Coordination Chemistry of Polynitriles, Part XI. Influence of 4,4′-Bipyridine and Solvent on the Crystal and Molecular Structures of Alkaline Earth Pentacyanocyclopentadienides
Abstract
1. Introduction
2. Materials and Methods
- MgCl2∙6H2O (51 mg, 250 µmol and [Ag(PCC)(4,4′-bipy)(H2O)1.25] (235 mg, 556 µmol)
- Product data: 1H-NMR (270 MHz, CD3OD): δ = 8.69 (m, 4H), 7.83 (m, 4H); 13C{1H} (69 MHz, CD3OD): δ = 151.2, 147.4, 123.2 (4,4′-bipy), 114.0, 103.4 (PCC).
- CaCl2 (28 mg, 250 µmol) and [Ag(PCC)(4,4′-bipy)(H2O)1.25] (235 mg, 556 µmol )
- Product data: 1H-NMR (270 MHz, CD3OD): δ = 8.69 (m, 4H), 7.83 (m, 4H); 13C{1H} (69 MHz, CD3OD): δ = 151.2, 147.4, 123.2 (4,4′-bipy), 114.0, 103.4 (PCC).
- SrCl2∙6H2O (49 mg, 185 µmol) and [Ag(PCC)(4,4′-bipy)(H2O)1.25] (177 mg, 371 µmol)
- BaCl2∙2H2O (61 mg, 250 µmol) and [Ag(PCC)(4,4′-bipy)(H2O)1.25] (235 mg, 556 µmol)
- Product data: 1H-NMR (270 MHz, CD3OD): δ = 8.69 (m, 4H), 7.83 (m, 4H); 13C{1H} (69 MHz, CD3OD): δ = 151.2, 147.4, 123.2 (4,4′-bipy), 113.9, 103.4 (PCC).
- [Mg(PCC)2(MeOH)(H2O)6]: (37 mg, 67 µmol) and 4,4′-bipy (14 mg, 90 µmol)
- Product Data: MS (FAB+): m/z = 157.0 (C10H8N2); (FAB-): m/z = 190.3 (C10N5)
- [Ca(PCC)2(H2O)6]: (16 mg, 30 µmol) and 4-4′-bipy (12 mg, 77 µmol)
- Product Data: MS (FAB+): m/z = 157.0 (C10H8N2); (FAB-): m/z = 190.3 (C10N5)
- [Sr(PCC)2(MeOH)0.5(H2O)2]: [35 mg, 67µmol) and 4,4′-bipy (29 mg, 186 µmol)
- Product Data: MS (FAB+): m/z = 157.0 (C10H8N2); (FAB-): m/z = 190.3 (C10N5)
- [Ba(PCC)2(H2O)3]: (15 mg, 27 µmol) and 4,4′-bipy (15 mg, 96 µmol).
- Product Data: MS (FAB+): m/z = 157.0 (C10H8N2); (FAB-): m/z = 190.3 (C10N5)
- Compound 1. Structure solution with shelxs. Refinement with omission of two low-angle reflections.
- Compound 2: Structure solution with shelxs. The crystal turned out to be a racemic twin. Refinement was performed with omission of six low-angle reflections and using the twin card with a final basf value of 0.45459. It was also necessary to restrain the water molecules (O–H and H–H distances). When applying the program subroutine addsym of platon, a non-crystallographic inversion center was detected. Refinement in a higher symmetric spacegroup was, however, not possible.
- Compound 3: Structure solution with sir97. Although there was some indication of twinning, the results of the twin-search routine of platon were not conclusive. Instead, a rather large number (16) of low-angle reflections was omitted from the refinement.
- Compound 4: Structure solution with dirdif. Platon analysis showed the presence of 4.7% solvent accessible voids, and actually it was possible to locate a disordered butanol molecule with low occupancy. Refinement with omission of six low-angle reflections. It was also necessary to restrain the water molecules (O–H and H–H distances) and the disordered solvent molecule.
- Compound 5: Structure solution with shelxt. The crystal turned out to be a racemic twin. Refinement was performed with shelxl 2018/3 with omission of 22 low-angle reflections and using the twin card with a final basf value of 0.34643. Since there was severe disorder within the ethanol molecules, a large number of restraints had to be applied on them.
- Compound 6: Structure solution with sir97. Refinement with omission of ten low-angle reflections. Since there was severe disorder within two butanol molecules, a large number of restraints had to be applied on them.
3. Results
3.1. Synthesis
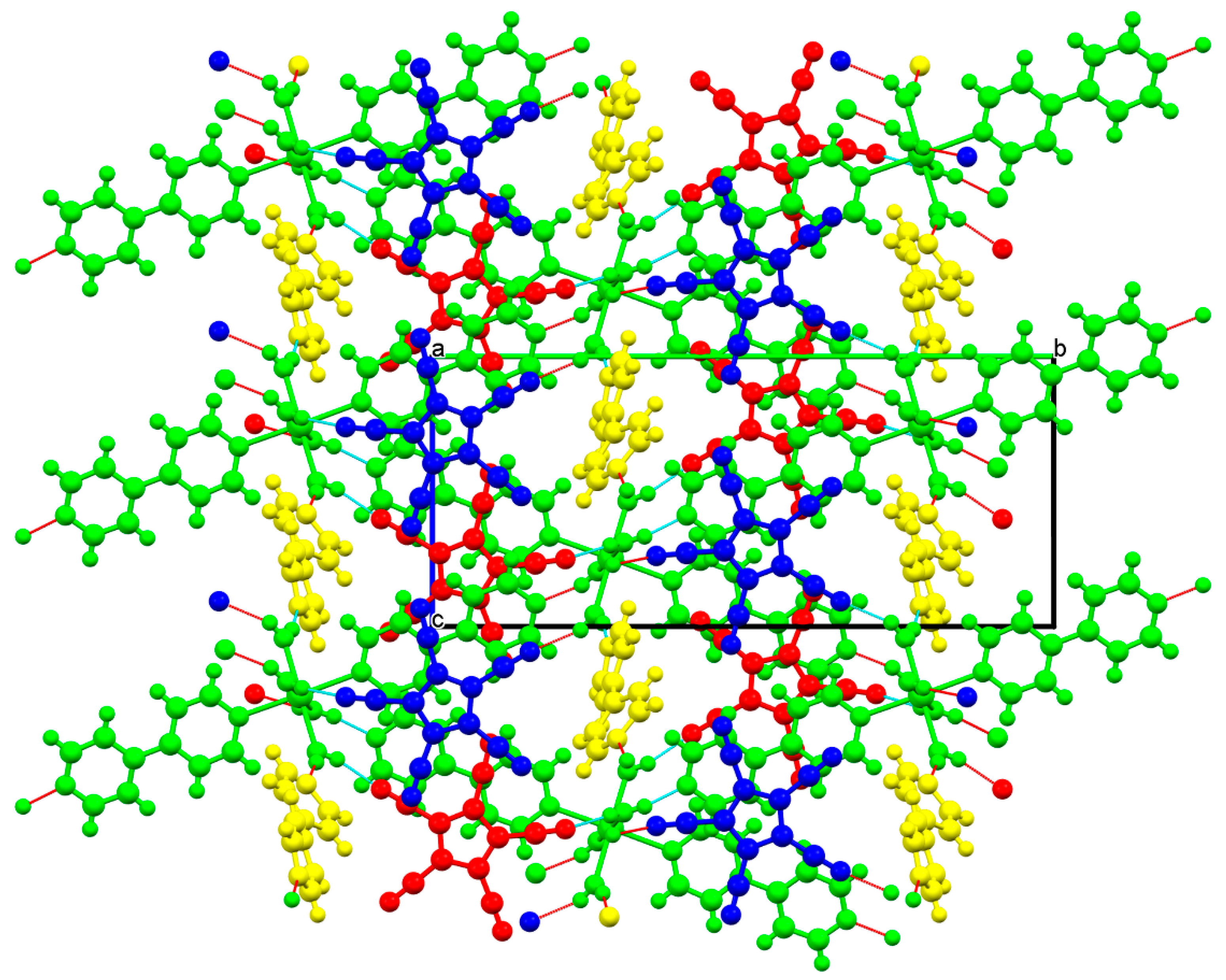
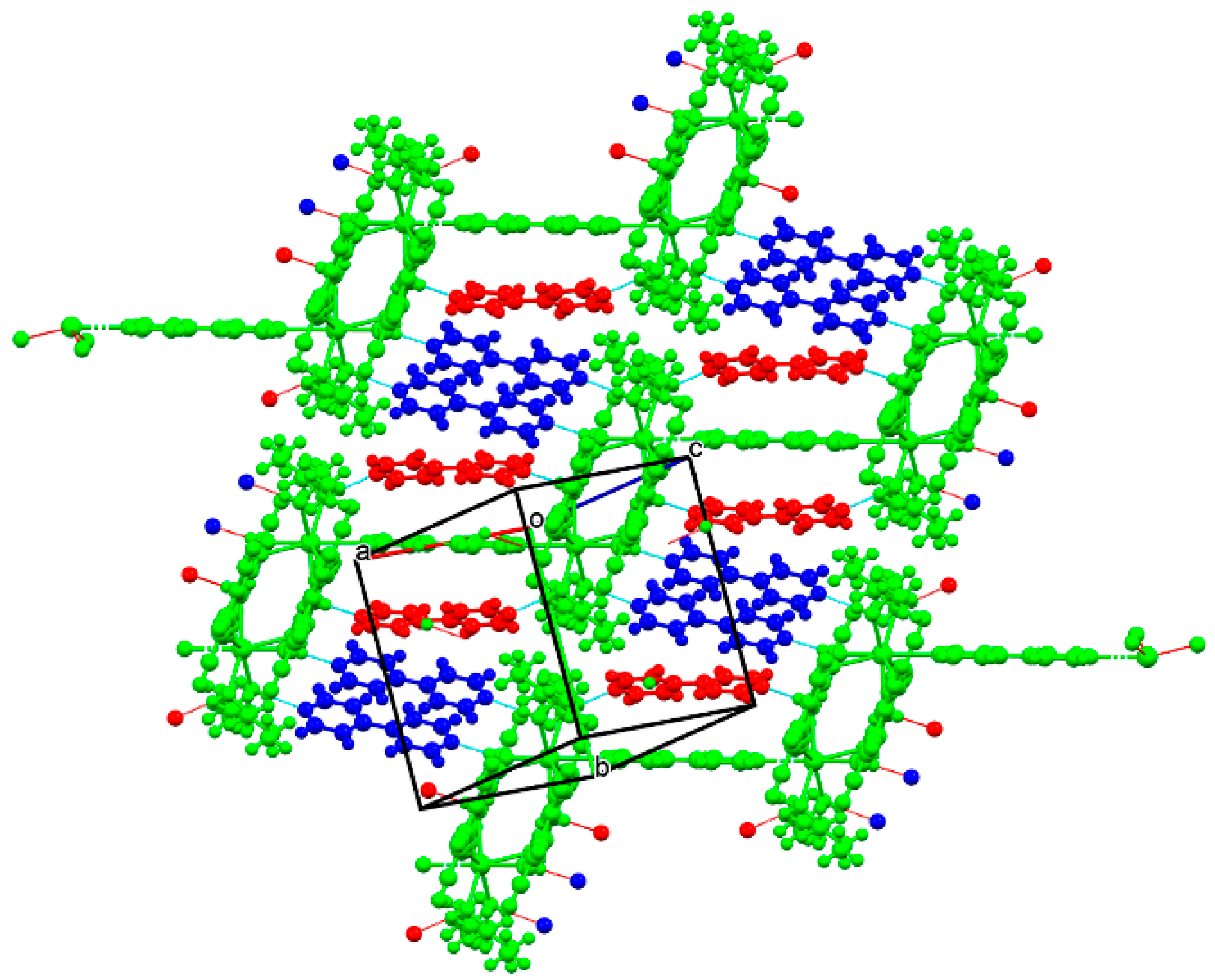
3.2. Crystal and Molecular Structures
3.2.1. Magnesium Pentacyanocyclopentadienides
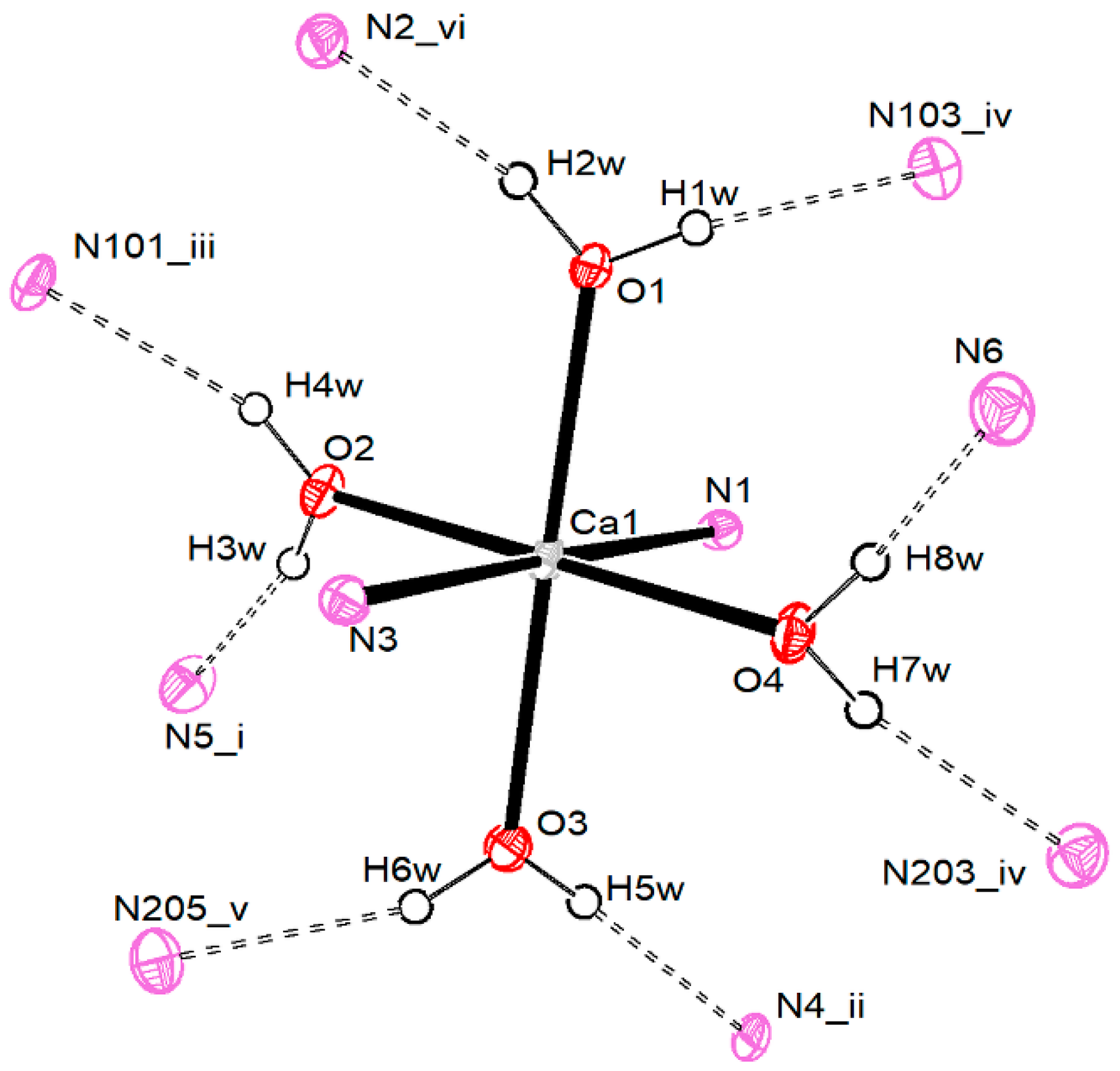
3.2.2. Calcium Pentacyanocyclopentadienides
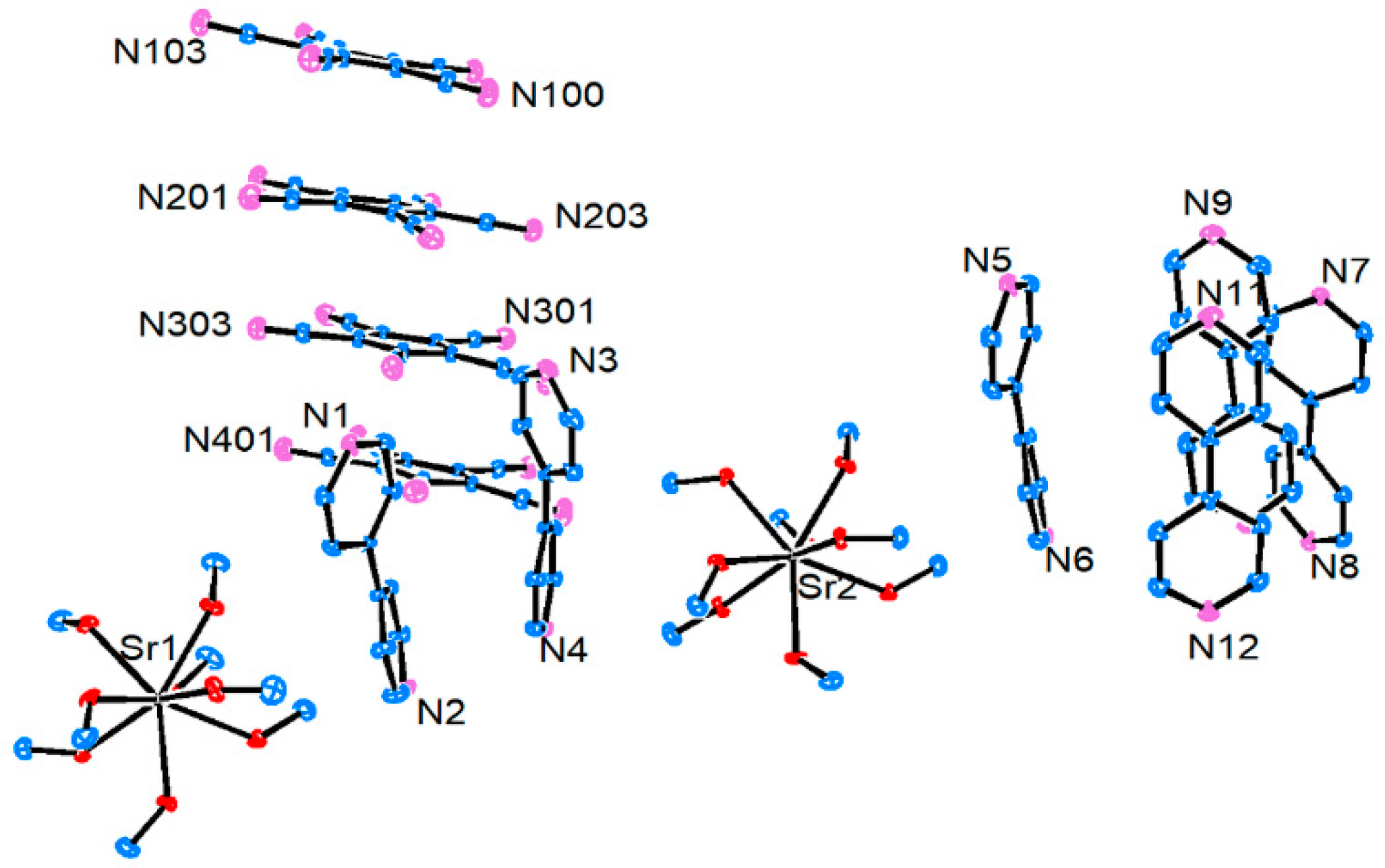
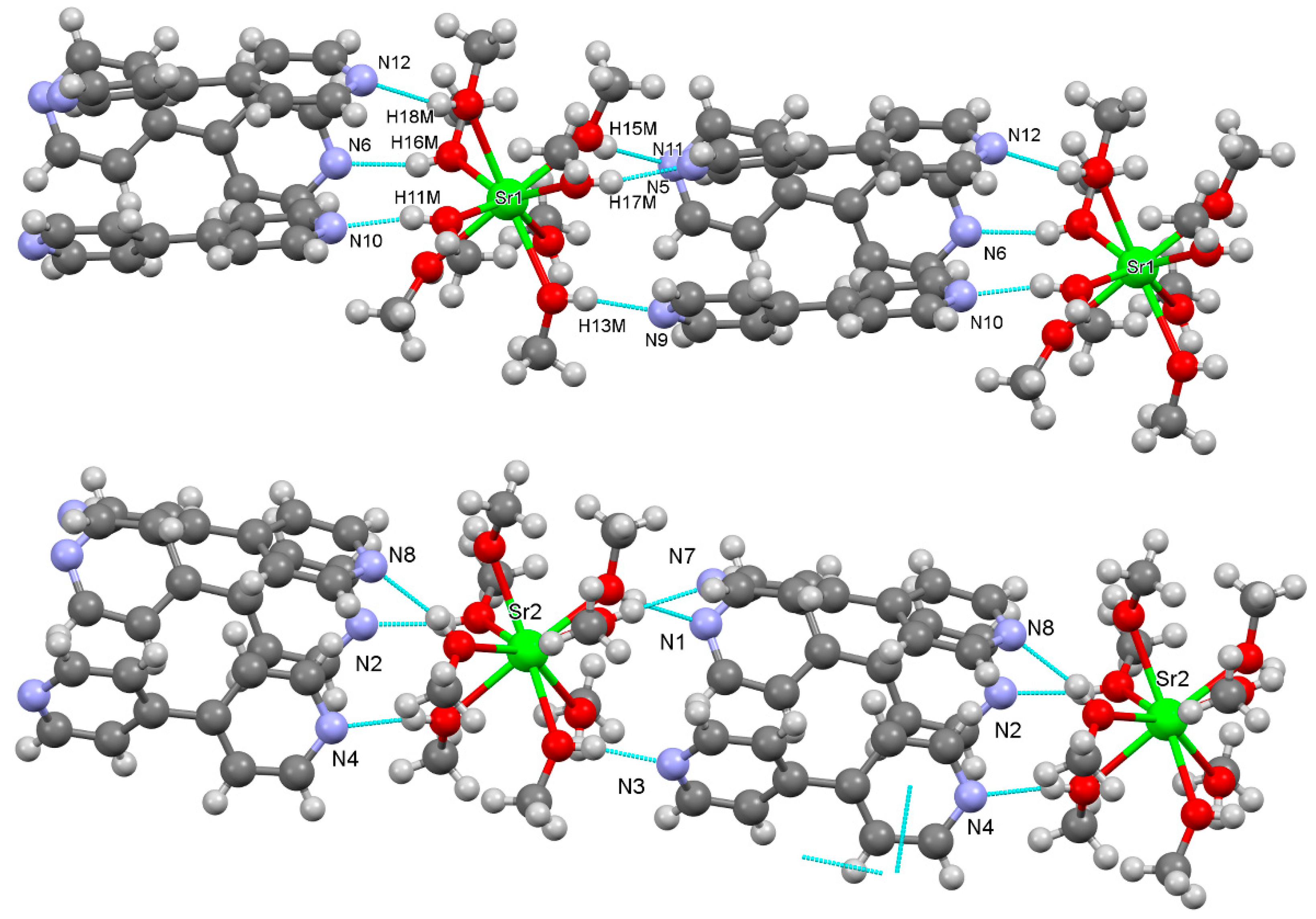
3.2.3. Strontium Pentacyanocyclopentadienides
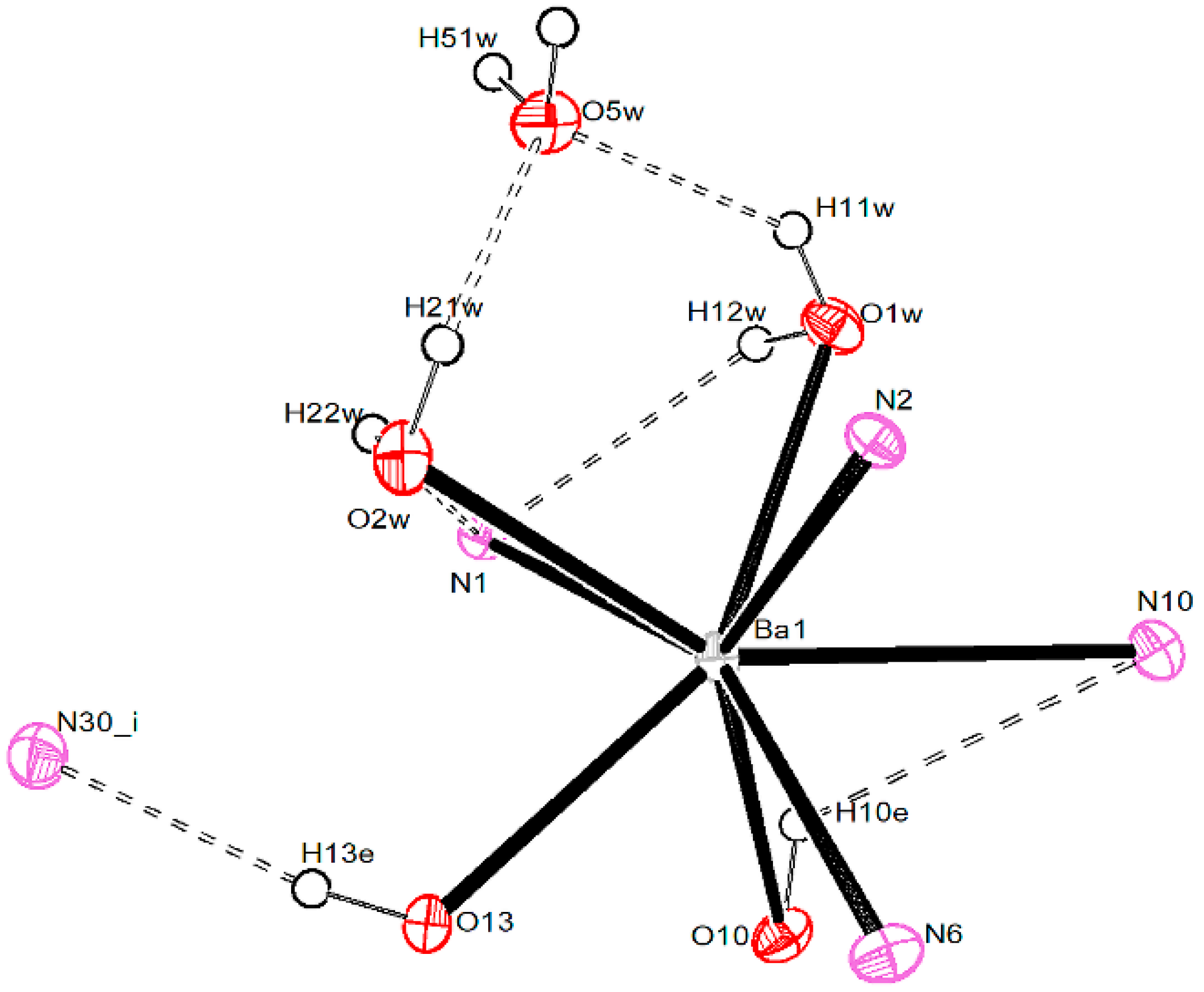
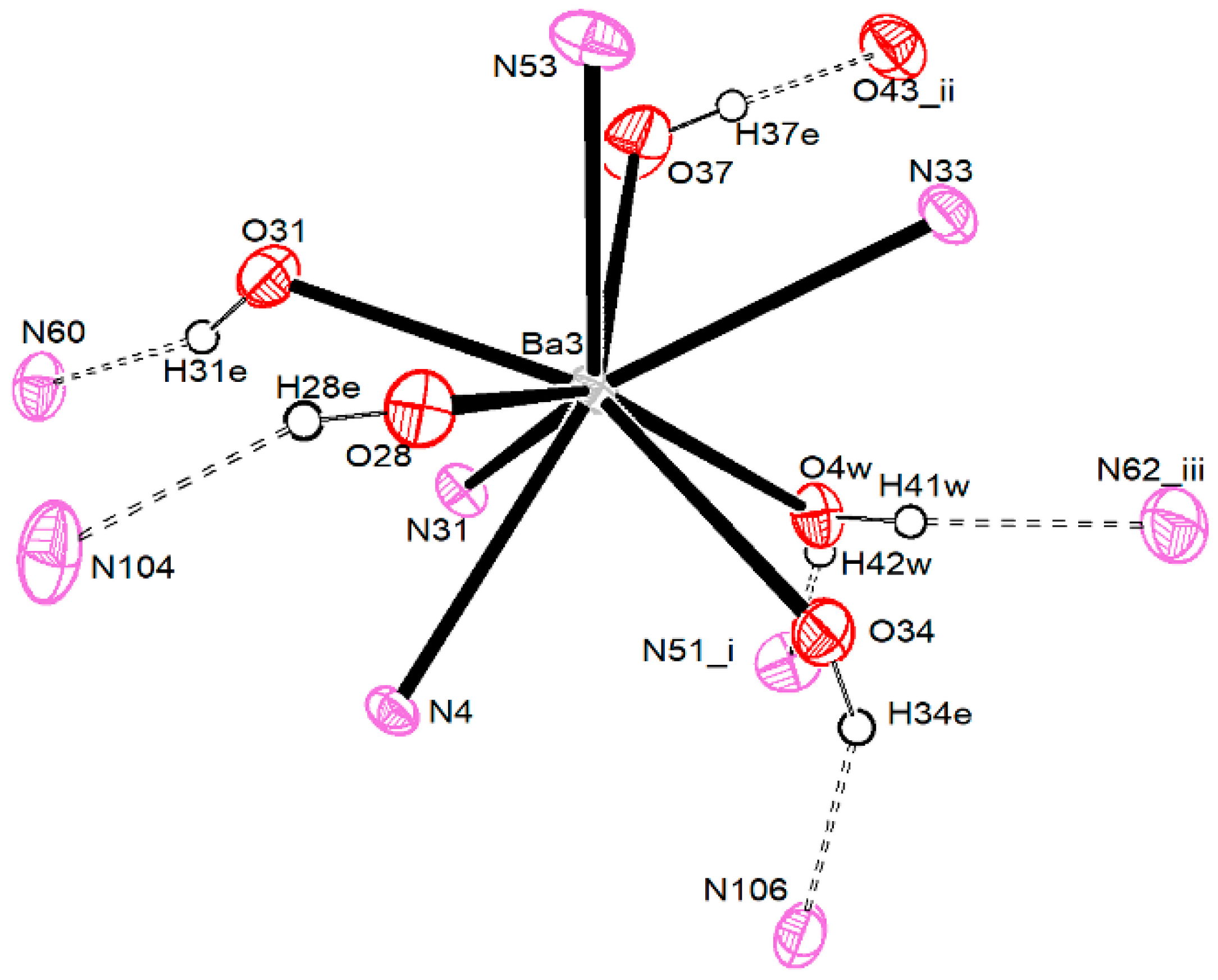
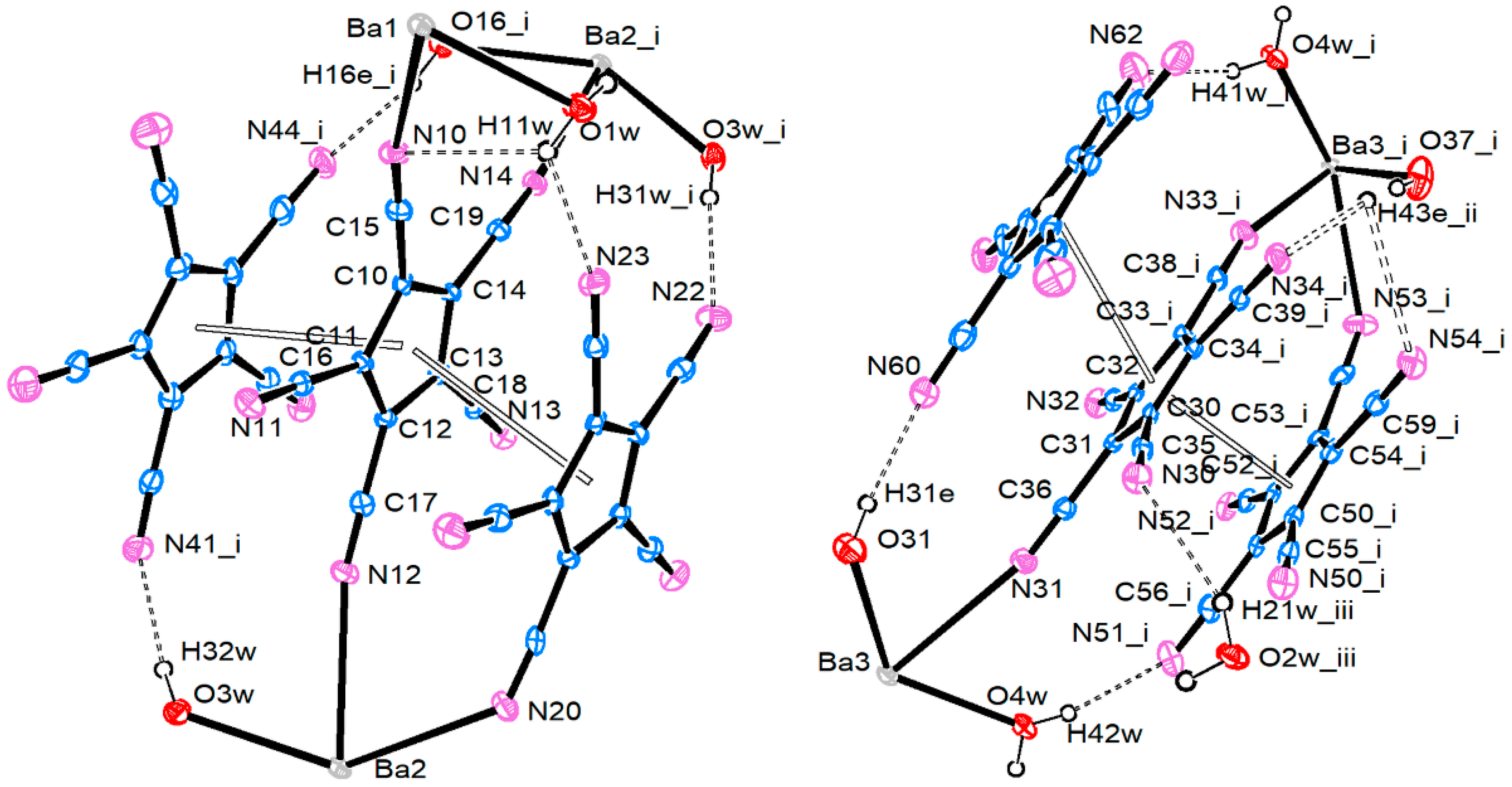
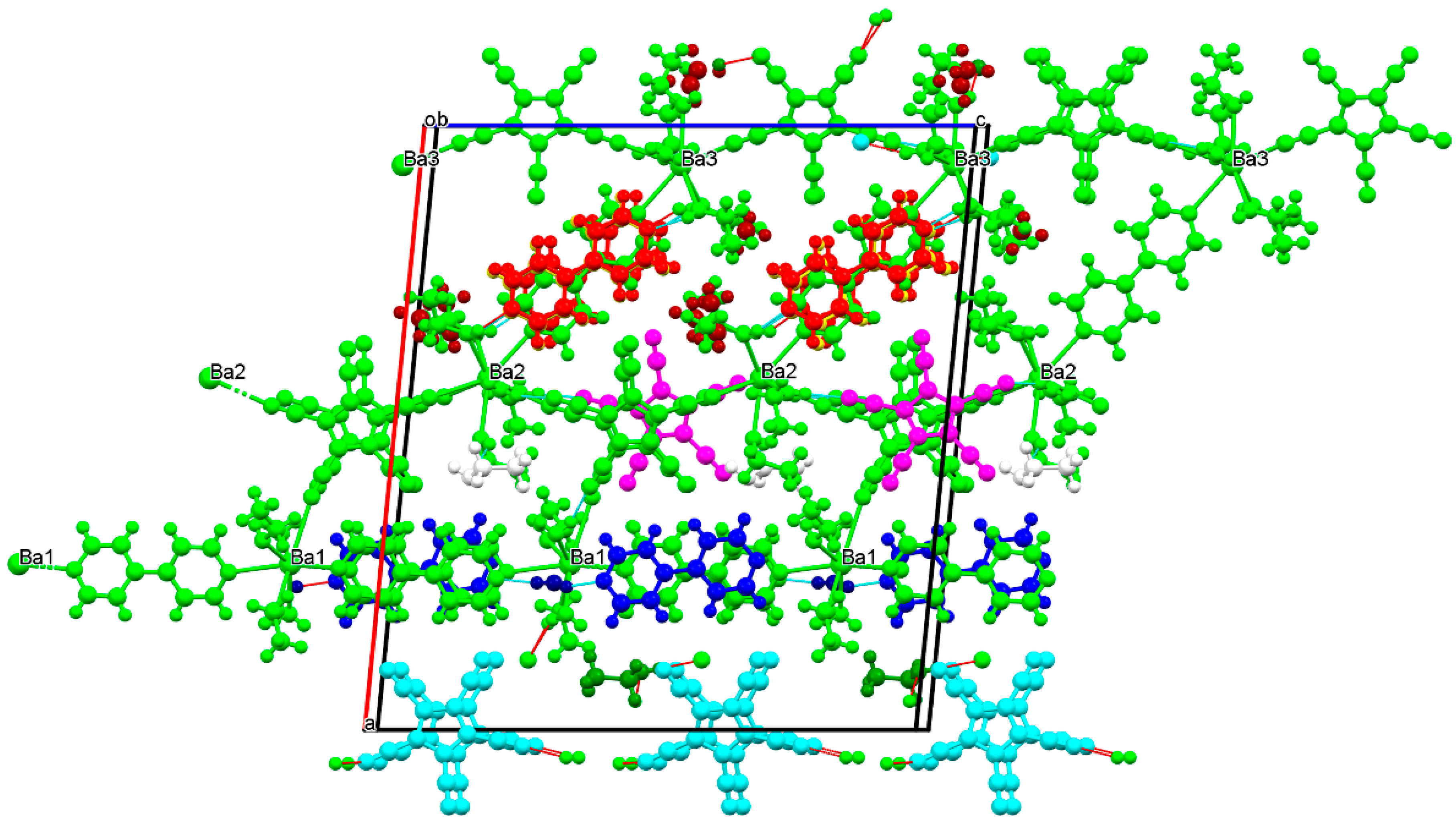
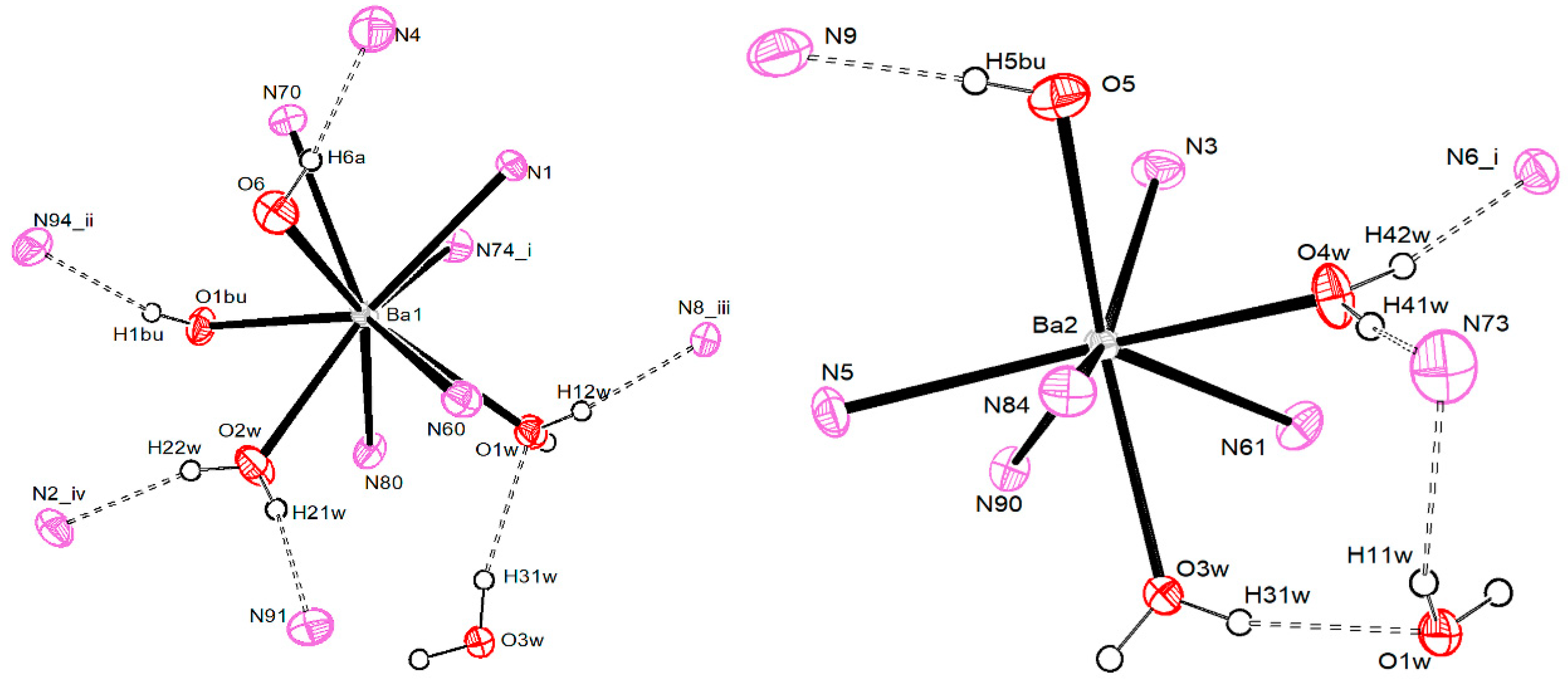
3.2.4. Barium Pentacyanocyclopentadienides
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Grepioni, F.; Casali, L.; Fiore, C.; Mazzei, L.; Sun, R.; Shemchuk, O.; Braga, D. Steps towards a nature inspired inorganic crystal engineering. Dalton Trans. 2022, 51, 7390–7400. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, D.J.; Bajpai, A.; Zaworotko, M.J. The “Chemistree” of Porous Coordination Networks: Taxonomic Classification of Porous Solids to Guide Crystal Engineering Studies. Small 2021, 17, 2006351. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, W.; Zhang, X.; Li, L.; Dong, H.; Hu, W. Creating Organic Functional Materials beyond Chemical Bond Dynthesis by Organic Cocrystal Engineering. J. Am. Chem. Soc. 2021, 143, 19243–19256. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.F. The Geometrical Basis of Crystal Chemistry I. Acta Cryst. 1954, 7, 535–544. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three-dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Biradha, K.; Sarkar, M. Rajput Crystal engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms. Chem. Commun. 2006, 40, 4169–4179. [Google Scholar] [CrossRef] [PubMed]
- Darwish, S.; Wang, S.-Q.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Comparison of Mechanochemistry vs Solution Methods for Synthesis of 4,4′-Bipyridine-Based Coordination Chemistry. ACS Sustain. Chem. Eng. 2019, 7, 19505–19512. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Sironi, A. Extended networks via hydrogen bond cross-linkages of [M(bipy)] (M= Zn2+ or Fe2+; bipy = 4,4′-bipyridyl) linear coordination polymers. Dalton Trans. 1997, 11, 1801–1803. [Google Scholar] [CrossRef]
- Roesky, H.W.; Andruh, M. The interplay of coordinative, hydrogen bonding and π-π stacking interactions in sustaining supramolecular solid-state architectures. A study case of bis(4-pyridyl)- amd bis(4-pyridyl-N-oxide) tectons. Coord. Chem. Rev. 2003, 236, 91–119. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, Y.-L.; Zeng, M.-H.; Kurmoo, M. The concept of mixed organic ligands in metal-organic frameworks: Design, tuning and functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Fromm, K.M. Coordination polymer networks with s-block metal ions. Coord. Chem. Rev. 2008, 252, 856–885. [Google Scholar]
- Benmansour, S.; Armani, C.; Setifi, F.; Triki, S.; Marchivie, M.; Gomez-Garcia, C.J. Polynitrile anions as ligands: From magnetic polymeric architectures to spin crossover materials. Coord. Chem. Rev. 2010, 254, 1468–1478. [Google Scholar] [CrossRef]
- Sünkel, K.; Reimann, D. Coordination Chemistry of Polynitriles, I. Syntheses and Crystal Structures of [Ag(PCC)(DMF)], [Ni(DMF)6](PCC)2 and [Co(DMF)6](PCC)2 (PCC = [C5(CN)5]−, DMF = N,N-dimethylformamide). Z. Nat. B 2013, 68, 546–550. [Google Scholar] [CrossRef][Green Version]
- Sünkel, K.; Nimax, P.R. Structural diversity in the alkaline earth metal compounds of tetra- and pentacyanocyclopentadienide. Dalton Trans. 2018, 47, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT- integrated space group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON squeeze: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C: Struct. Chem. 2015, C71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Skelton, B.W.; Waters, A.F.; Whitaker, C.R.; White, A.H. Magnesium iodide-4,4‘-bipyridine-water (1/3/4) and strontium iodide-4,4‘-bipyridine-propan-1-ol (1/2.5/2). Acta Crystallogr. Sect. C. 2003, 59, m435–m438. [Google Scholar] [CrossRef] [PubMed]
- Kepert, D.L.; Waters, A.F.; White, A.H. Synthesis and Structural Systematics of Nitrogen Base Adducts of Group 2 Salts. VIII. Some Mixed-Ligand Complexes of Group 2 Metal Halides with Aromatic N,N’-Bidentate and Oxygen Donors. Aust. J. Chem. 1996, 49, 117–135. [Google Scholar] [CrossRef]
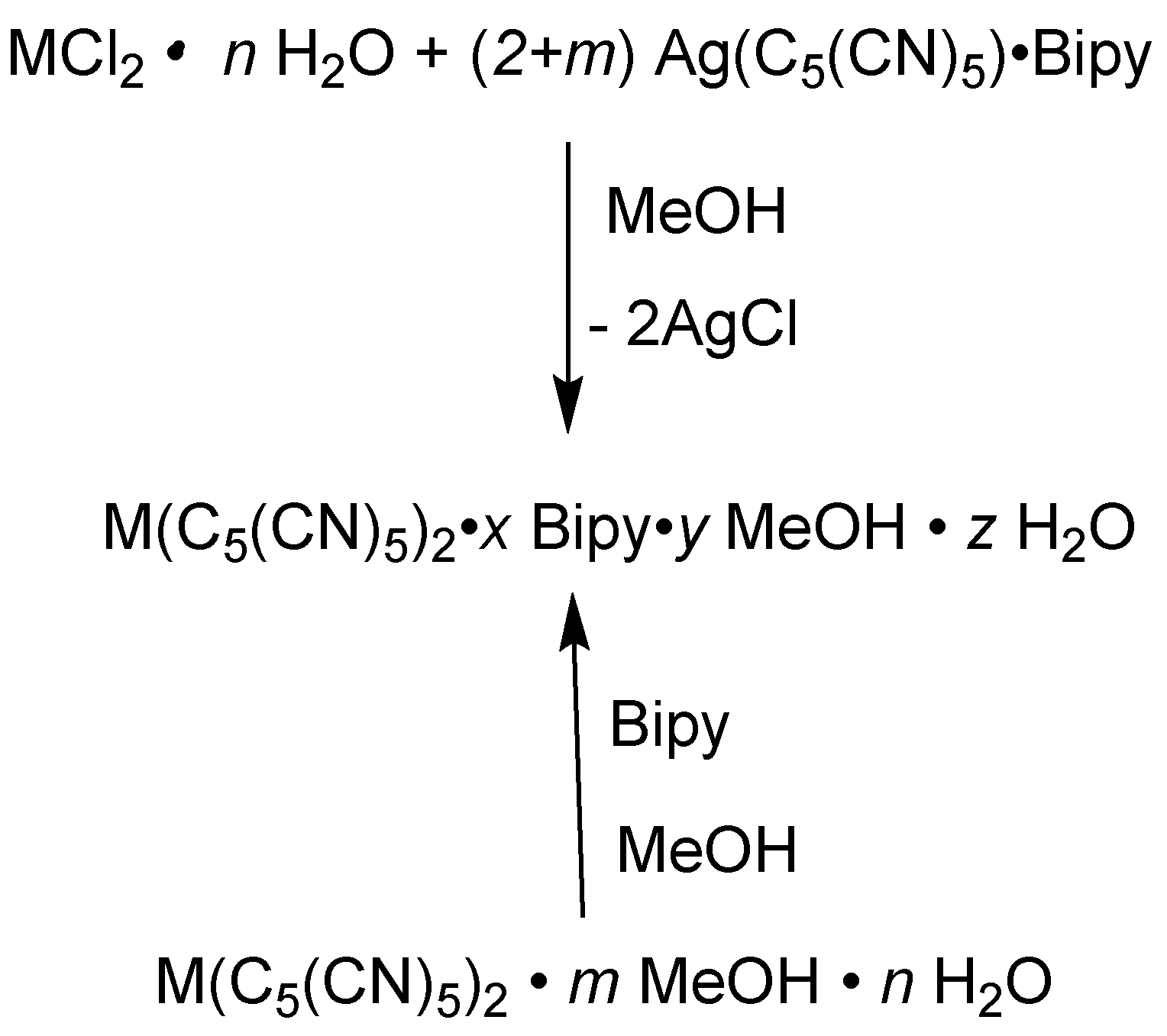
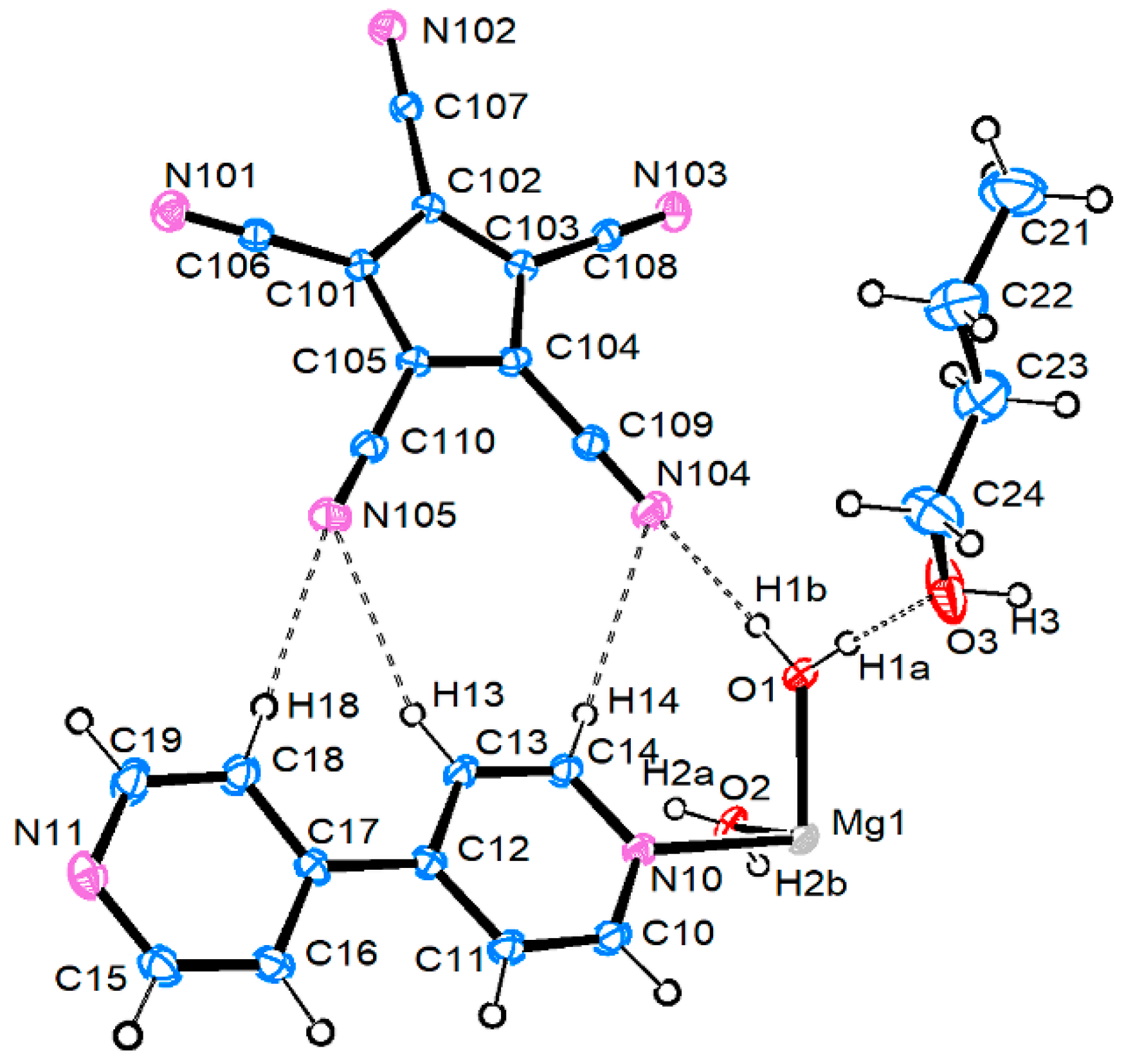
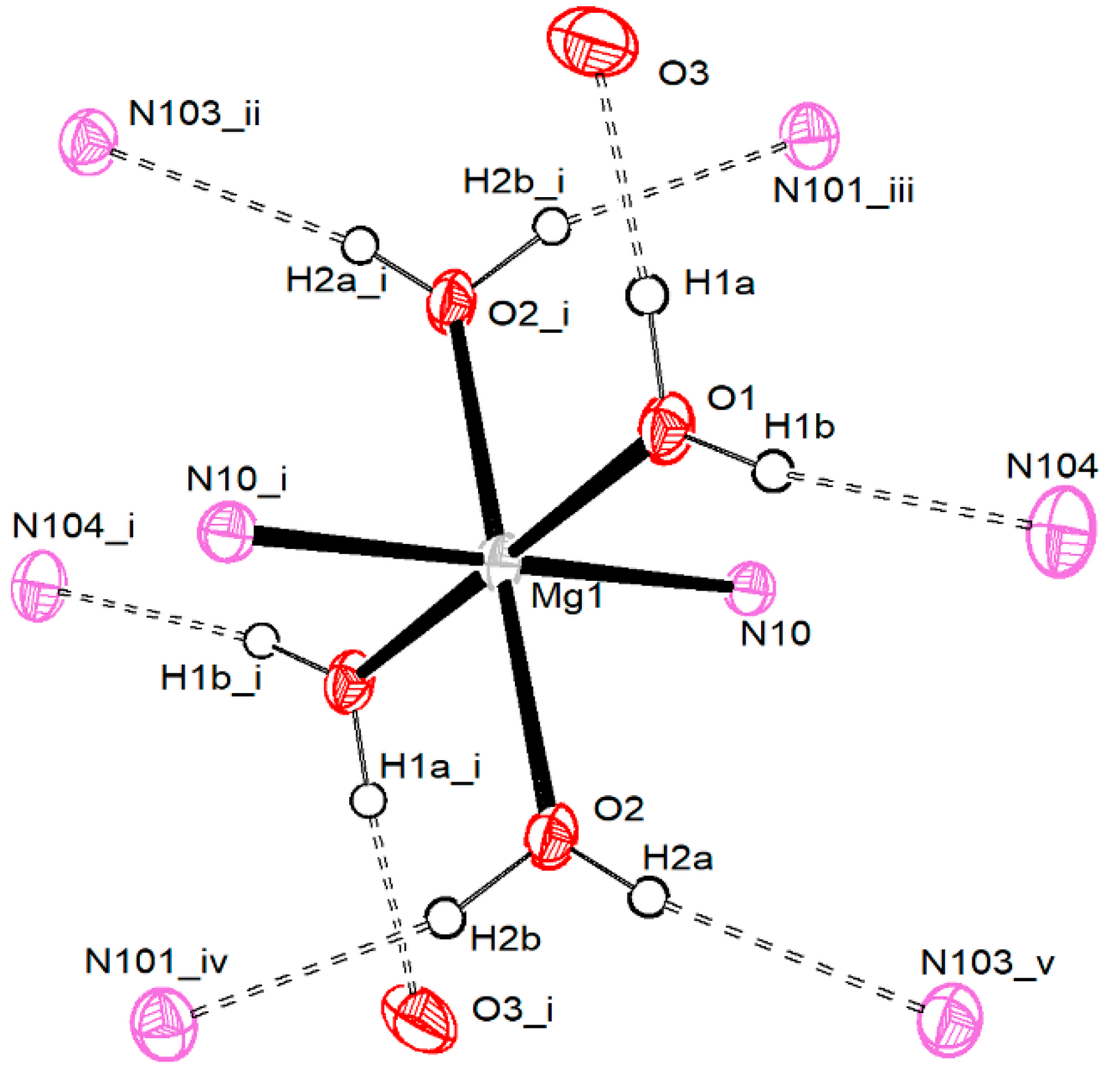
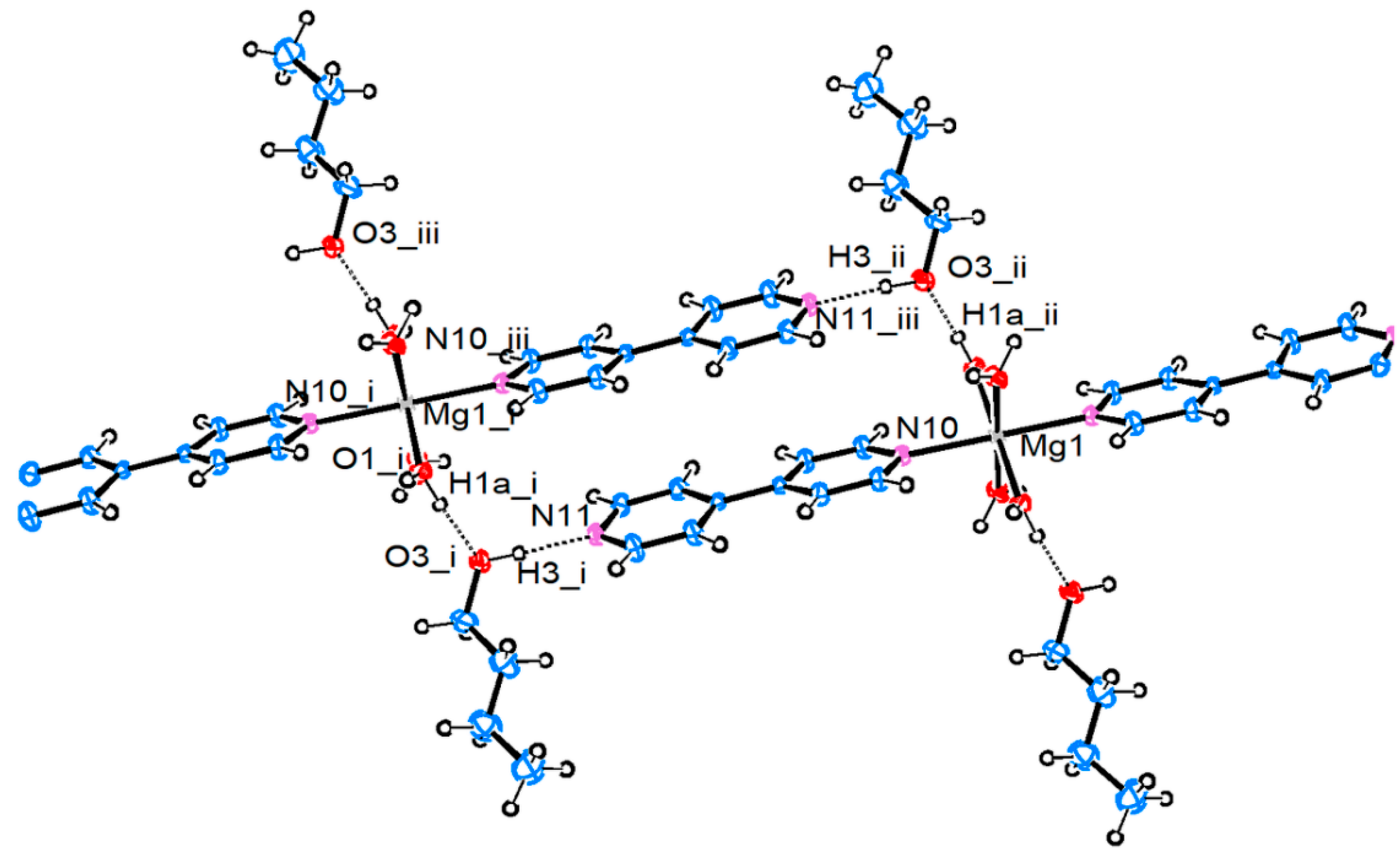




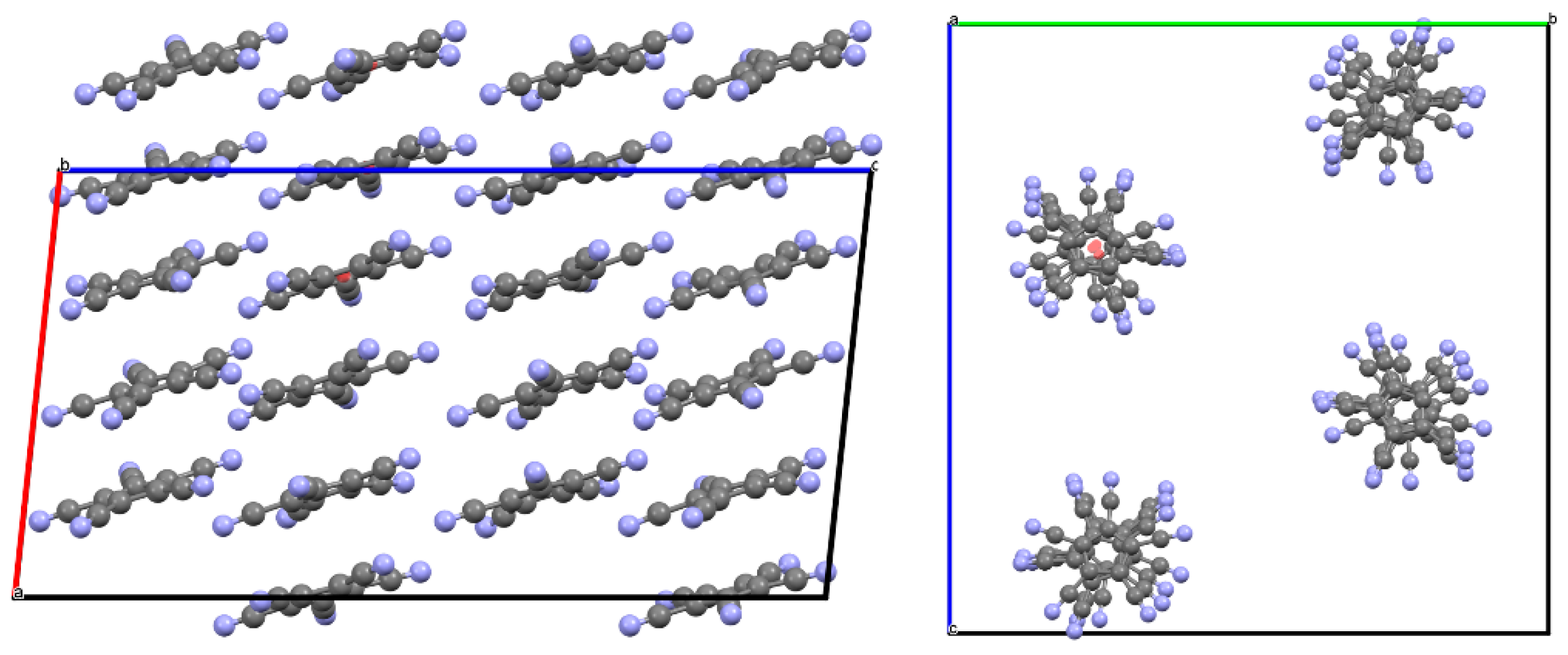

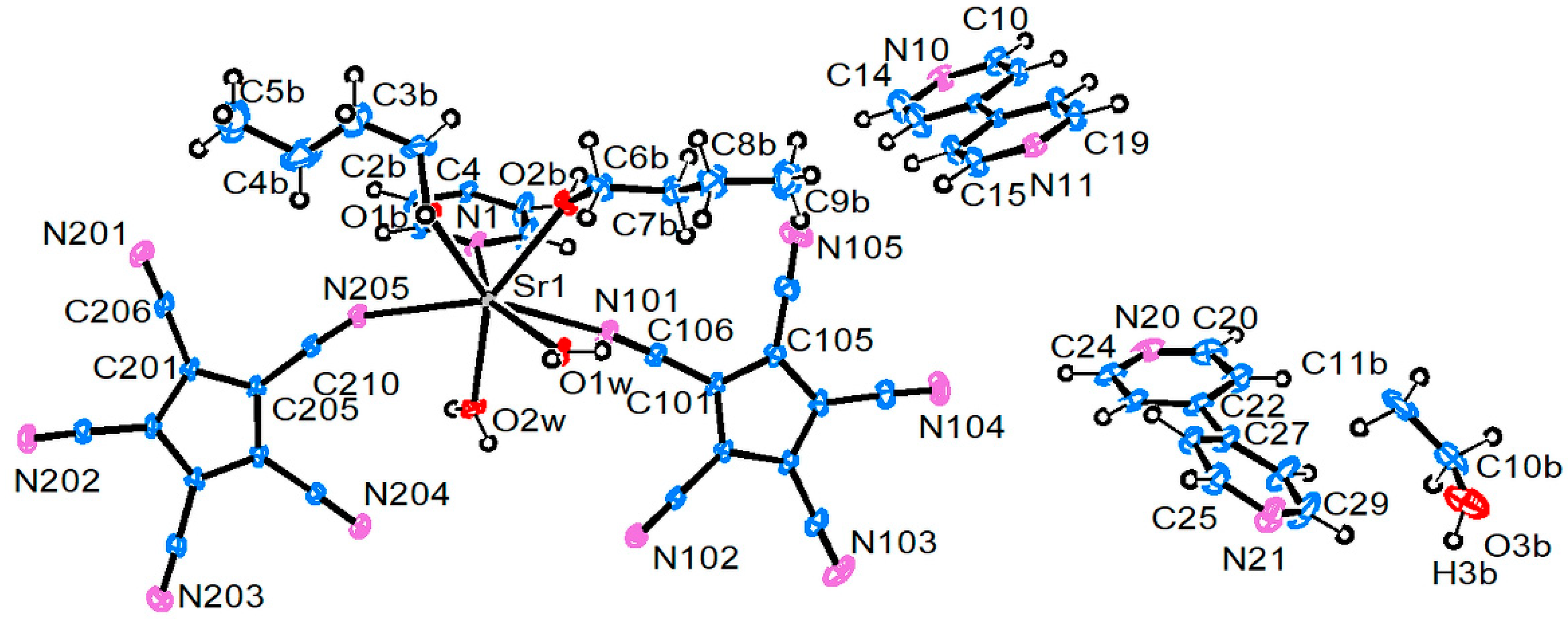
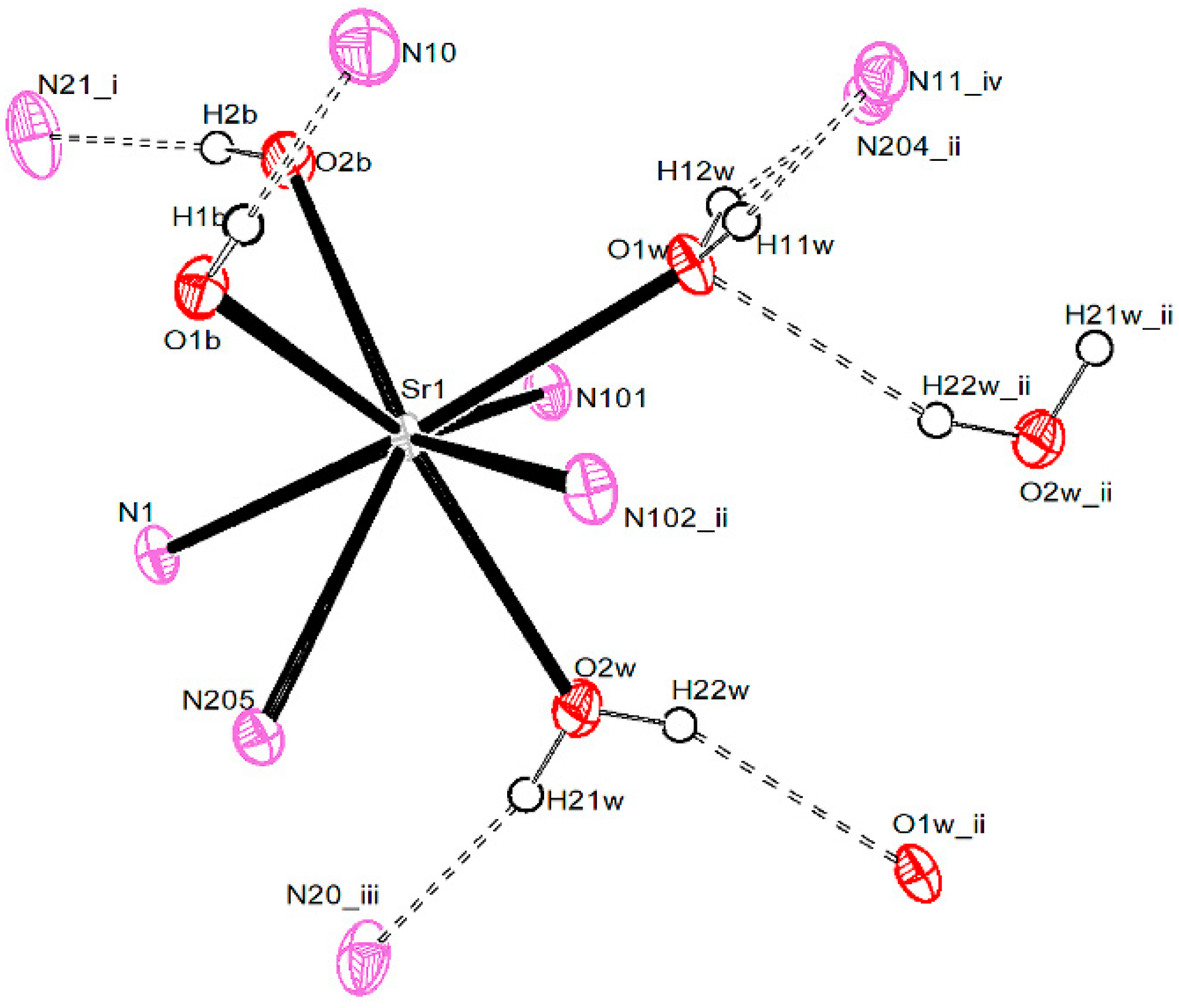
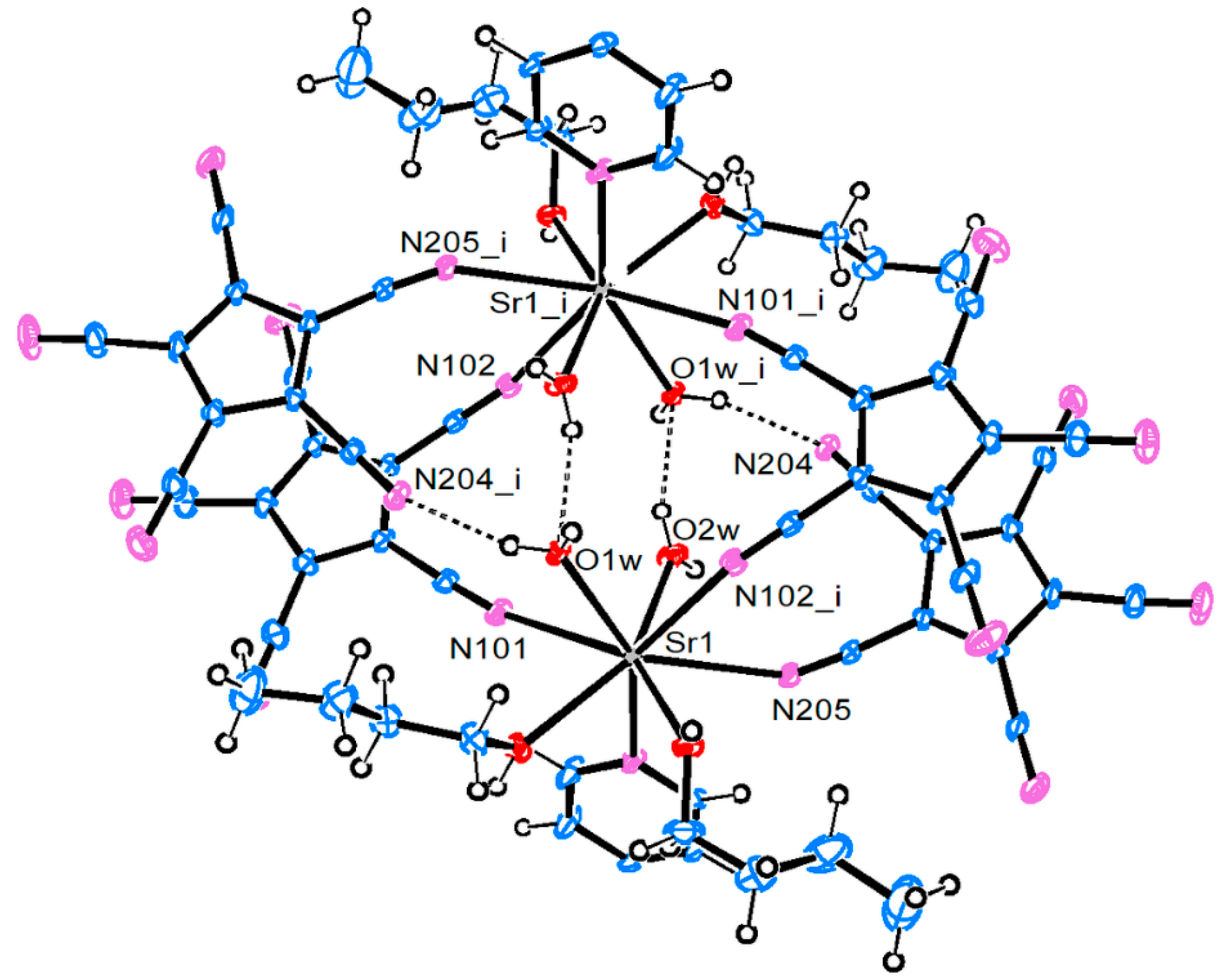






| Compound 1 | |||
| Mg1–O1 | 2.030 (2) | N10–Mg1–N10′ | 180 |
| Mg1–O2 | 2.060 (2) | C11-C12-C17-C16 | 5.9 (4) |
| Mg1–N10 | 2.224 (2) | Mg1–N10…C12 | 178 |
| C12–C17 | 1.490 (4) | ||
| Compound 2 | |||
| Ca1–O1 | 2.322 (3) | C13–C18 | 1.484 (6) |
| Ca1–O2 | 2.318 (3) | C23–C28 | 1.485 (6) |
| Ca1–O3 | 2.329 (3) | N1–Ca1–N3 | 177.70 (12) |
| Ca1–O4 | 2.325 (3) | Ca1-N1…C3 | 176 |
| Ca1–N1 | 2.516 (4) | Ca1-N3…C13 | 176 |
| Ca1–N3 | 2.490 (4) | C2-C3-C8-C9 | 17.2 (6) |
| C3–C8 | 1.499 (6) | C12-C13-C18-C19 | −15.0 (6) |
| C22-C23-C28-C29 | −27.8 (7) | ||
| Compound 3 | |||
| Sr1–O11 | 2.555 (2) | Sr2–O21 | 2.595 (2) |
| Sr1–O12 | 2.641 (2) | Sr2–O22 | 2.558 (2) |
| Sr1–O13 | 2.620 (2) | Sr2–O23 | 2.579 (2) |
| Sr1–O14 | 2.602 (2) | Sr2–O24 | 2.616 (2) |
| Sr1–O15 | 2.589 (2) | Sr2–O25 | 2.562 (2) |
| Sr1–O16 | 2.608 (2) | Sr2–O26 | 2.569 (2) |
| Sr1–O17 | 2.591 (2) | Sr2–O27 | 2.581 (2) |
| Sr1–O18 | 2.611 (2) | Sr2–O28 | 2.587 (2) |
| C3–C8 | 1.477 (4) | C33–C38 | 1.480 (4) |
| C13–C18 | 1.477 (4) | C43–C48 | 1.479 (4) |
| C23–C28 | 1.481 (4) | C53–C58 | 1.473 (4) |
| C2-C3-C8-C7 | −32.4 (4) | C32-C33-C38-C37 | 28.5 (4) |
| C12-C13-C18-C17 | 39.5 (4) | C42-C43-C48-C47 | −31.1 (4) |
| C22-C23-C28-C27 | −22.4( 4) | C52-C53-C58-C57 | −32.6 (4) |
| Compound 4 | |||
| Sr1–O1W | 2.505 (2) | Sr1–N1 | 2.704 (2) |
| Sr1–O2W | 2.518 (2) | Sr1–N101 | 2.817 (3) |
| Sr1–O1B | 2.526 (2) | Sr1–205 | 2.782 (2) |
| Sr1–O2B | 2.567 (2) | Sr1–N102′ | 2.815 (3) |
| C4–C4′ | 1.486 (5) | C3-C4-C4′-C5′- | 0.1 (4) |
| C12–C17 | 1.488 (3) | C11-C12-C17-C18 | −1.5 (4) |
| C22–C27 | 1.482 (4) | C21-C22-C27-C28 | −32.1 (4) |
| Sr1-N1…C4 | 179 | ||
| Sr1–N101–C106 | 164.8 (2) | Sr1–N205–C210 | 152.8 (2) |
| N101–Sr1–N205 | 135.72 (7) | N101–Sr1–N102′ | 129.10 (6) |
| Ba1–O1W | 2.694 (3) | Ba2–O3W | 2.733 (4) | Ba3–O4W | 2.741 (4) |
| Ba1–O2W | 2.759 (3) | Ba2–O16 | 2.754 (4) | Ba3–O28 | 2.777 (4) |
| Ba1–O10 | 2.710 (4) | Ba2–O19 | 2.826 (4) | Ba3–O31 | 2.744 (4) |
| Ba1–O13 | 2.741 (3) | Ba2–O22 | 2.751 (4) | Ba3–O34 | 2.804 (4) |
| Ba1–N1 | 2.960 (4) | Ba2–O25 | 2.818 (4) | Ba3–O37 | 2.712 (4) |
| Ba1–N2′ | 3.017 (4) | Ba2–N3 | 2.920 (4) | Ba3–N4 | 2.961 (4) |
| Ba1–N6 | 2.929 (4) | Ba2–N12 | 3.120 (5) | Ba3–N31 | 3.035 (5) |
| Ba1–N10 | 3.189 (4) | Ba2–N14′ | 3.061 (4) | Ba3–N33 | 3.085 (5) |
| Ba2–N20 | 2.935 (5) | Ba3–N53 | 2.998 (5) | ||
| C102–C107 | 1.483 (7) | C112–C117 | 1.472 (9) | C122–C127 | 1.489 (7) |
| C202–C207 | 1.480 (6) | C212–C217 | 1.476 (6) | C222–C227 | 1.477 (6) |
| Ba1-N10-C15 | 160.9 (4) | Ba2-N12-C17 | 166.2 (4) | Ba2′-N14-C19 | 169.1 (4) |
| Ba2-N20-C25 | 135.8 (4) | Ba3-N33-C38 | 162.0 (4) | Ba3-N53-C58 | 143.9 (4) |
| C101-C102-C107-C108 | −29.5 (5) | C111-C112-C117-C116 | 30.8 (8) | C121-C122-C127-C126 | 28.7 (7) |
| C201-C202-C207-C206 | −28.7 (6) | C211-C212-C217-C218 | 31.2 (7) | C221-C222-C227-C228 | 30.2 (7) |
| Ba1-N1…C202 | 170 | Ba1-N6…C227 | 138 | Ba2-N3…C212 | 177 |
| Ba3-N4…C217 | 174 |
| Ba1–O1W | 2.798 (3) | Ba2–O3W | 2.663 (3) |
| Ba1–O2W | 2.649 (3) | Ba2–O4W | 2.617 (3) |
| Ba1–O1Bu | 2.753 (3) | Ba2–O5 | 2.777 (3) |
| Ba1–O6 | 2.740 (3) | Ba2–N3 | 2.919 (4) |
| Ba1–N1 | 2.895 (3) | Ba2–N5 | 2.892 (3) |
| Ba1–N60 | 2.968 (4) | Ba2–N61 | 2.986 (4) |
| Ba1–N70 | 2.937 (4) | Ba2–N84 | 2.956 (4) |
| Ba1–N74′ | 2.967 (4) | Ba2–N90 | 2.931 (4) |
| Ba1–N80 | 3.006 (4) | ||
| C12–C17 | 1.481 (5) | C22–C27 | 1.476 (6) |
| C32–C37 | 1.479 (6) | C42–C47 | 1.479 (6) |
| C52–C52′ | 1.488 (11) | ||
| Ba1-N60-C65 | 173.9 (3) | Ba2-N61-C66 | 153.5 (4) |
| Ba1-N70-C75 | 159.3 (4) | Ba2-N84-C89 | 164.7 (3) |
| Ba1′-N74-C79 | 145.4 (4) | Ba2-N90-C95 | 140.3 (3) |
| Ba1-N80-C85 | 163.1 (4) | Ba2-N3…C22 | 143 |
| Ba1-N1…C12 | 178 | Ba2-N5…C32 | 166 |
| C11-C12-C17-C18 | 28.8 (6) | C21-C22-C27-C26 | −31.2 (7) |
| C31-C32-C37-C38 | 35.0 (6) | C41-C42-C47-C46 | 26.0 (4) |
| C51-C52-C52′-C53′ | −0.4 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimax, P.R.; Sünkel, K. Coordination Chemistry of Polynitriles, Part XI. Influence of 4,4′-Bipyridine and Solvent on the Crystal and Molecular Structures of Alkaline Earth Pentacyanocyclopentadienides. Chemistry 2022, 4, 1524-1545. https://doi.org/10.3390/chemistry4040101
Nimax PR, Sünkel K. Coordination Chemistry of Polynitriles, Part XI. Influence of 4,4′-Bipyridine and Solvent on the Crystal and Molecular Structures of Alkaline Earth Pentacyanocyclopentadienides. Chemistry. 2022; 4(4):1524-1545. https://doi.org/10.3390/chemistry4040101
Chicago/Turabian StyleNimax, Patrick R., and Karlheinz Sünkel. 2022. "Coordination Chemistry of Polynitriles, Part XI. Influence of 4,4′-Bipyridine and Solvent on the Crystal and Molecular Structures of Alkaline Earth Pentacyanocyclopentadienides" Chemistry 4, no. 4: 1524-1545. https://doi.org/10.3390/chemistry4040101
APA StyleNimax, P. R., & Sünkel, K. (2022). Coordination Chemistry of Polynitriles, Part XI. Influence of 4,4′-Bipyridine and Solvent on the Crystal and Molecular Structures of Alkaline Earth Pentacyanocyclopentadienides. Chemistry, 4(4), 1524-1545. https://doi.org/10.3390/chemistry4040101






