Honey Discrimination Using Fourier Transform-Infrared Spectroscopy
Abstract
:1. Introduction
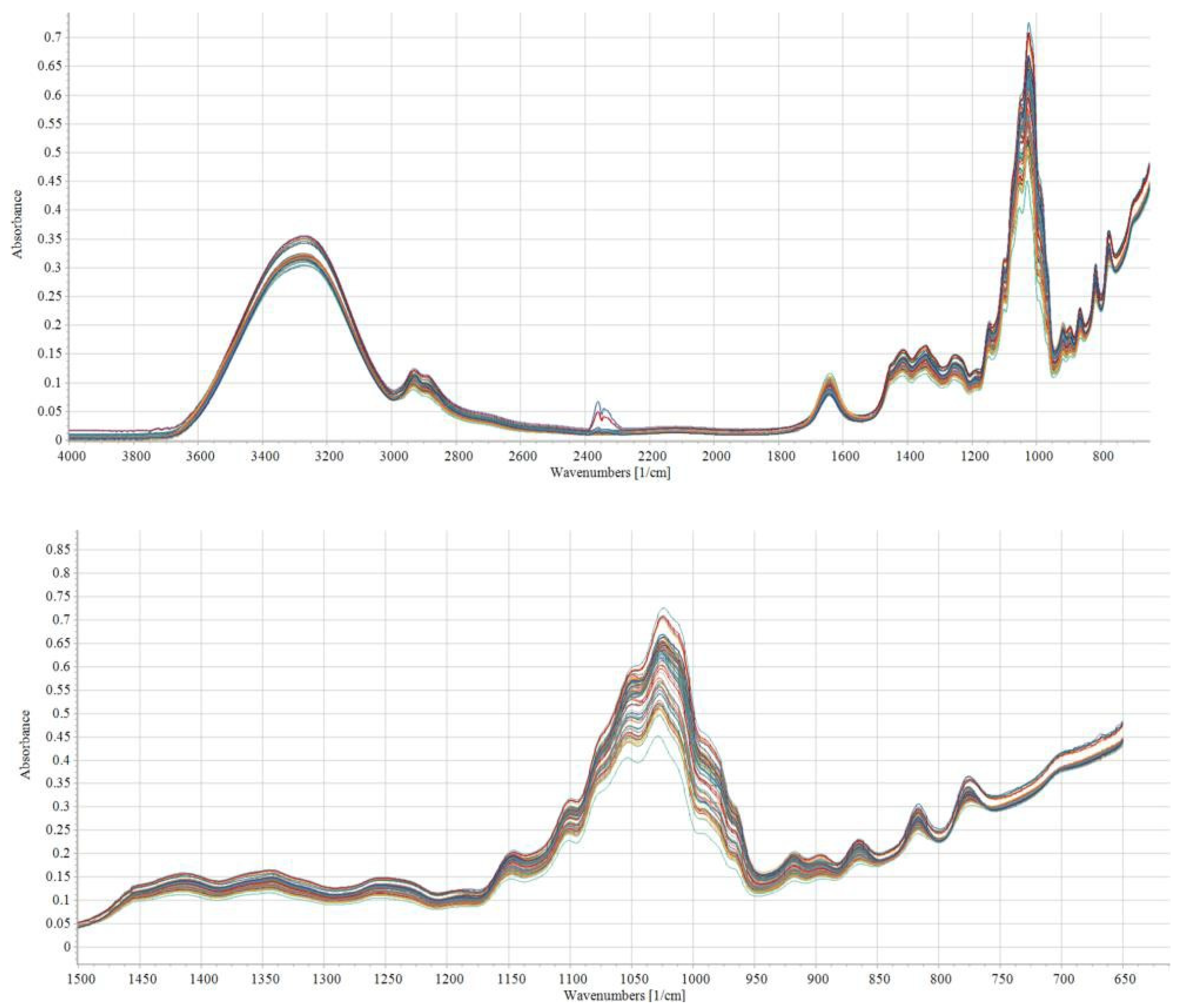
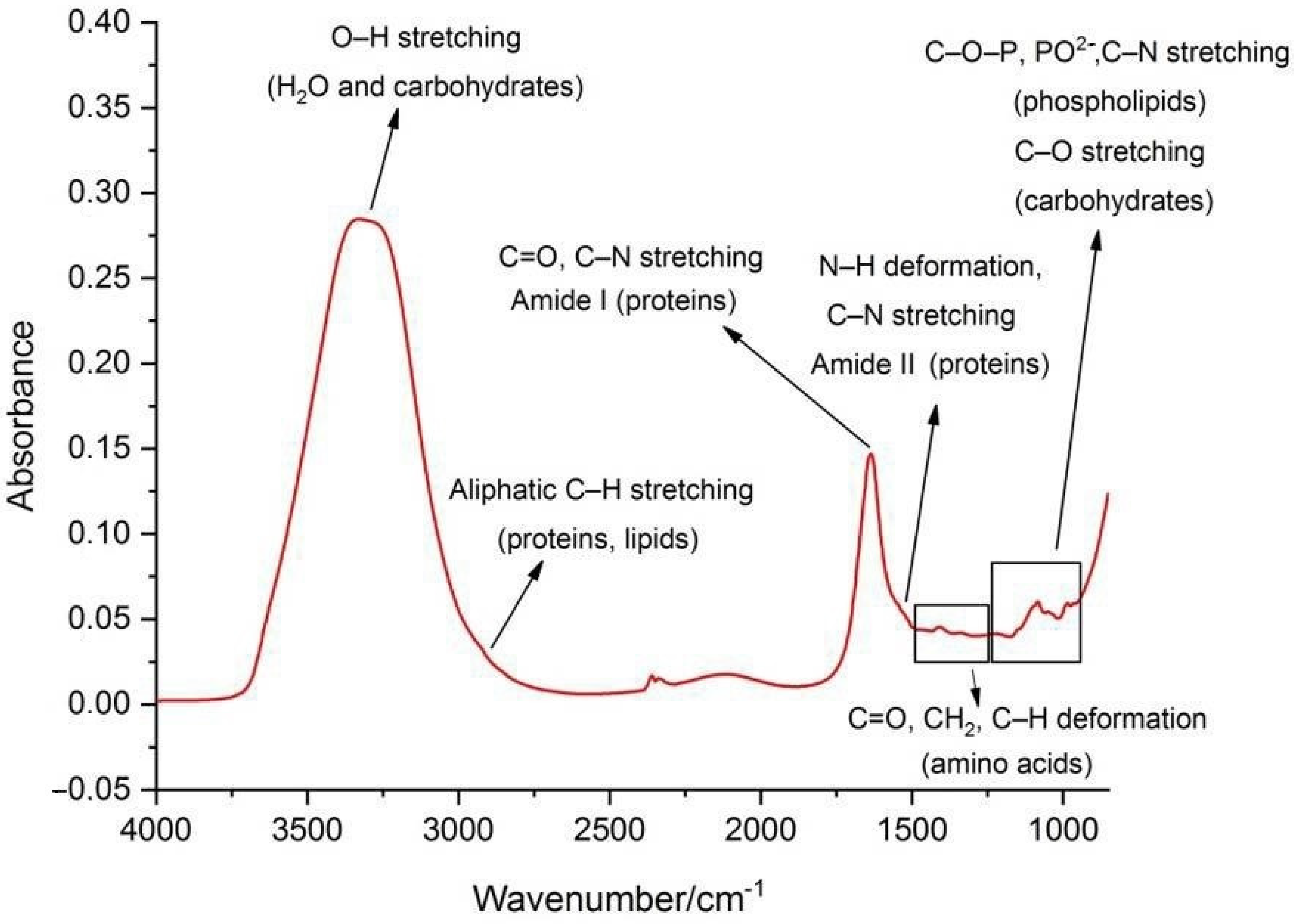
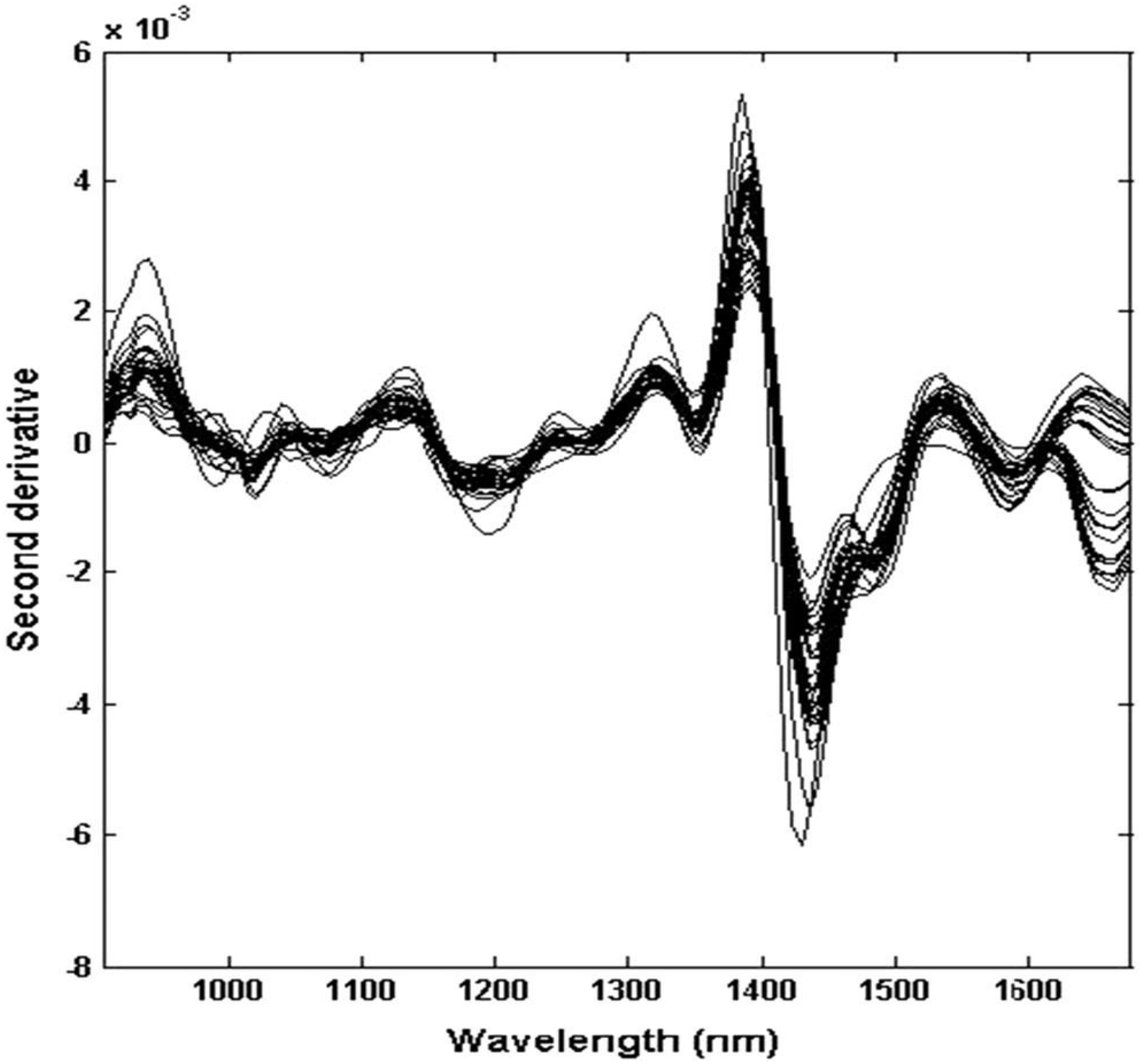
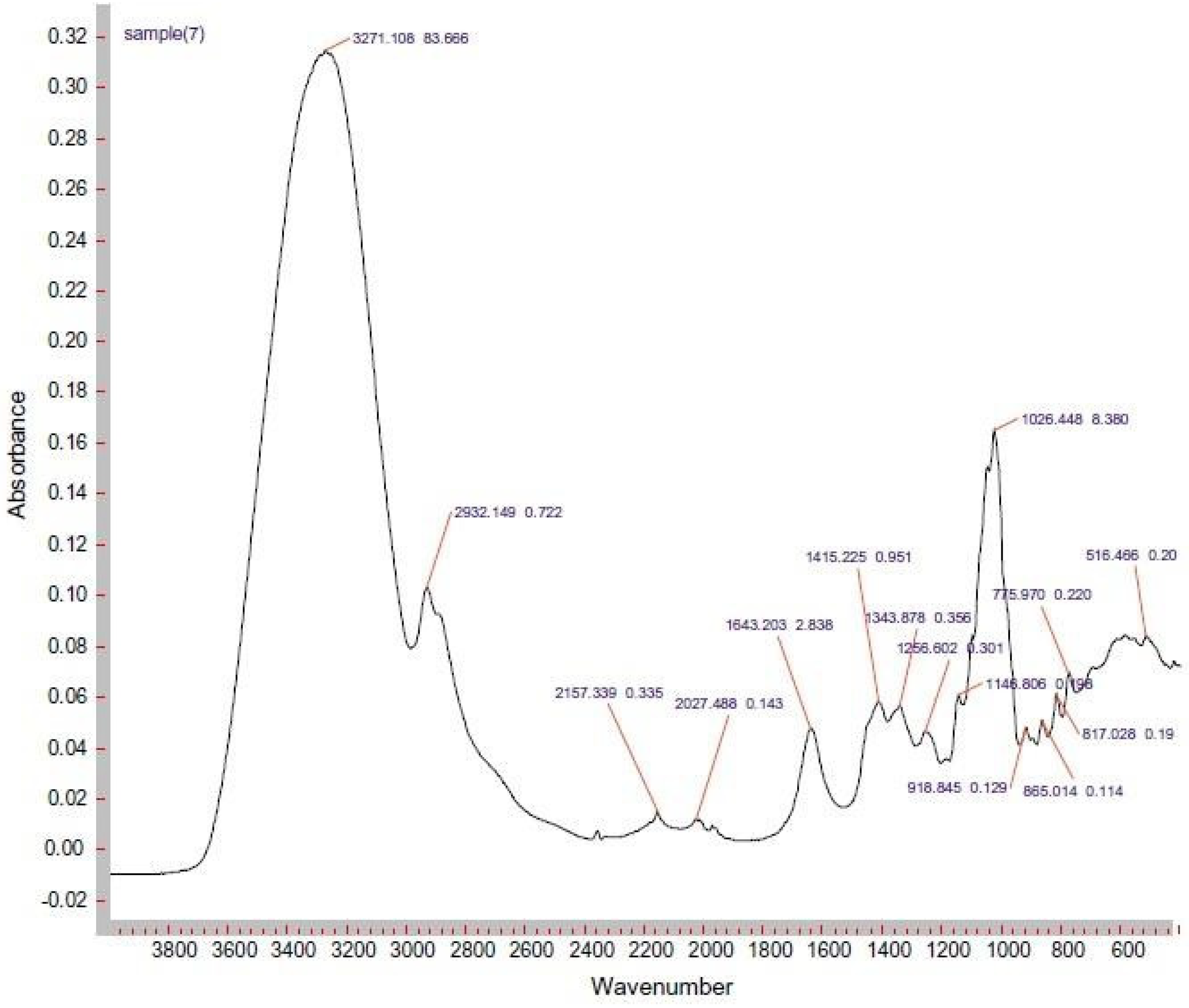
2. Applications of Fourier Transform Infrared Spectroscopy
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Codex Alimentarius Commission Standards. Draft Revised Standard for Honey; 33rd Session; Food Standards Agency: Geneva, Switzerland, 2010; pp. 19–26. [Google Scholar]
- Council of the European Union. Council Directive, 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Commun. 2002, L10, 47–52. [Google Scholar]
- Global Industry Analysts Inc. Honey: A Global Strategic Business Report; Global Industry Analysts Inc.: San Jose, CA, USA, 2016; Available online: http://www.strategyr.com/MarketResearch/Honey_Market_Trends.asp (accessed on 1 July 2022).
- Conti, M.E.; Stripeikis, J.; Campanella, L.; Cucina, D.; Tudino, M.B. Characterization of Italian honeys (Marche Region) on the basis of their mineral content and some typical quality parameters. Chem. Cen. J. 2007, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P. Determination of metal content in honey by atomic absorption and emission spectrometries. TrAC Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey innutrition and human health: A review. Medit. J. Nutr. Met. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Conti, M.E.; Finoia, M.G.; Fontana, L.; Mele, G.; Botrè, F.; Iavicoli, I. Characterization of Argentine honeys on the basis of their mineral content and some typical quality parameters. Chem. Cent. J. 2014, 8, 44. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.X. Chemical composition, characterization, and differentiation of honey botanical and geographical origins. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 62, pp. 89–137. [Google Scholar] [CrossRef]
- Cozzolino, D.; Corbella, E.; Smyth, H.E. Quality control of honey using infrared spectroscopy: A review. Appl. Spectrosc. Rev. 2011, 46, 523–538. [Google Scholar] [CrossRef]
- Odeh, I.; Abu-Lafi, S.; Dewik, H.; Al-Najjar, I.; Imam, A.; Dembitsky, V.M.; Hanuš, L.O. A variety of volatile compounds as markers in Palestinian honey from Thymus capitatus, Thymelaea hirsuta, and Tolpis virgata. Food Chem. 2007, 101, 1393–1397. [Google Scholar] [CrossRef]
- Svečnjak, L.; Biliškov, N.; Bubalo, D.; Barišić, D. Application of infrared spectroscopy in honey analysis. Agric. Conspec. Sci. 2011, 76, 191–195. [Google Scholar]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-chemical methods for the characterization of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. Recent developments in honey characterization. RSC Adv. 2015, 5, 59696–59714. [Google Scholar] [CrossRef]
- Perez-Arquillue, C.; Conchello, P.; Arino, A.; Juan, T.; Herrera, A. Physicochemical attributes and pollen spectrum of some unifloral Spanish honeys. Food Chem. 1995, 54, 167–172. [Google Scholar] [CrossRef]
- Wang, J.; Kliks, M.; Qu, W.Y.; Jun, S.J.; Shi, G.Y.; Li, Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009, 57, 10081–10088. [Google Scholar] [CrossRef] [PubMed]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Application of carbohydrate analysis to verify honey authentication. J. Chromatogr. A 2003, 1021, 145–155. [Google Scholar] [CrossRef]
- Ohe, W.; Von der Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35 (Suppl. S1), S18–S25. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiouc, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Adamchuk, L.; Sukhenko, V.; Akulonok, O.; Bilotserkivets, T.; Vyshniak, V.; Lisohurska, D.; Lisohurska, O.; Slobodyanyuk, N.; Shanina, O.; Galyasnyj, I. Methods for determining the botanical origin of honey. Potravin. Slovak J. Food Sci. 2020, 14, 483–493. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Mid-infrared (MIR) spectroscopy for quality analysis of liquid foods. Food Eng. Rev. 2019, 11, 142–158. [Google Scholar] [CrossRef]
- Guyon, F.; Enora Logodin, E.; Magdas, D.A.; Gaillard, L. Potential of FTIR-ATR diamond in discriminating geographical and botanical origins of honeys from France and Romania. Talanta Open 2021, 3, 100022. [Google Scholar] [CrossRef]
- Stuart, B.H. Biological Applications of Infrared Spectroscopy; Ando, D.J., Ed.; ACOL Series; Wiley: Chichester, UK, 1997; 212p, ISBN 978-0-471-97414-7. [Google Scholar]
- Gallardo-Velazquez, T.; Osorio-Revilla, G.; Loa, M.Z.; Rivera-Espinoza, Y. Application of FTIR-HATR spectroscopy and multivariate analysis to the quantification of adulterants in Mexican honeys. Food Res. Int. 2009, 42, 313–3318. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Kosmalska, A.; Zaborski, M. Characteristics of compounds in hops using cyclic voltammetry, UV–VIS, FTIR and GC–MS analysis. Food Chem. 2014, 156, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kraspryzk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Control 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Pauliuc, D.; Ciursă, P.; Ropciuc, S.; Dranca, F.; Oroian, M. Physicochemical parameters prediction and authentication of different monofloral honeys based on FTIR spectra. J. Food Compos. Anal. 2021, 102, 104021. [Google Scholar] [CrossRef]
- Gok, S.; Severcan, M.; Goormaghtigh, E.; Kandemir, I.; Severcan, F. Differentiation of Anatolian honey samples from different botanical origins by ATR-FTIR spectroscopy using multivariate analysis. Food Chem. 2015, 170, 232–240. [Google Scholar] [CrossRef]
- Tewari, J.; Irudayaraj, J. Quantification of saccharides in multiplefloral honeys using Fourier transform infrared micro attenuated total reflectance spectroscopy. J. Agric. Food Chem. 2004, 52, 3237–3243. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef]
- Horvatinec, J.; Svečnjak, L. Infrared (FTIR) spectral features of honey bee (Apis mellifera L.) hemolymph. J. Cent. Eur. Agric. 2020, 21, 37–41. [Google Scholar] [CrossRef]
- Freire, K.R.L.; Lins, A.C.S.; Dórea, M.C.; Santos, F.A.R.; Camara, C.A.; Silva, T.M.S. Palynological origin, phenolic content, and antioxidant properties of honey bee-collected pollen from Bahia, Brazil. Molecules 2012, 17, 1652–1664. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Leme, L.M.; Montenegro, H.R.; da Rochados Santos, L.; Sereia, M.J.; Valderrama, P.; Março, P.H. Relation between near-infrared spectroscopy and physicochemical parameters for discrimination of honey samples from Jatai weyrauchi and Jatai angustula bees. Food Anal. Methods 2018, 11, 1944–1950. [Google Scholar] [CrossRef]
- Cariou, V.; Qannari, E.M.; Rutledge, D.N.; Vigneau, E. ComDim: From multiblock data analysis to path modeling. Food Qual. Prefer. 2016, 67, 27–34. [Google Scholar] [CrossRef]
- Jouan-Rimbaud Bouveresse, D.; Pinto, R.C.; Schmidtke, L.M.; Locquet, N.; Rutledge, D.N. Identification of significant factors by an extension of ANOVA-PCA based on multi-block analysis. Chemom. Intell. Lab. Syst. 2011, 106, 173–182. [Google Scholar] [CrossRef]
- Nayik, G.A.; Dar, B.N.; Nanda, V. Physico-chemical, rheological and sugar profile of different unifloral honeys from Kashmir. valley of India. Arab. J. Chem. 2019, 12, 3151–3162. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunaciu, A.A.; Aboul-Enein, H.Y. Honey Discrimination Using Fourier Transform-Infrared Spectroscopy. Chemistry 2022, 4, 848-854. https://doi.org/10.3390/chemistry4030060
Bunaciu AA, Aboul-Enein HY. Honey Discrimination Using Fourier Transform-Infrared Spectroscopy. Chemistry. 2022; 4(3):848-854. https://doi.org/10.3390/chemistry4030060
Chicago/Turabian StyleBunaciu, Andrei A., and Hassan Y. Aboul-Enein. 2022. "Honey Discrimination Using Fourier Transform-Infrared Spectroscopy" Chemistry 4, no. 3: 848-854. https://doi.org/10.3390/chemistry4030060
APA StyleBunaciu, A. A., & Aboul-Enein, H. Y. (2022). Honey Discrimination Using Fourier Transform-Infrared Spectroscopy. Chemistry, 4(3), 848-854. https://doi.org/10.3390/chemistry4030060






