Scholar] [CrossRef]


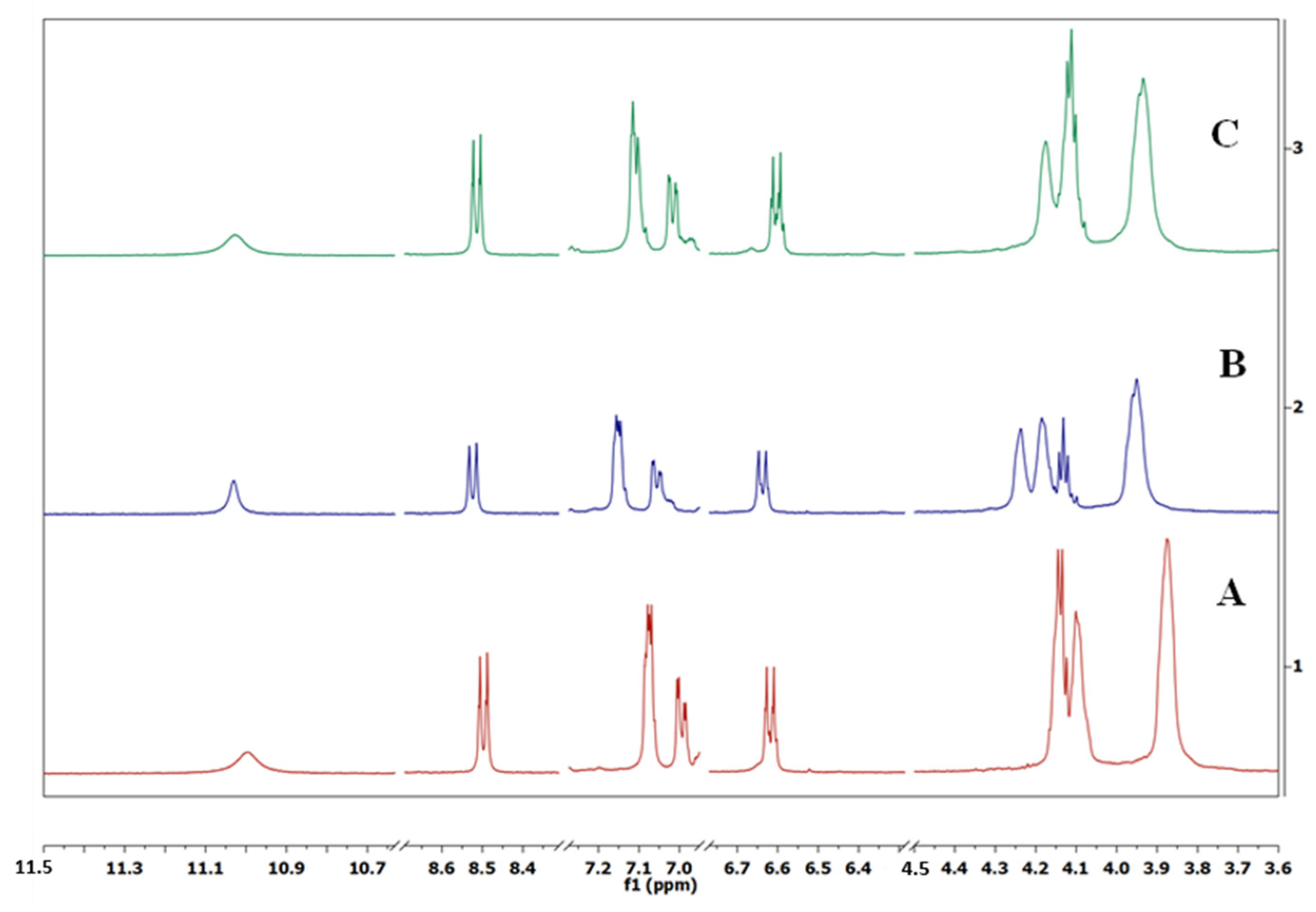
| Compound | Chemical Shifts (δ ppm) | ||||||
|---|---|---|---|---|---|---|---|
| HNH | HAr | HAr | HAr | HAr | Hether | Hether | |
| bis-NBD-DB18-C-6 | 11.00 | 8.50 | 7.08 | 6.99 | 6.62 | 4.17–4.07 | 3.88 |
| bis-NBD-DB18-C-6 + K+ | 11.03 | 8.53 | 7.15 | 7.06 | 6.64 | 4.29–4.11 | 3.95 |
| bis-NBD-DB18-C-6 + Na+ | 11.03 | 8.52 | 7.11 | 7.02 | 6.60 | 4.21–4.09 | 3.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujor, A.; Tecuceanu, V.; Hanganu, A.; Ionita, P. Synthesis and Structural Analysis of a Nitrobenzofurazan Derivative of Dibenzo-18-Crown-6 Ether. Chemistry 2022, 4, 1696-1701. https://doi.org/10.3390/chemistry4040110
Bujor A, Tecuceanu V, Hanganu A, Ionita P. Synthesis and Structural Analysis of a Nitrobenzofurazan Derivative of Dibenzo-18-Crown-6 Ether. Chemistry. 2022; 4(4):1696-1701. https://doi.org/10.3390/chemistry4040110
Chicago/Turabian StyleBujor, Alexandru, Victorita Tecuceanu, Anamaria Hanganu, and Petre Ionita. 2022. "Synthesis and Structural Analysis of a Nitrobenzofurazan Derivative of Dibenzo-18-Crown-6 Ether" Chemistry 4, no. 4: 1696-1701. https://doi.org/10.3390/chemistry4040110
APA StyleBujor, A., Tecuceanu, V., Hanganu, A., & Ionita, P. (2022). Synthesis and Structural Analysis of a Nitrobenzofurazan Derivative of Dibenzo-18-Crown-6 Ether. Chemistry, 4(4), 1696-1701. https://doi.org/10.3390/chemistry4040110







