A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal–Semiconductor Heterostructures: A Review
Abstract
:1. Introduction
2. Plasmonic Dynamics
3. Synthesis of Plasmonic Metal–Semiconductor Photocatalyst
4. Factors Affecting Plasmonic Nanostructure
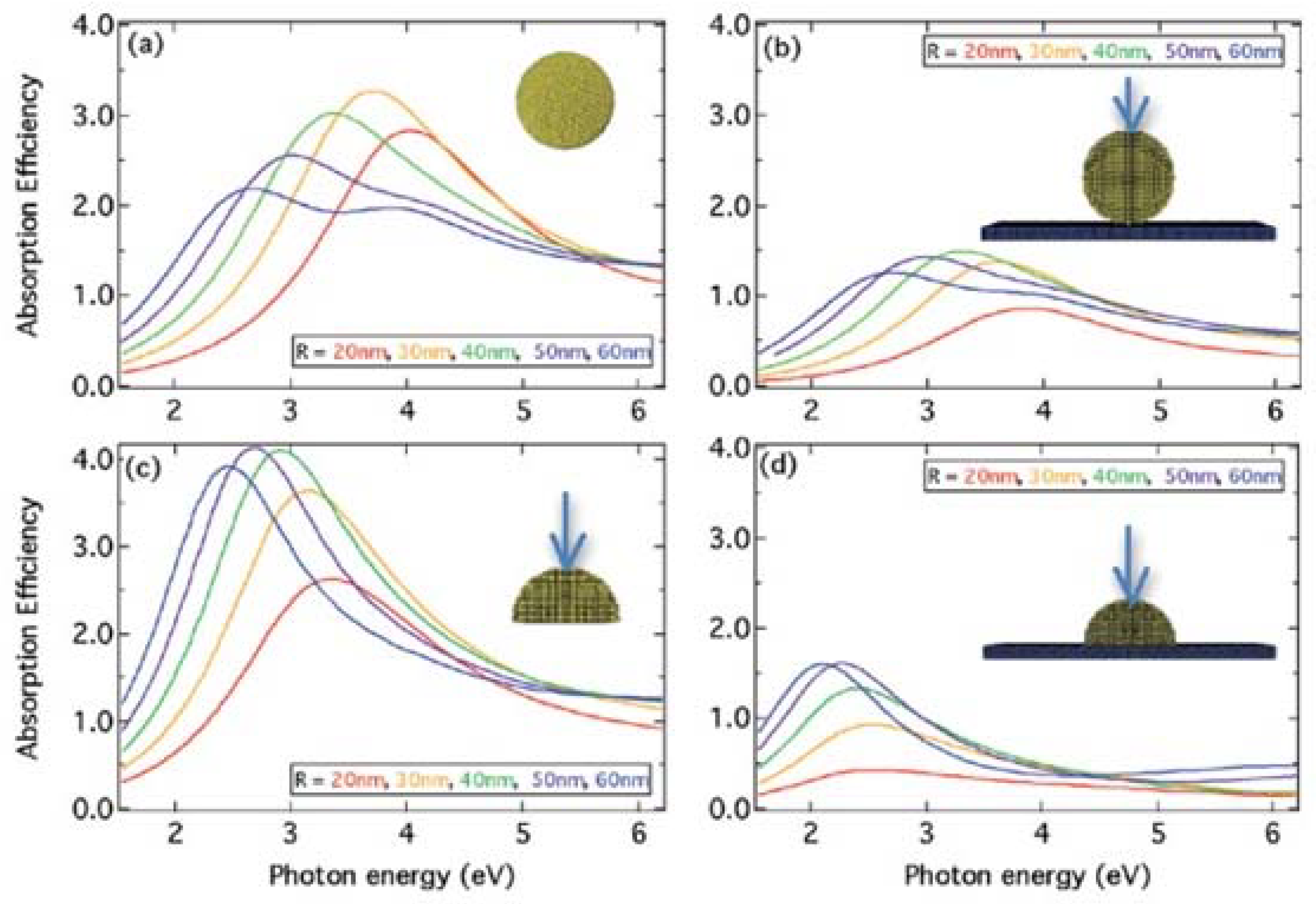
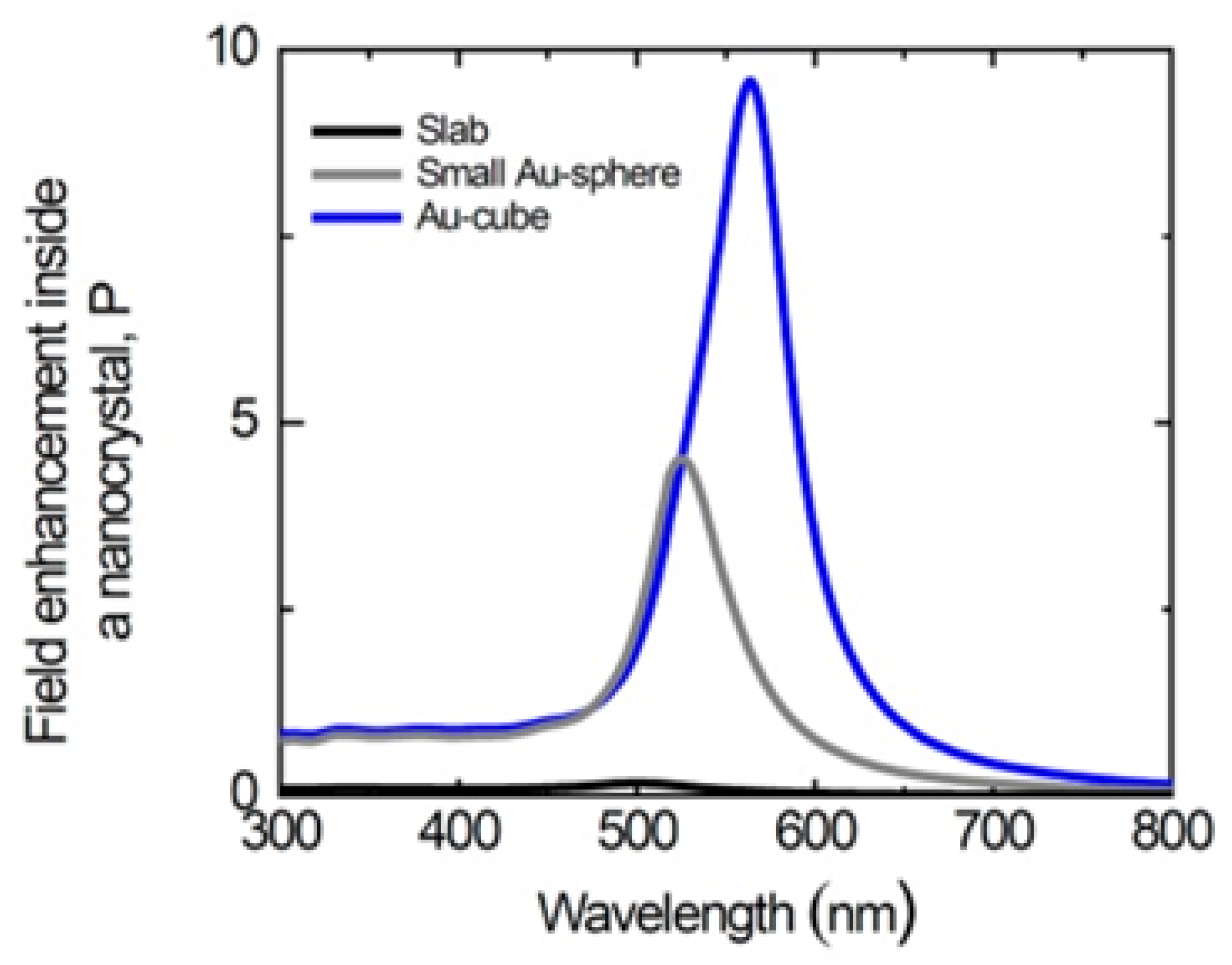
4.1. Size and Shape
4.2. Material Composition
4.3. Ratio of Noble Metal to the Semiconductor
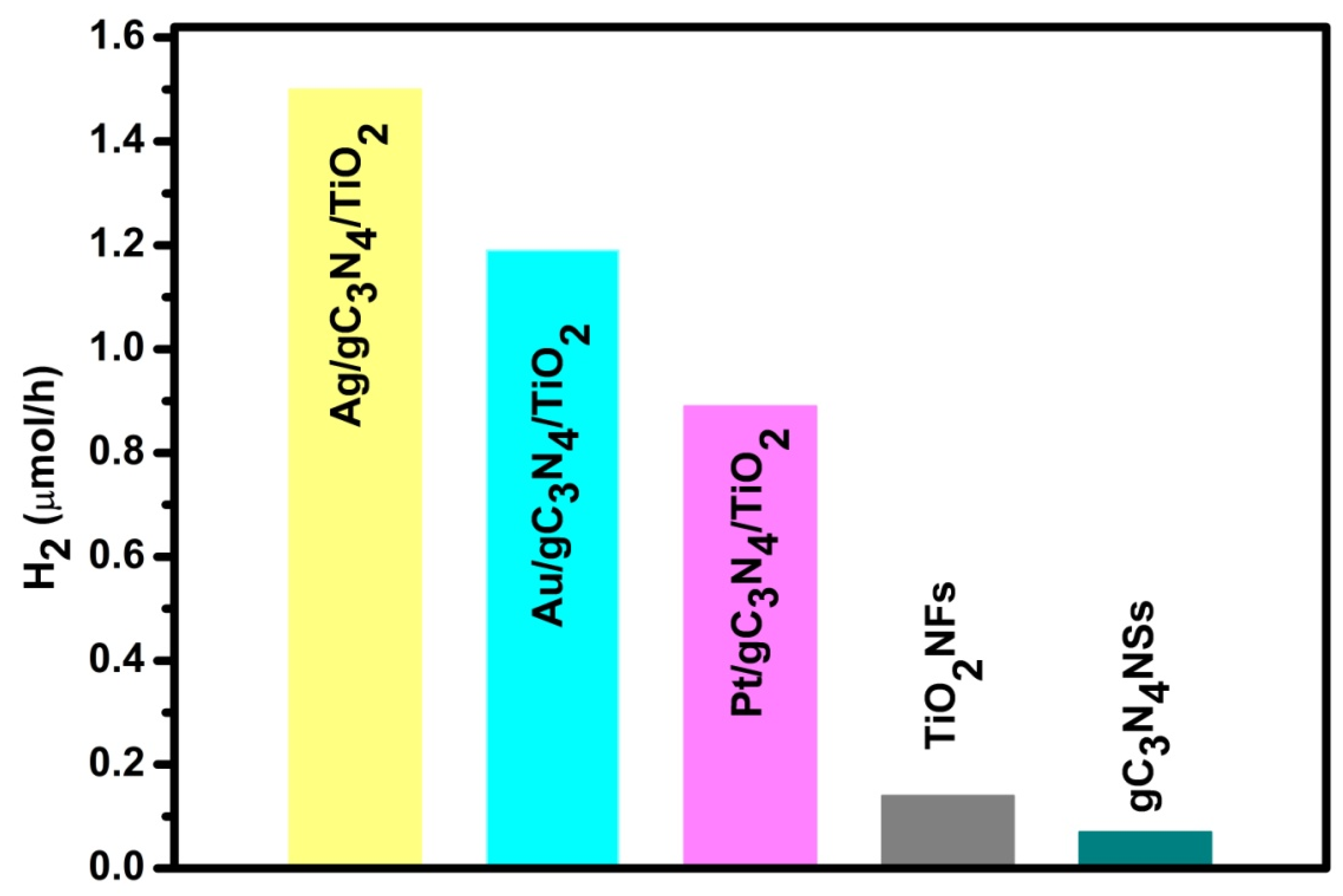
5. Plasmonic Metal–Semiconductor Heterostructure for Photocatalytic Hydrogen Evolution from Ammonium Borane
6. Plasmonic Metal–Semiconductor Heterostructure for Dye Water Treatment
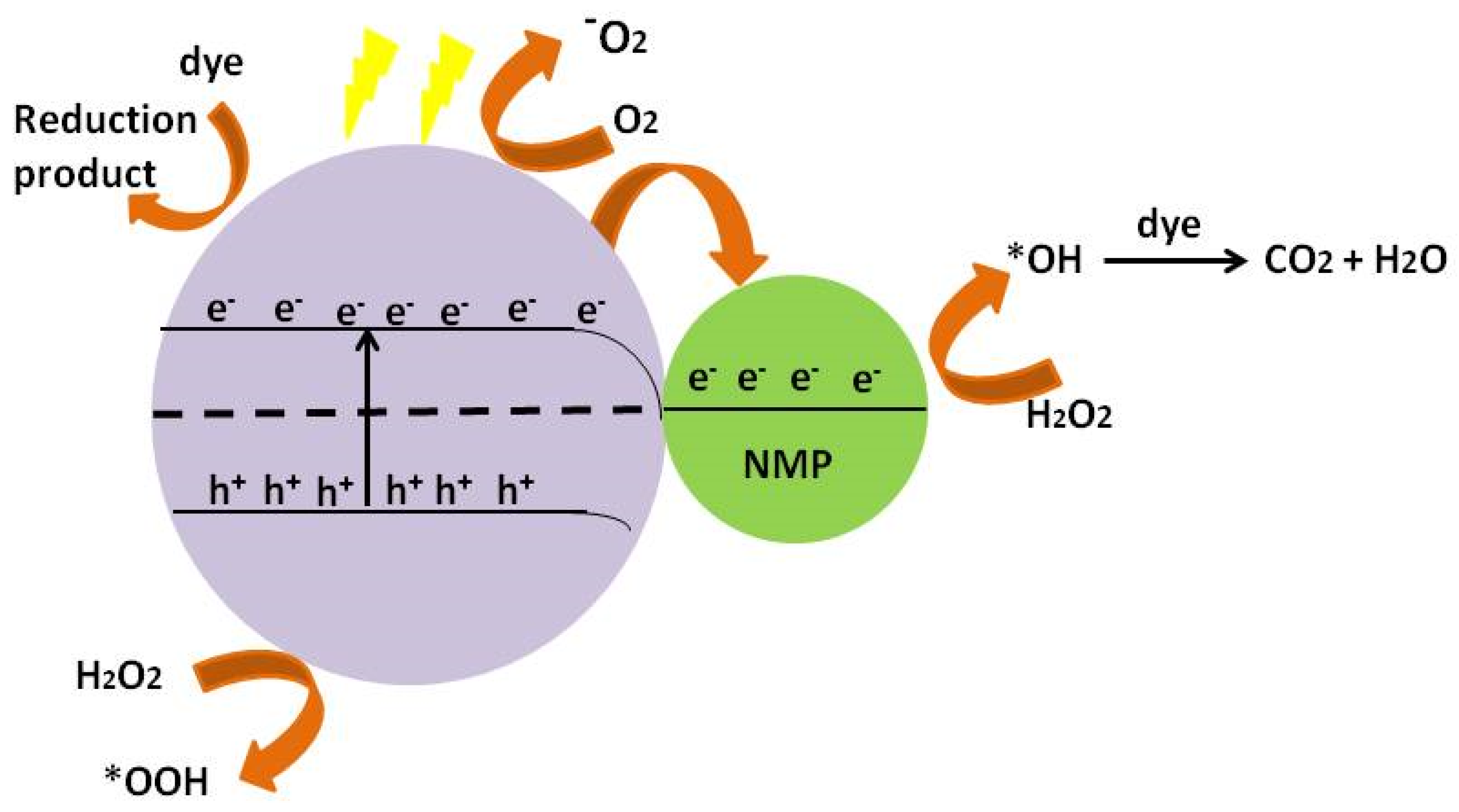
7. Proposed Mechanism
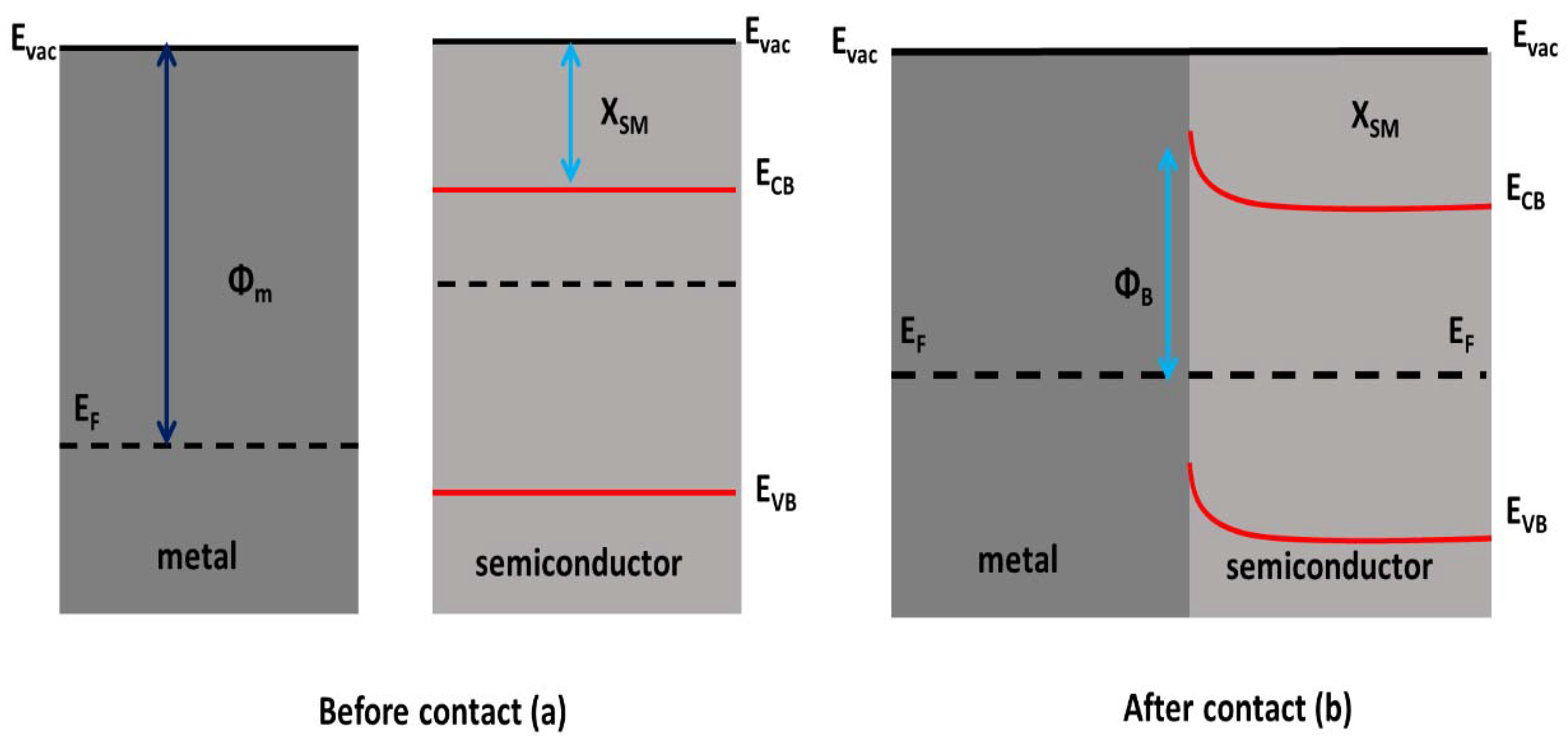
7.1. Schottky Barrier
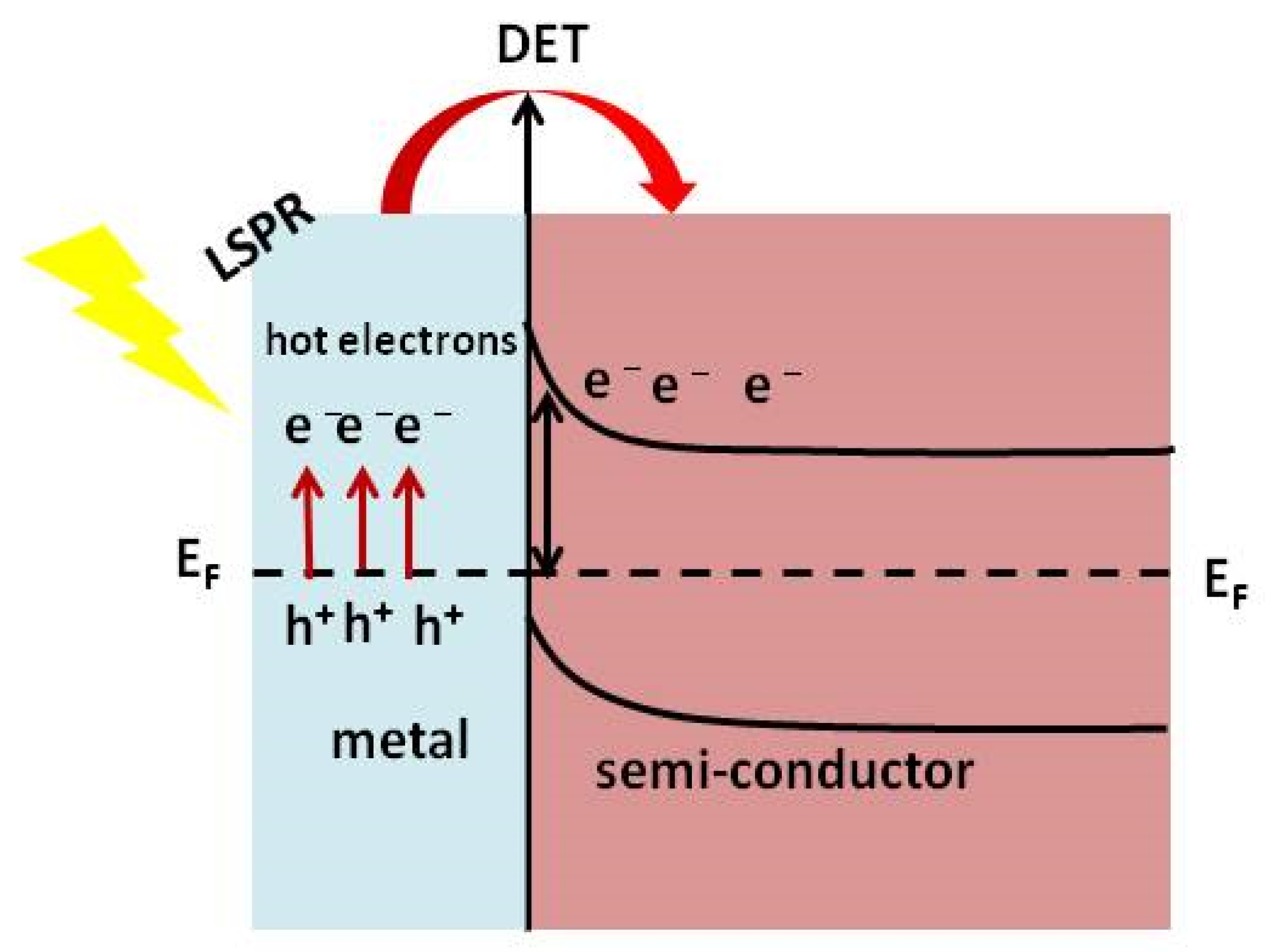
7.2. Direct Electron Transfer
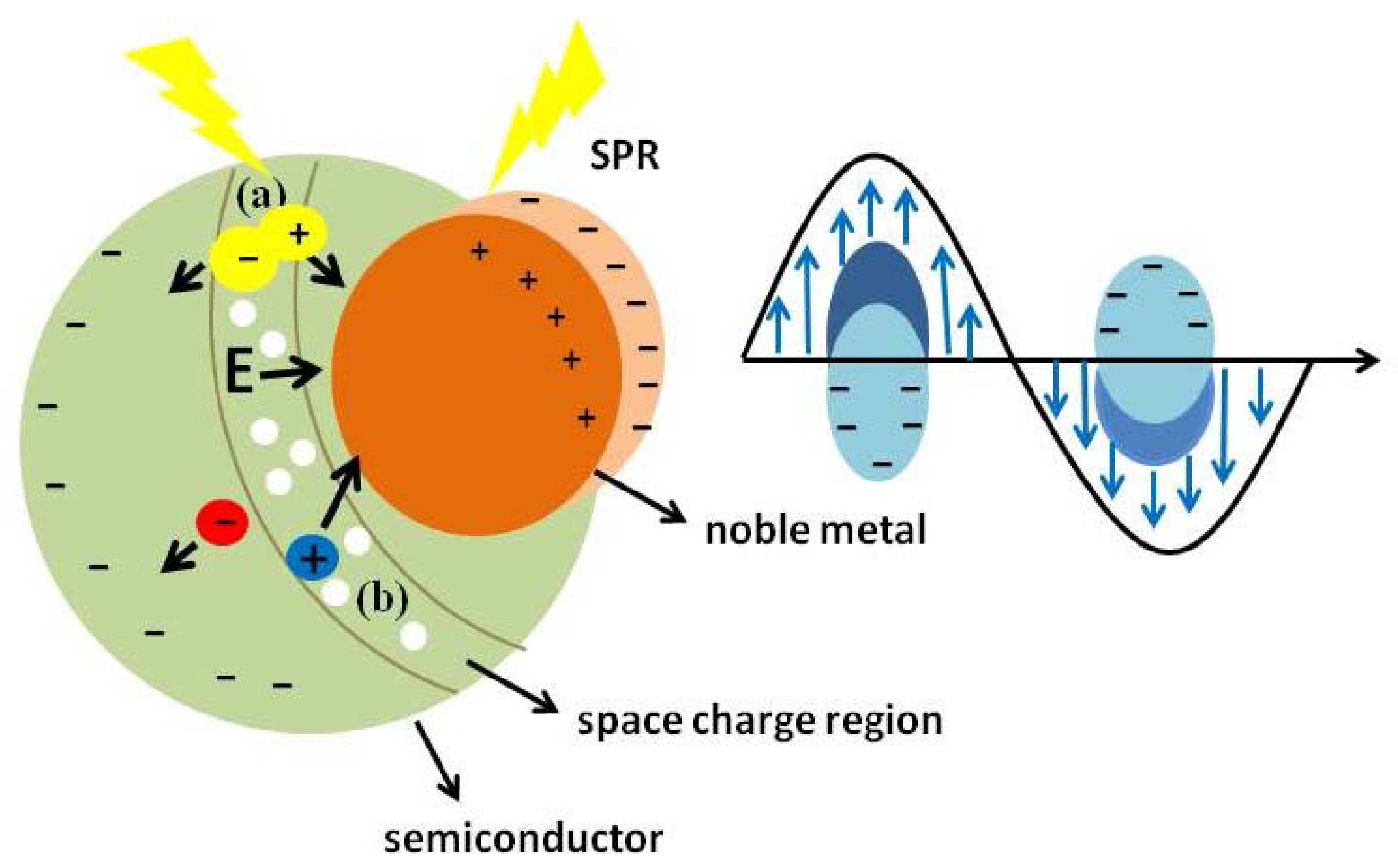
7.3. Enhanced Local Electric Field
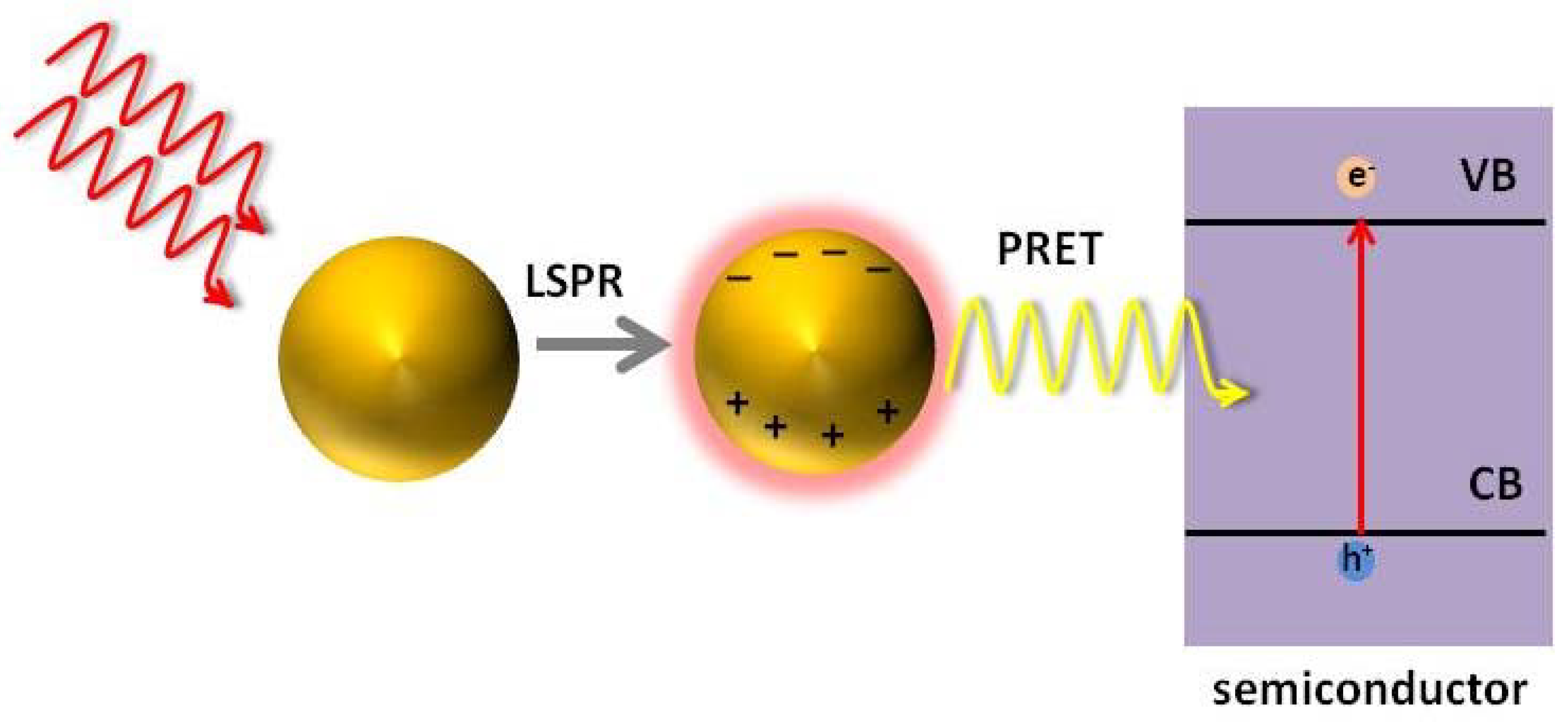
7.4. Plasmon Resonant Energy Transfer
8. Prospects and Future
8.1. Plasmonics as Energy Inputs
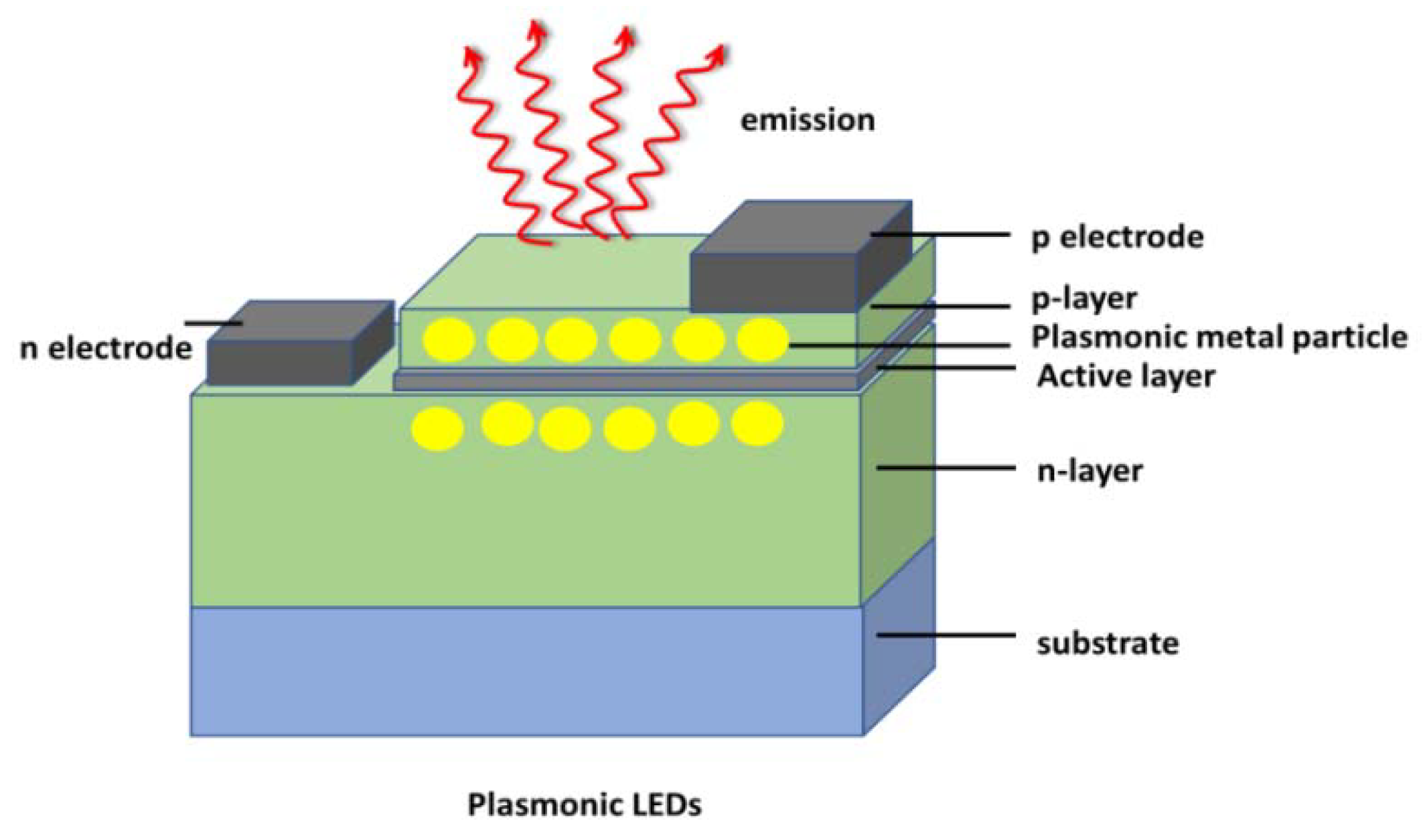
8.2. Plasmonic LED and Quantum Dots
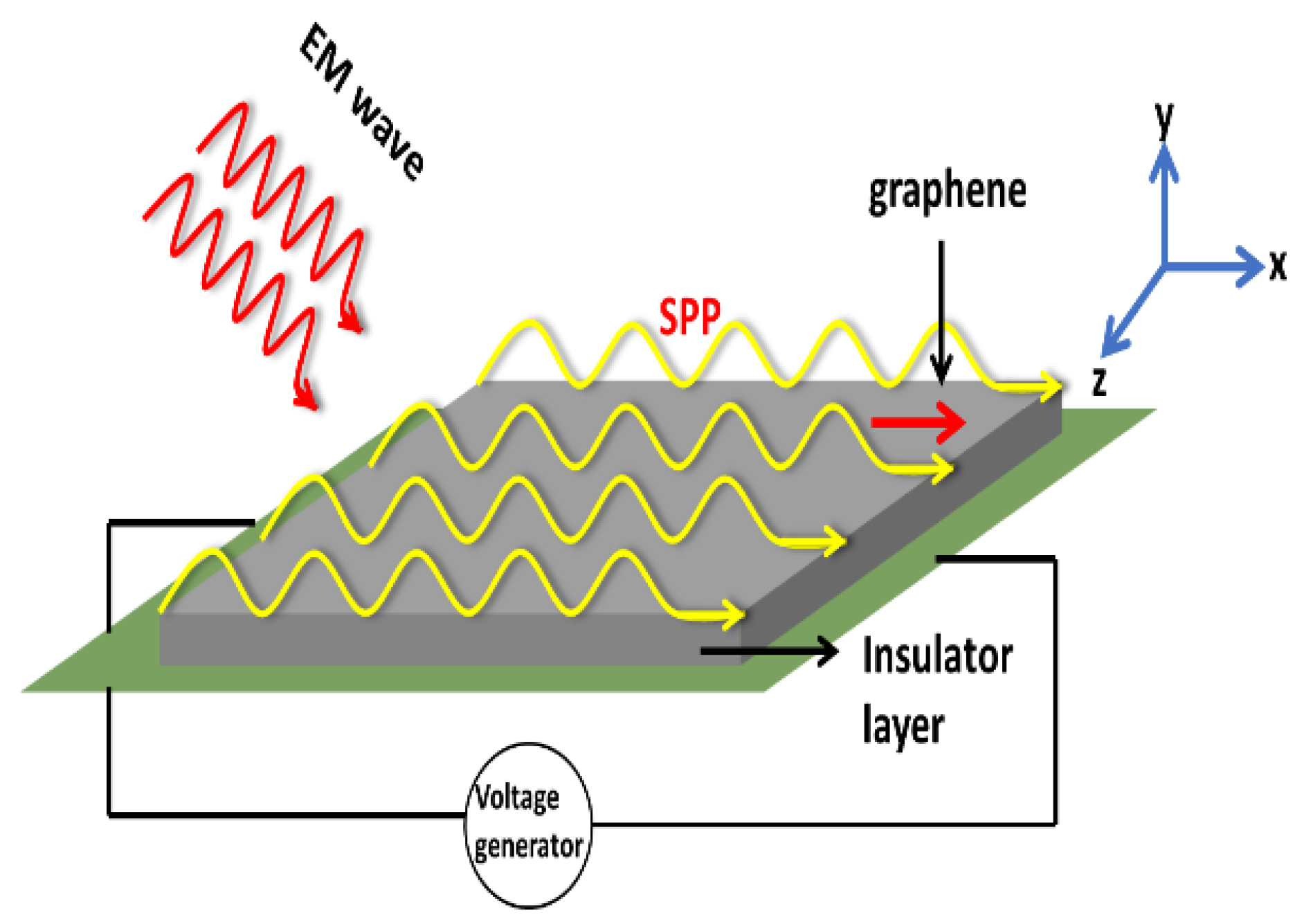
8.3. Communication with Plasmonics
8.4. Plasmonic Nanoparticles in the Treatment of Cancer
8.5. Plasmonic in Desalination of Water
8.6. Plasmonic Invisible Photodetector
9. Limitations and Challenges
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leitao, E.M.; Jurca, T.; Manners, I. Catalysis in service of main group chemistry offers a versatile approach to p-block molecules and Materials. Nat. Chem. 2013, 5, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Eberle, U.; Felderhoff, M.; Schüth, F. Hydrogen Storage Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Chem. Rev. 2004, 104, 1283–1315. [Google Scholar] [CrossRef]
- Ramachandran, R.A.M. An overview of industrial uses of hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Chen, P. Amides and Borohydrides for High-Capacity Solid-State Hydrogen Storage—Materials Design and Kinetic Improvements. MRS Bull. 2013, 38, 480–487. [Google Scholar] [CrossRef]
- Akbayrak, S.; Ozkar, S. ScienceDirect Ammonia Borane as Hydrogen Storage Materials. Int. J. Hydrogen Energy 2018, 40, 18592–18606. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; El-Newehy, M.H.; Ahmed, M.; Kim, H.Y. Catalytic hydrolysis of ammonia borane for hydrogen generation using Cu(0) nanoparticles supported on TiO2 nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 194–201. [Google Scholar] [CrossRef]
- Denney, M.C.; Pons, V.; Hebden, T.J.; Heinekey, D.M.; Goldberg, K.I. Efficient Catalysis of Ammonia Borane Dehydrogenation. J. Am. Chem. Soc. 2006, 128, 12048–12049. [Google Scholar] [CrossRef]
- Stephens, F.H.; Baker, R.T.; Matus, M.H.; Grant, D.J.; Dixon, D.A. Acid Initiation of Ammonia–Borane Dehydrogenation for Hydrogen Storage. Angew. Chem. Int. Ed. 2007, 46, 746–749. [Google Scholar] [CrossRef]
- Zhang, X.; Kam, L.; Williams, T.J. Dehydrogenetion of ammonia borane through the third equivalent of hydrogen. Dalton Trans. 2016, 45, 7672–7677. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Chandra, M. A Portable Hydrogen Generation System: Catalytic Hydrolysis of Ammonia—Borane. J. Alloys Compd. 2007, 447, 729–732. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Q. Catalytic Hydrolysis of Ammonia Borane for Chemical Hydrogen Storage. Catal. Today 2011, 170, 56–63. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Indirani, M.; Jagirdar, B.R. First Row Transition Metal Ion-Assisted Ammonia—Borane Hydrolysis for Hydrogen Generation. Inorg. Chem. 2008, 47, 7424–7429. [Google Scholar] [CrossRef]
- Tian, H.; Wan, C.; Xue, X.; Hu, X.; Wang, X. Effective Electron Transfer Pathway of the Ternary TiO2/RGO/Ag Nanocomposite with Enhanced Photocatalytic Activity under Visible Light. Catalysts 2017, 7, 156. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Z.; Zhang, M.; Guo, L. Piezotronics Enhanced Photocatalytic Activities of Ag-BaTiO3 Plasmonic Photocatalysts. J. Alloys Compd. 2019, 801, 483–488. [Google Scholar] [CrossRef]
- Kisielewska, A.; Batory, D.; Spilarewicz-Stanek, K.; Jakimi, A. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials 2020, 13, 4653. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K. Applied Surface Science Novel and e Ffi Cient Synthesis of Ag-ZnO Nanoparticles for the Sunlight- Induced Photocatalytic Degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Naik, G.; Kim, J.; Kinsey, N.; Boltasseva, A. Alternative Plasmonic Materials. Adv. Mater. 2014, 4, 189–221. [Google Scholar] [CrossRef]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.; Chang, W.; Link, S.; Olson, J.; Dominguez-Medina, S. Optical Characterization of Single Plasmonic Nanoparticles. Chem. Soc. Rev. 2014, 44, 40–57. [Google Scholar] [CrossRef] [Green Version]
- Sathiyapriya, R.; Hariharan, V.; Prabakaran, K.; Durairaj, M.; Aroulmoji, V.; Sathiyapriya, R.; Hariharan, V.; Prabakaran, K.; Durairaj, M.; Ma, V.A.N.; et al. Nanotechnology in Materials and Medical Sciences to Cite This Version: HAL Id: Hal-03093587 Nanotechnology in Materials and Medical Sciences. Int. J. Adv. Sci. Eng. 2019, 5, 1077–1084. [Google Scholar] [CrossRef]
- Zhang, S.; Wong, C.L.; Zeng, S.; Bi, R.; Tai, K.; Dholakia, K.; Olivo, M. Metasurfaces for Biomedical Applications: Imaging and Sensing from a Nanophotonics Perspective. Nanophotonics 2021, 10, 259–293. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, X.; Song, J.; Yang, H. Plasmonic Gold Nanoagents for Cancer Imaging and Therapy. View 2021, 2, 20200149. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.; Park, J.; Yip, A.C.K. Photocatalysts for Degradation of Dyes in Industrial Effluents: Opportunities and Challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Lau, G.E.; Azurahanim, C.; Abdullah, C.; Amir, W.; Wan, N.; Assaw, S.; Lim, A.; Zheng, T. Eco-Friendly Photocatalysts for Degradation of Dyes. Catalysts 2020, 10, 1129. [Google Scholar] [CrossRef]
- Liao, C.; Huang, C.; Wu, J.C.S. Hydrogen Production from Semiconductor-Based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef] [Green Version]
- Kudo, A.; Kato, Ã.H.; Tsuji, I. Strategies for the Development of Visible-Light-Driven Photocatalysts for Water Splitting. Chem. Lett. 2004, 33, 1534–1539. [Google Scholar] [CrossRef]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast Plasmon-Induced Electron Transfer from Gold Nanodots into TiO2 Nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.; Tong, Y.; Zhang, J.Z.; Hsu, Y.; Li, Y. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity Across Entire UV-Visible Region for Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Metal/Semiconductor Hybrid Nanostructures for Plasmon- Enhanced Applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef]
- Zhuang, T.T. A Unique Ternary Semiconductor-(Semiconductor/Metal) Nano-Architecture for Efficient Photocatalytic Hydrogen Evolution; Springer: Singapore, 2018; pp. 53–75. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. Applied Surface Science A Review on Plasmonic Metal—TiO2 Composite for Generation, Trapping, Storing and Dynamic Vectorial Transfer of Photogenerated Electrons across the Schottky Junction in a Photocatalytic System. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Kawamura, G.; Matsuda, A. Synthesis of Plasmonic Photocatalysts for Water Splitting. Catalysts 2019, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.-Y.; Su, K.-Y.; Peng, X.-Y.; Wu, R.-J.; Chavali, M.; Chang, W.-C. Hydrogen production by photocatalytic water-splitting on Pt-doped TiO2–ZnO under visible light. J. Taiwan Inst. Chem. Eng. 2017, 70, 161–167. [Google Scholar] [CrossRef]
- Taylor, P.; Katyal, J.; Soni, R.K. Size- and Shape-Dependent Plasmonic Properties of Aluminum Nanoparticles for Nanosensing Applications. J. Mod. Opt. 2013, 60, 37–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2−xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper (I) Sulfides. J. Am. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Bunluesak, T.; Phuruangrat, A.; Thongtem, S. Visible-light-driven heterostructure Ag/Bi2WO6 nanocomposites synthesized by photodeposition method and used for photodegradation of rhodamine B dye. Res. Chem. Intermed. 2021, 47, 3079–3092. [Google Scholar] [CrossRef]
- Jo, S.; Verma, P.; Kuwahara, Y.; Mori, k.; Choi, W.; Yamashita, H. Enhanced hydrogen production from ammonia borane using controlled plasmonic performance of Au nanoparticles deposited on TiO2. J. Mater. Com. 2017, 5, 21883–21892. [Google Scholar] [CrossRef]
- Simagina, V.; Komova, O.; Ozerova, A.; Netskina, O.; Odegova, G.; Kayl, N.; Filippov, T. TiO2-based photocatalysts for controllable hydrogen evolution from ammonia borane. Catal. Today 2020, 379, 149–158. [Google Scholar] [CrossRef]
- Yin, H.; Kuwahara, Y.; Mori, K.; Cheng, H.; Wen, M.; Huo, Y.; Yamashita, H. Localized Surface Plasmon Resonances in Plasmonic Molybdenum Tungsten Oxide Hybrid for Visible-Light-Enhanced Catalytic Reaction. J. Phys. Chem. C 2017, 121, 23531–23540. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of Ag-Based Plasmonic Photocatalysis in Hydrogen Production from Ammonia Borane by the Assistance of Single-Site Ti-Oxide Moieties within a Silica Framework. Chem. A Eur. J. 2017, 23, 3616–3622. [Google Scholar] [CrossRef]
- Cheng, H.; Kamegawa, T.; Mori, K.; Yamashita, H. Angewandte Surfactant-Free Nonaqueous Synthesis of Plasmonic Molybdenum Oxide Nanosheets with Enhanced Catalytic Activity for Hydrogen Generation from Ammonia Borane under Visible Light. Angew. Chem. 2014, 126, 2954–2958. [Google Scholar] [CrossRef]
- Online, V.A.; Friedrich, M.; Kempe, R. Mesoporous silica nanosphere supported platinum nanoparticles (Pt@MSN): One pot synthesis and catalytic hydrogen generation. RSC Adv. 2016, 6, 10438–10441. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Chen, C.J.; Cheng, L.C.; Wu, P.C.; Cheng, B.H.; Ho, Y.Z.; Tseng, M.L.; Hsu, Y.Y.; Chan, T.S.; et al. Plasmon Inducing Effects for Enhanced Photoelectrochemical Water Splitting: X-Ray Absorption Approach to Electronic Structures. ACS Nano 2012, 6, 7362–7372. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Yang, F.; Li, Z.; Zeng, J.; Zhang, X.; Chen, Z.; Ziang, J.; Tan, Y.; Peng, R.; He, H.; et al. Applied Surface Science Mechanisms in Photoluminescence Enhancement of ZnO Nanorod Arrays by the Localized Surface Plasmons of Ag Nanoparticles. Appl. Surf. Sci. 2012, 258, 8548–8551. [Google Scholar] [CrossRef]
- Mahanti, M.; Basak, D. Highly Enhanced UV Emission Due to Surface Plasmon Resonance in Ag—ZnO Nanorods. Chem. Phys. Lett. 2012, 542, 110–116. [Google Scholar] [CrossRef]
- Wu, X.-F.; Song, H.-Y.; Yoon, J.-M.; Yu, Y.-T.; Chen, Y.-F. Synthesis of Core−Shell Au@TiO2 Nanoparticles with Truncated Wedge-Shaped Morphology and Their Photocatalytic Properties. Langmuir 2009, 25, 6438–6447. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.; The, A.; Au, M.T.M. Synthesis of M@TiO2 (M = Au, Pd, Pt) Core À Shell Nanocomposites with Tunable Photoreactivity. J. Phys. Chem. C 2011, 2, 9136–9145. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Li, C. Multifunctional Fe3O4@Ag/SiO2/Au Core–Shell Microspheres as a Novel SERS-Activity Label via Long-Range Plasmon Coupling. Langmuir 2012, 29, 690–695. [Google Scholar] [CrossRef]
- Zhang, Q.; Lima, D.Q.; Lee, I.; Zaera, F.; Chi, M.; Yin, Y. A Highly Active Titanium Dioxide Based Visible-Light Photocatalyst with Nonmetal Doping and Plasmonic Metal Decoration. Angew. Chem. Int. Ed. 2011, 50, 7088–7092. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, H.; Zhang, T.; Zhang, Y.; Shao, Y. Preparation of SiO2@Au@TiO2 core–shell nanostructures and their photocatalytic activities under visible light irradiation. Chem. Eng. J. 2013, 226, 209–216. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Chen, D.; Meng, X.; Liu, T.; Fu, C.; Hao, N.; Zhang, Y.; Wu, X.; Ren, J.; et al. Multifunctional Fe3O4@P(St/MAA)@Chitosan@Au Core/Shell Nanoparticles for Dual Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2013, 5, 4966–4971. [Google Scholar] [CrossRef]
- Albella, P.; Garcia-Cueto, B.; Gonz, F.; Moreno, F.; Wu, P.C.; Kim, T.; Brown, A.; Yang, Y.; Everitt, H.O.; Videen, G. Shape Matters: Plasmonic Nanoparticle Shape Enhances Interaction. Nano Lett. 2011, 11, 3531–3537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Govorov, A.O. Optical Generation of Hot Plasmonic Carriers in Metal Nanocrystals: The Effects of Shape and Field Enhancement. J. phys. Chem. C 2014, 118, 7606–7614. [Google Scholar] [CrossRef]
- Wei, X.; Shao, C.; Li, X.; Lu, N.; Wang, K.; Zhang, Z.; Liu, Y. Facile in situ synthesis of plasmonic nanoparticles-decorated g-C3N4/TiO2 heterojunction nanofibers and comparison study of their photosynergistic effects for efficient photocatalytic H2evolution. Nanoscale 2016, 8, 11034–11043. [Google Scholar] [CrossRef] [PubMed]
- Varapragasam, S.J.P.; Mia, S.; Wieting, C.; Balasanthiran, C.; Hossan, Y.; Baride, A.; Rioux, R.M.; Hoefelmeyer, J.D. Ag–TiO2 Hybrid Nanocrystal Photocatalyst: Hydrogen Evolution under UV Irradiation but Not under Visible-Light Irradiation. ACS Appl. Energy Mater. 2019, 2, 8274–8282. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Zhao, J.; Osterloh, F.E. Photochemical Charge Separation in Nanocrystal Photocatalyst Films: Insights from Surface Photovoltage Spectroscopy. J. Phys. Chem. Lett. 2014, 5, 782–786. [Google Scholar] [CrossRef]
- Commentary, G. Boosting the E Ffi Ciency of Suspended Photocatalysts for Overall Water Splitting. J. Phys. Chem. Lett. 2014, 5, 2510–2511. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Q.; Su, Z.; Zhao, Z.; Wang, Y.; Li, Y.; Lu, X.; Wei, D.; Feng, G.; Yu, Q.; et al. Efficient Solar Water-Splitting Using a Nanocrystalline CoO Photocatalyst. Nat. Nanotechnol. 2013, 9, 69–73. [Google Scholar] [CrossRef]
- Frueh, S.; Kellett, R.; Mallery, C.; Molter, T.; Willis, W.S.; King, C.; Suib, S.L. Pyrolytic Decomposition of Ammonia Borane to Boron Nitride. Chem. Phys. Chem. 2011, 11, 783–792. [Google Scholar] [CrossRef]
- Graham, T.W.; Tsang, C.; Chen, X.; Guo, R.; Jia, W.; Lu, S.; Sui-Seng, C.; Ewart, C.B.; Lough, A.; Amoroso, D.; et al. Catalytic Solvolysis of Ammonia Borane. Angew. Chem. Int. Ed. 2010, 49, 8708–8711. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Duchene, J.S.; Sweeny, B.C.; Johnston-Peck, A.C.; Su, D.; Stach, E.A.; Wei, W.D. Prolonged Hot Electron Dynamics in Plasmonic-Metal/Semiconductor Heterostructures with Implications for Solar Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 7887–7891. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, Y.; Gautam, A.; Li, Y.; Amal, R. Exploring the Origin of Enhanced Activity and Reaction Pathway for Photocatalytic H2 Production on Au/B-TiO2 Catalysts. ACS Catal. 2014, 4, 1451–1457. [Google Scholar] [CrossRef]
- Xing, M.; Yang, B.; Yu, H.; Tian, B.; Bagwasi, S.; Zhang, J.; Gong, X. Enhanced Photocatalysis by Au Nanoparticle Loading on TiO2 Single-Crystal (001) and (110) Facets. J. Phys. Chem. Lett. 2013, 4, 3910–3917. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting E Ffi Cient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Sheldon, M.T.; Trudeau, P.; Mokari, T.; Wang, L.; Alivisatos, A.P. Enhanced Semiconductor Nanocrystal Conductance via Solution Grown Contacts. Nano Lett. 2009, 9, 3676–3682. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.W.; Han, R.; Dong, B.; Vela, J.; Fang, N. Super-Resolution Mapping of Photogenerated Electron and Hole Separation in Single Metal − Semiconductor Nanocatalysts. J. Am. Chem. Soc. 2014, 136, 1398–1408. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Shevchenko, E.V.; Talapin, D.V. Au-PbS Core-Shell Nanocrystals: Plasmonic Absorption Enhancement and Electrical Doping via Intra-Particle Charge Transfer. J. Am. Chem. Soc. 2008, 130, 9673–9675. [Google Scholar] [CrossRef]
- Qi, X.; Li, X.; Chen, B.; Lu, H.; Wang, L.; He, G. Highly Active Nanoreactors: Patchlike or Thick Ni Coating on Pt Nanoparticles Based on Con Fi Ned Catalysis. ACS Appl. Mater. Interface 2016, 8, 1922–1928. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Fang, Y.; Liu, B.; Huang, J.; Miao, F.; Bao, Y.; Dong, B. Applied Catalysis B: Environmental IR-Driven Strong Plasmonic-Coupling on Ag Nanorices/W18O49 Nanowires Heterostructures for Photo/Thermal Synergistic Enhancement of H2 Evolution from Ammonia Borane. Appl. Catal. B Environ. 2019, 252, 164–173. [Google Scholar] [CrossRef]
- Liu, P.; Wen, M.; Tan, C.; Navlani-García, M.; Kuwahara, Y.; Mori, K.; Yamashita, H.; Chen, L. Surface plasmon resonance enhancement of production of H2 from ammonia borane solution with tunable Cu2−xS nanowired decorated by Pd nanoparticles. Nano Energy 2017, 31, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Kuwahara, Y.; Mori, K.; Yamashita, H. Plasmonic metal MoxW1−xO3−y for visible-light-enhanced H2 production from ammonia borane. J. Mat. Chem. A 2018, 6, 10932–10938. [Google Scholar] [CrossRef]
- Kamegawa, T.; Nakaue, T. Complete hydrogen release from aqueous ammonia borane over a platinum loaded titanium dioxide photocatalyst. Chem. Commun. 2015, 51, 16802–16805. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M.; Moaaed, M.; Taha, A.; Nassar, M.M.; Mahmoud, M.S. Super Effective Zn-Fe-Doped TiO2 Nanofibers as Photocatalyst for Ammonia Borane Hydrolysis. Int. J. Green Energy 2016, 13, 642–649. [Google Scholar] [CrossRef]
- Nitride, C.; Zhang, M.; Xiao, X.; Wu, Y.; An, Y.; Xu, L.; Wan, C. Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite. Catalysts 2019, 9, 1009. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Cai, W.; Wang, J.; Liu, H.; Chen, W. Gold nanorods@ metal organic framework core shell nanostructure as contrast agent for photoacoustic imagingand its biocompatibility. J. Alloys Compd. 2018, 748, 193–198. [Google Scholar] [CrossRef]
- Lee, Y.; Schade, N.B.; Sun, L.; Fan, J.A.; Bae, D.R.; Mariscal, M.M.; Lee, G.; Capasso, F.; Sacanna, S.; Manoharan, V.N.; et al. Ultrasmooth, Highly Spherical Monocrystalline Gold Particles for Precision Plasmonics. ACS Nano 2013, 7, 11064–11070. [Google Scholar] [CrossRef]
- Tan, T.; Ngoc, L.; Ngoc, H.; Thi, T.; Luong, K.; Ngoc, T. Encapsulating Gold Nanoparticles in Zeolitic Imidazolate Framework Crystal for Novel Optical Response. Polyhedron 2018, 148, 171–177. [Google Scholar] [CrossRef]
- Shang, W.; Zeng, C.; Du, Y.; Hui, H.; Liang, X.; Chi, C.; Wang, K.; Wang, Z.; Tian, J. Core—Shell Gold Nanorod @ Metal—Organic Framework Nanoprobes for Multimodality Diagnosis of Glioma. Adv. Mater. 2017, 29, 1604381. [Google Scholar] [CrossRef]
- Bavykin, B.D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Joaquin, J.; Zhao, R. Photocatalytic Purification of Volatile Organic Compounds in Indoor Air: A Literature Review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H.; Light, V. Ag@AgCl: A Highly Efficient and Stable Photocatalyst Active Under. Angew. Chem. Int. Ed. 2008, 41, 7931–7933. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of Highly Efficient Ag@AgCl Plasmonic Photocatalysts with various structure. Chem. Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Tian, B.; Zeng, C.; Li, T.; Wang, T.; Zhang, J. Ecofriendly Synthesis and Photocatalytic Activity of Uniform Cubic Ag@AgCl Plasmonic Photocatalyst. J. Phys. Chem. C 2012, 117, 213–220. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Porous AgCl/Ag Nanocomposites with Enhanced Visible Light Photocatalytic Properties. J. Phys. Chem. C 2010, 114, 3175–3179. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, B.; Wang, P.; Wang, Z.; Qin, X.; Zhang, X.; Cheng, H. The Synthesis of the Near-Spherical AgCl Crystal for Visible Light Photocatalytic Applications. Dalton Trans. 2011, 40, 4104–4110. [Google Scholar] [CrossRef]
- Links, D.A. One-Step Synthesis of AgCl Concave Cubes by Preferential Overgrowth along <111> and <110> Directions. Chem. Commun. 2012, 2, 3488–3490. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.; Higgins, D.; Li, H.; Wang, H.; Chen, Z. Highly Active and Durable Core–Corona Structured Bifunctional Catalyst for Rechargeable Metal–Air Battery Application. Nano Lett. 2012, 12, 1946–1952. [Google Scholar] [CrossRef]
- Zhu, M.; Cheng, P.; Liu, M. Sunlight-Driven Plasmonic Photocatalysts Based on Ag/AgCl Nanostructures Morphology Selection. J. Mater. Chem. 2011, 21, 16413–16419. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Zhu, Z. Ag/Bi2WO6 Plasmonic Composites with Enhanced Visible Photocatalytic Activity. Ceram. Int. 2014, 40, 6495–6501. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Lu, Y.; Liu, J.-K. An efficient photocatalyst for degradation of various organic dyes: Ag@Ag2MoO4–AgBr composite. J. Hazard. Mater. 2016, 307, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Begildayeva, T.; Jin, H.; Koutavarapu, R.; Yu, Y.; Choi, M.; Yong, M. Chemosphere Plasmonic ZnO/Au/g-C3N4 Nanocomposites as Solar Light Active Photocatalysts for Degradation of Organic Contaminants in Wastewater. Chemosphere 2021, 263, 128262. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Ramjan, M.M.; Suresh, P.; Muthuraj, V. Fabrication of Highly Efficient Visible Light Driven Ag/CeO2 Photocatalyst for Degradation of Organic Pollutants. J. Alloys Compd. 2016. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Li, G.; Nie, X.; Zhao, H.; Wong, P.; An, T. Synthesis and Characterization of Novel Plasmonic Ag/AgX-CNTs (X = Cl, Br, I) Nanocomposite Photocatalysts and Synergetic Degradation of Organic Pollutant under Visible Light. ACS Appl. Mater. Interfaces 2013, 5, 6959–6967. [Google Scholar] [CrossRef]
- Liu, J.Y.; Bai, Y.; Wang, P.Q. Photocatalytic Degradation of Phenol Using Au/Bi2WO6 Composite Microspheres under Visible-Light Irradiation. Micro Nano Lett. 2013, 8, 90–93. [Google Scholar] [CrossRef]
- Khanam, S. Decolourization of Rhodamine B and Methylene Blue Dyes in the Presence of Bismuth Tungstates: A Detailed Investigation on the Effect of Grain Size. Bull. Mater. Sci. 2021, 44, 2. [Google Scholar] [CrossRef]
- Khanam, S.; Rout, S.K. Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6. Chemistry 2022, 4, 22. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Plasmon-Induced Photoelectrochemistry at Metal Nanoparticles Supported on Nanoporous TiO2. Chem. Commun. 2004, 16, 1810–1811. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Transfer from Metal to Semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Boriskina, S.V.; Ghasemi, H.; Chen, G. Plasmonic Materials for Energy: From Physics to Applications. Biochem. Pharm. 2013, 16, 375–386. [Google Scholar] [CrossRef]
- Pillai, S.; Green, M.A. Solar Energy Materials & Solar Cells Plasmonics for Photovoltaic Applications. Sol. Energy Mater. Sol. Cells 2010, 94, 1481–1486. [Google Scholar] [CrossRef]
- Neumann, O.; Urban, A.S.; Day, J.; Lal, S.; Nordlander, P.; Halas, N.J. Solar Vapor Generation Enabled by Nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef]
- Fang, Z.; Zhen, Y.; Neumann, O.; Polman, A.; Garc, F.J. Evolution of Light-Induced Vapor Generation at a Liquid-Immersed Metallic Nanoparticle. Nano Lett. 2013, 13, 1736–1742. [Google Scholar] [CrossRef] [Green Version]
- Polman, A. Solar Steam Nanobubbles. ACS Nano 2013, 15–18. [Google Scholar] [CrossRef]
- Atre, A.C.; García-Etxarri, A.; Alaeian, H.; Dionne, J.A. Toward high-efficiency solar upconversion with plasmonic nanostructures. J. Opt. 2012, 14, 024008. [Google Scholar] [CrossRef]
- Garcia, G.; Runnerstrom, E.L.; Mendelsberg, R.J.; Llordes, A.; Anders, A.; Richardson, T.J.; Milliron, D.J. Dynamically Modulating the Surface Plasmon Resonance of Doped Semiconductor Nanocrystals. Nano Lett. 2011, 11, 4415–4420. [Google Scholar] [CrossRef]
- Flatte, M.E.; Kornyshev, A.A.; Urbakh, M. Electrovariable Nanoplasmonics and Self-Assembling Smart Mirrors. J. Phys. Chem. C 2010, 114, 1735–1747. [Google Scholar] [CrossRef]
- Zijlstra, P.; Chon, J.W.M.; Gu, M. Five-Dimensional Optical Recording Mediated by Surface Plasmons in Gold Nanorods. Nature 2009, 459, 410–413. [Google Scholar] [CrossRef]
- Joulain, K.; Mulet, J. Surface Electromagnetic Waves Thermally Excited: Radiative Heat Transfer, Coherence Properties and Casimir Forces Revisited in the near Field. Surf. Sci. Rep. 2005, 57, 59–112. [Google Scholar] [CrossRef] [Green Version]
- Bermel, P.; Boriskina, S.V.; Yu, Z.; Joulain, K. Control of Radiative Processes for Energy Conversion and Harvesting. Opt. Express 2015, 23, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot Electrons Do the Impossible: Plasmon-Induced Dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.H.; Aykol, M.; Valley, D.; Hou, W.; Cronin, S.B. Plasmon Resonant Enhancement of Carbon Monoxide Catalysis. Nano Lett. 2010, 10, 1314–1318. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous Catalysis Mediated by Plasmon Heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef] [Green Version]
- Chen, G. Nonlocal and Nonequilibrium Heat Conduction in the Vicinity of Nanoparticles. J. Heat. Transf. 1996, 118, 539–545. [Google Scholar] [CrossRef]
- Atanasov, P.A.; Nedyalkov, N.N.; Imamova, S.E.; Miyanishi, T.; Obara, M. Substrate Nanomodification Based on Heating and near Field Properties of Gold Nanoparticles. Int. J. Nnaoparticles 2010, 3, 206–219. [Google Scholar] [CrossRef]
- Richardson, H.H.; Hickman, Z.N.; Govorov, A.O.; Thomas, A.C.; Zhang, W.; Kordesch, M.E. Thermooptical Properties of Gold Nanoparticles Embedded in Ice: Characterization of Heat Generation and Melting. Nano Lett. 2006, 6, 783–788. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological Applications of Quantum Dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef]
- Tian, J.; Shen, T.; Liu, X.; Fei, C.; Lv, L.; Cao, G. Enhanced Performance of PbS- Quantum-Dot-Sensitized Solar Cells via Optimizing Precursor Solution and Electrolytes. Sci. Rep. 2016, 6, 23094. [Google Scholar] [CrossRef] [PubMed]
- Jornet, J.M.; Akyildiz, I.F. Graphene-Based Plasmonic Nano-Antenna for Terahertz Band Communication in Nanonetworks. IEEE J. Sel. Areas Commun. 2013, 31, 685–694. [Google Scholar] [CrossRef]
- Jornet, J.M.; Akyildiz, I.F.; York, N. Graphene-Based Plasmonic Nano-Transceiver for Terahertz Band Communication. In Proceedings of the 8th European Conference on Antennas and Propagation (EuCAP 2014), The Hague, The Netherlands, 6–11 April 2014; pp. 492–496. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Yin, W.; Zheng, X.; Zhang, X.; Yu, J.; Dong, X.; Yong, Y.; Gao, F.; Yan, L.; Gu, Z.; et al. One-pot synthesis of PEGylated plasmonic MoO3−x hollow nanospheres for photoacoustic imaging guided chemo-photothermal combinational therapy of cancer. Biomaterials 2016, 76, 11–24. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Jiang, C.; Li, Y.; Song, X.; Lu, L. Plasmonic titanium nitride nanoparticles for in vivo photoacoustic tomography imaging and photothermal cancer therapy. Biomaterials 2017, 132, 37–47. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S. 3D Self-Assembly of Aluminium Nanoparticles for Plasmon-Enhanced Solar Desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Fleury, R.; Alù, A. Cloaking and Invisibility: A Review. FERMAT 2014, 1. [Google Scholar]





| Catalysts | Source | Time | H2 (Evolution) | Ref. |
|---|---|---|---|---|
| Au/TiO2 | Visible light | 4 h | 85 μmol | [18] |
| Ag/TiO2 | LED (365 nm) | 3 h | 330 μmol | [38] |
| Ag/Ti/SBA | Visible light | 1 h | 65 μmol | [21] |
| MoxW1−xO3−y | LED (420 nm) | 1 h | 60 mol% | [39] |
| MoO3−x | LED (420 nm) | 1 h | 77 mol% | [41] |
| Cu/TiO2 | Without light | 1 h | 90 mol% | [7] |
| Pt-TiO2 | UV light | 4 h | 15 μmol | [74] |
| ZnO-Fe2O3-TiO2 | Visible light | 50 min | 2.7 equivalent of H2 | [75] |
| PtNi/g-C3N4 | Visible light | 60 min | 3 mL/min | [76] |
| PtNi@SiO2 | Visible light | 60 min | 4.9 mL/min | [41] |
| Pt@SiO2 | Visible light | 60 min | 1.1 mL/min | [41] |
| Ag/W18O49 | Visible light | 60 min | 0.18 μmol/min | [71] |
| Pd/Cu7S4 | Visible light | 240 min | 157.04 mmol/min | [72] |
| Pd/MoxW1−xO3−y | Visible light | 10 min | 6.72 mL/min | [73] |
| Catalyst | Organic Pollutant | Time | Degradation % | Reference |
|---|---|---|---|---|
| Ag@AgCl | Phenol | 50 min | 93 | [85] |
| Ag@AgCl | DCP | 60 min | 97 | [54] |
| Ag@AgCl | AO7 | 20 min | 97 | [54] |
| Ag/AgBr-CNTs | TBP | 50 min | 100 | [96] |
| AgBr | TBP | 50 min | 60 | [62] |
| CNTs | TBP | 50 min | 45 | [62] |
| Ag/AgCl-CNTs | TBP | 50 min | 39 | [62] |
| AgCl | TBP | 50 min | 26 | [62] |
| Ag/AgI-CNTs | TBP | 50 min | 42 | [62] |
| AgI | TBP | 50 min | 8 | [62] |
| Ag-BaTiO3 | RhB | 75 min | 83 | [15] |
| TiO3 | RhB | 75 min | 63 | [63] |
| Ag/Bi2WO6 | RhB | 140 min | 94.1 | [61] |
| Au/Bi2WO6 | Phenol | 60 min | 93.3 | [97] |
| Bi2WO6 | Phenol | 60 min | 65.2 | [64] |
| Bi2WO6 | RhB | 15 h | 90 | [98] |
| Bi2WO6 | MB | 8 h | 49 | [98] |
| Ag/Bi2WO6 | RhB | 10 min | 50 | [99] |
| Ag/Bi2WO6 | MB | 90 min | 68 | [99] |
| Ag@Ag2MoO4-AgBr | Bromophenol blue | 7 min | 100 | [93] |
| Ag@Ag2MoO4-AgBr | RhB | 7 min | 100 | [93] |
| Ag@Ag2MoO4-AgBr | Amino black 10b | 7 min | 100 | [93] |
| ZnO/Au15/gC3N430 | MB | 12 min | 99 | [94] |
| Ag/CeO2 | RhB | 70 min | 100 | [95] |
| Ag/CeO2 | MB | 60 min | 97 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, S.; Rout, S.K. A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal–Semiconductor Heterostructures: A Review. Chemistry 2022, 4, 454-479. https://doi.org/10.3390/chemistry4020034
Khanam S, Rout SK. A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal–Semiconductor Heterostructures: A Review. Chemistry. 2022; 4(2):454-479. https://doi.org/10.3390/chemistry4020034
Chicago/Turabian StyleKhanam, Shomaila, and Sanjeeb Kumar Rout. 2022. "A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal–Semiconductor Heterostructures: A Review" Chemistry 4, no. 2: 454-479. https://doi.org/10.3390/chemistry4020034
APA StyleKhanam, S., & Rout, S. K. (2022). A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal–Semiconductor Heterostructures: A Review. Chemistry, 4(2), 454-479. https://doi.org/10.3390/chemistry4020034







