Characterization of Biochar Produced from Greenhouse Vegetable Waste and Its Application in Agricultural Soil Amendment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatments
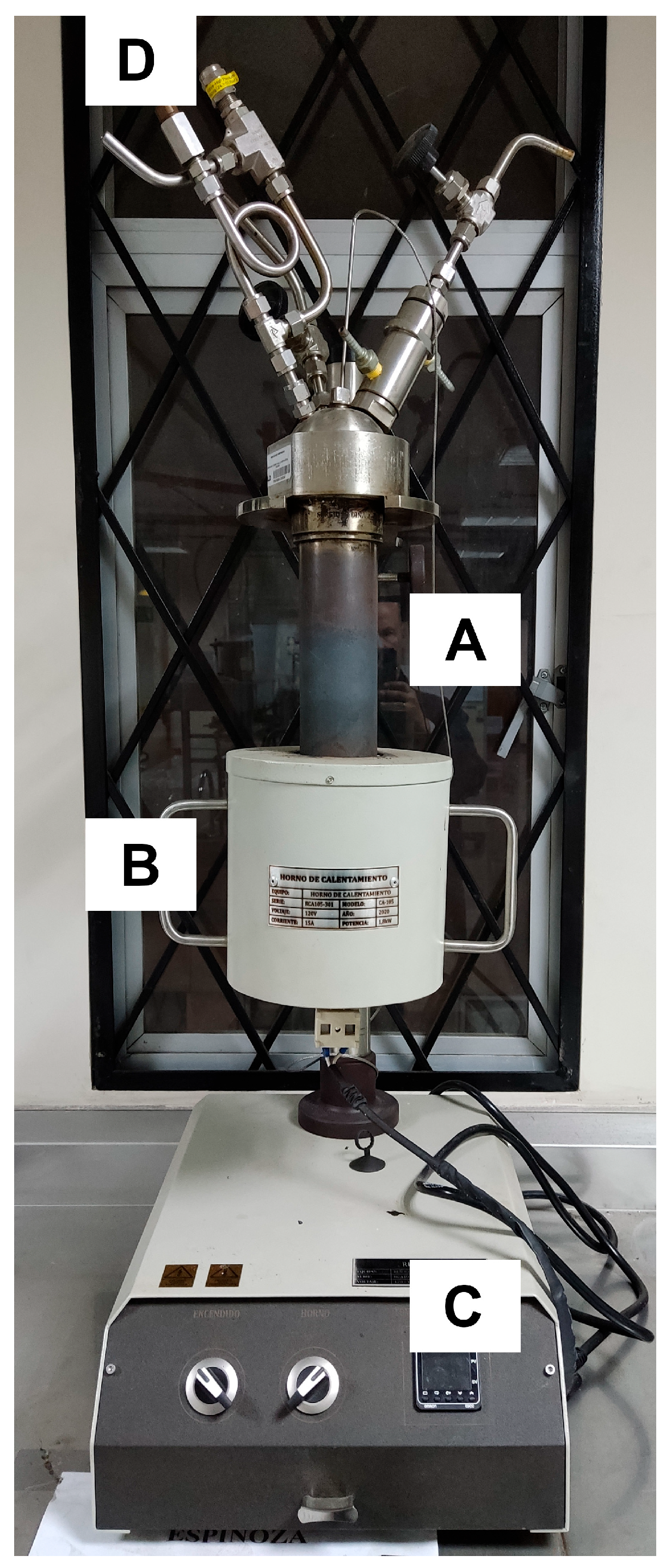
2.3. Biochar Production
2.4. Sample Characterization
2.5. Biochar Application
3. Results and Discussion
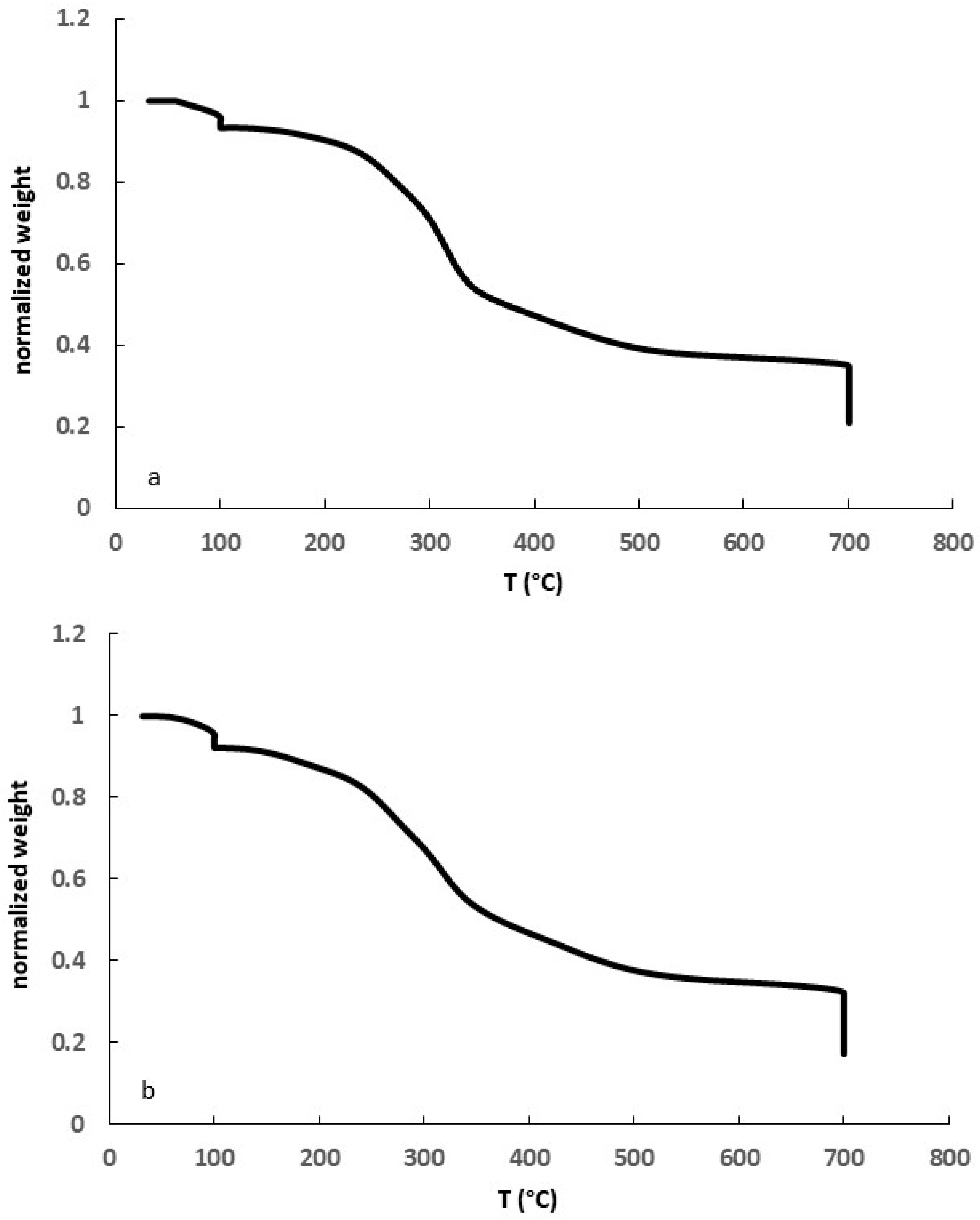
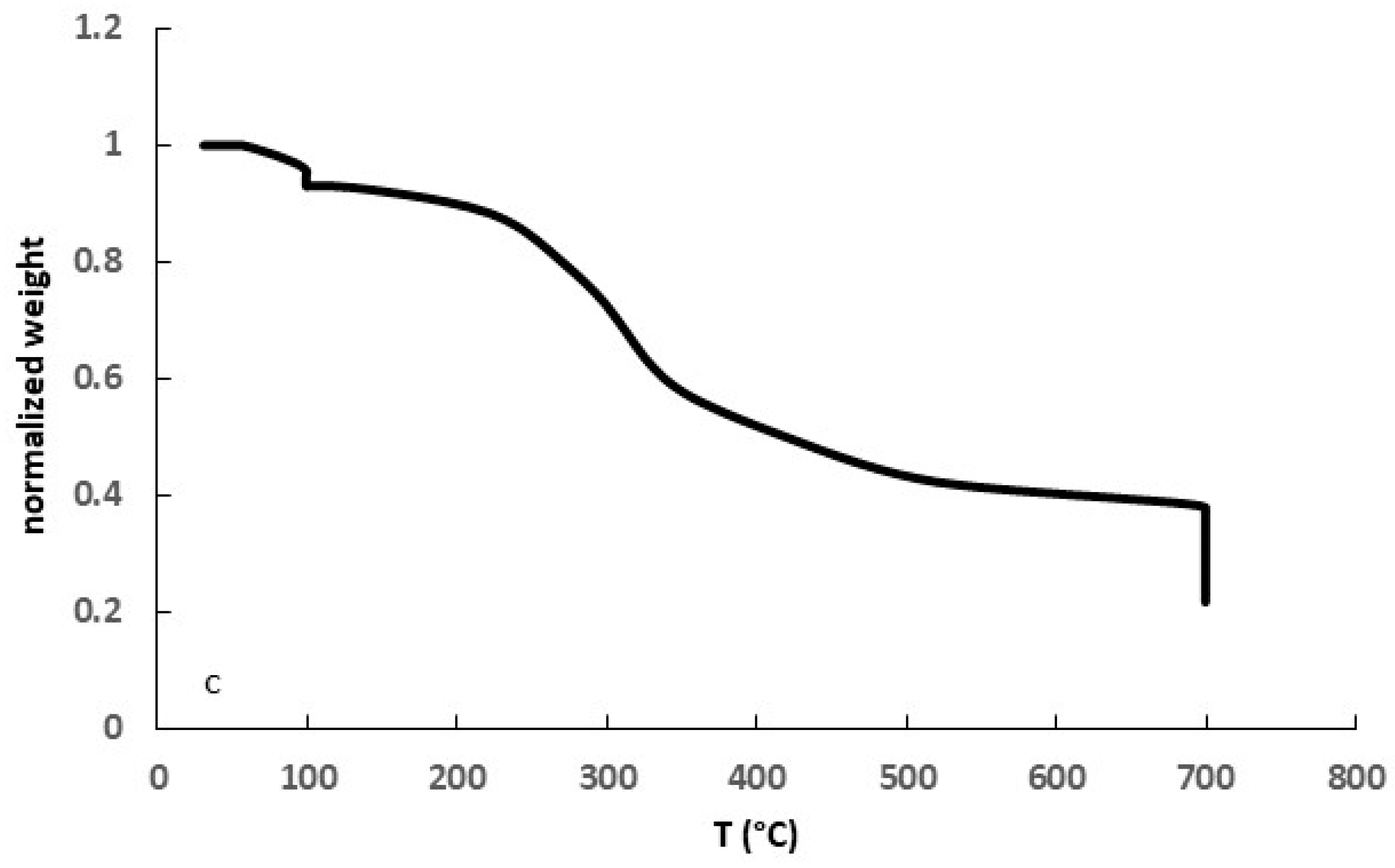
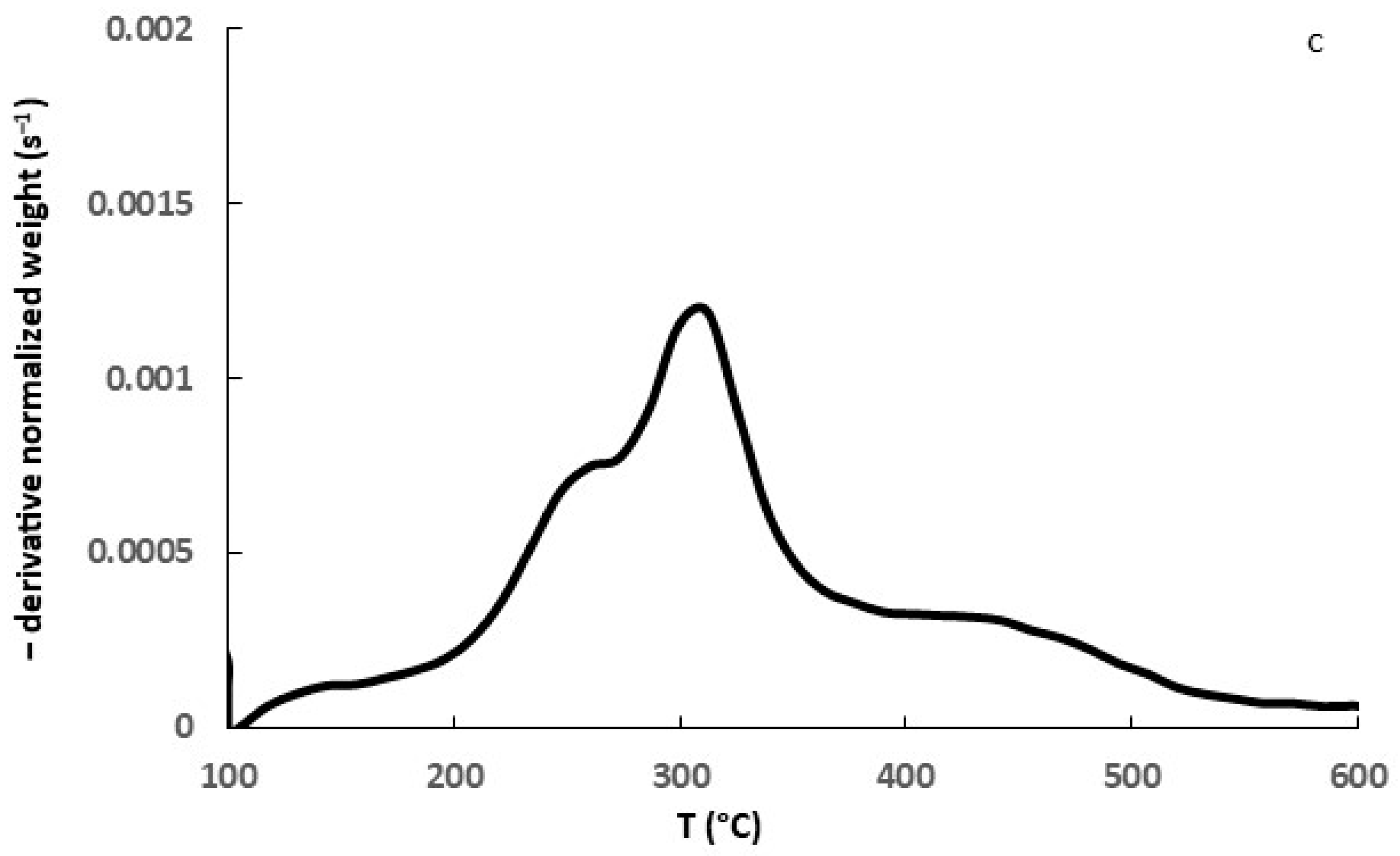
3.1. Biomass Characterization
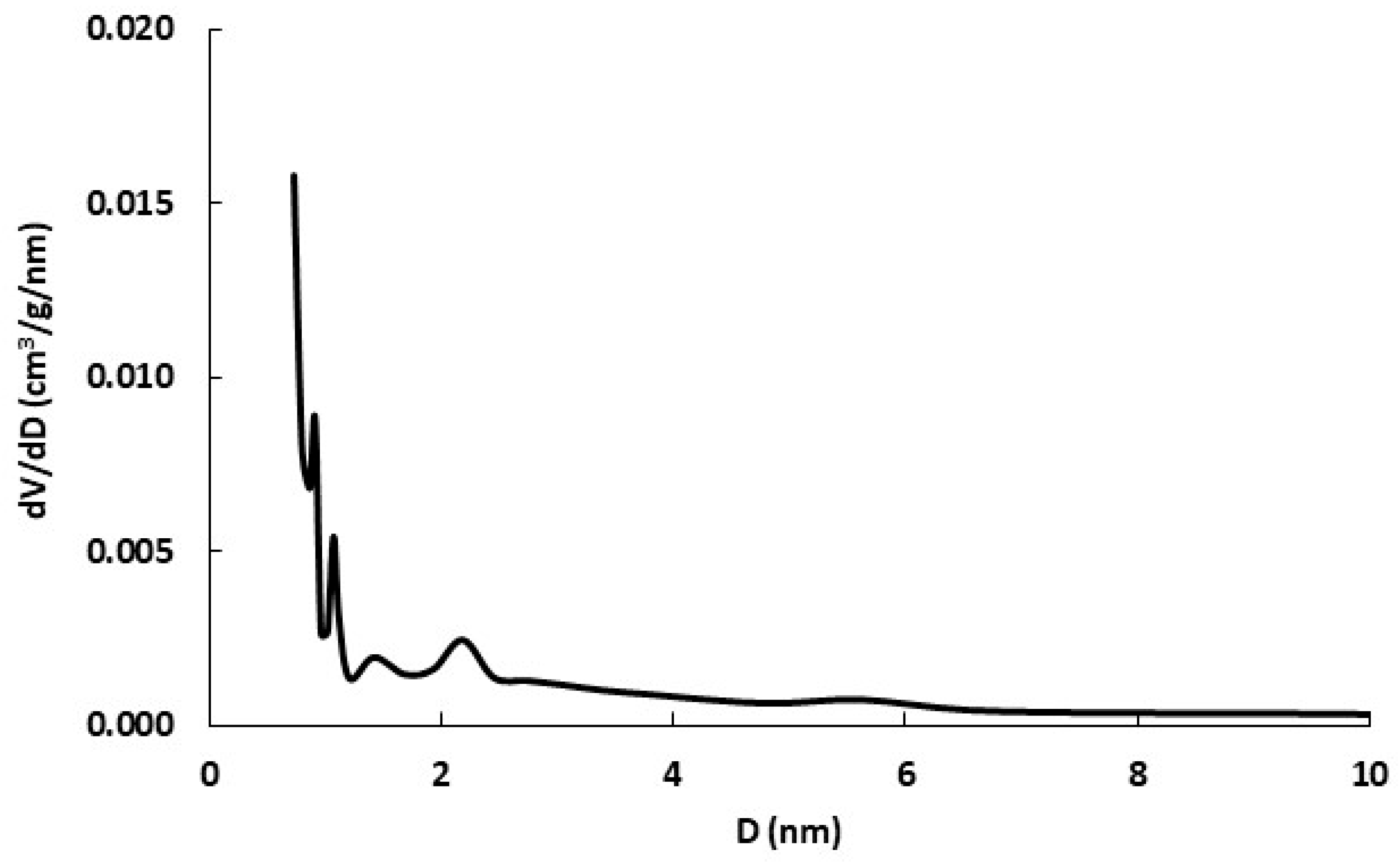
3.2. Biochar Characterization
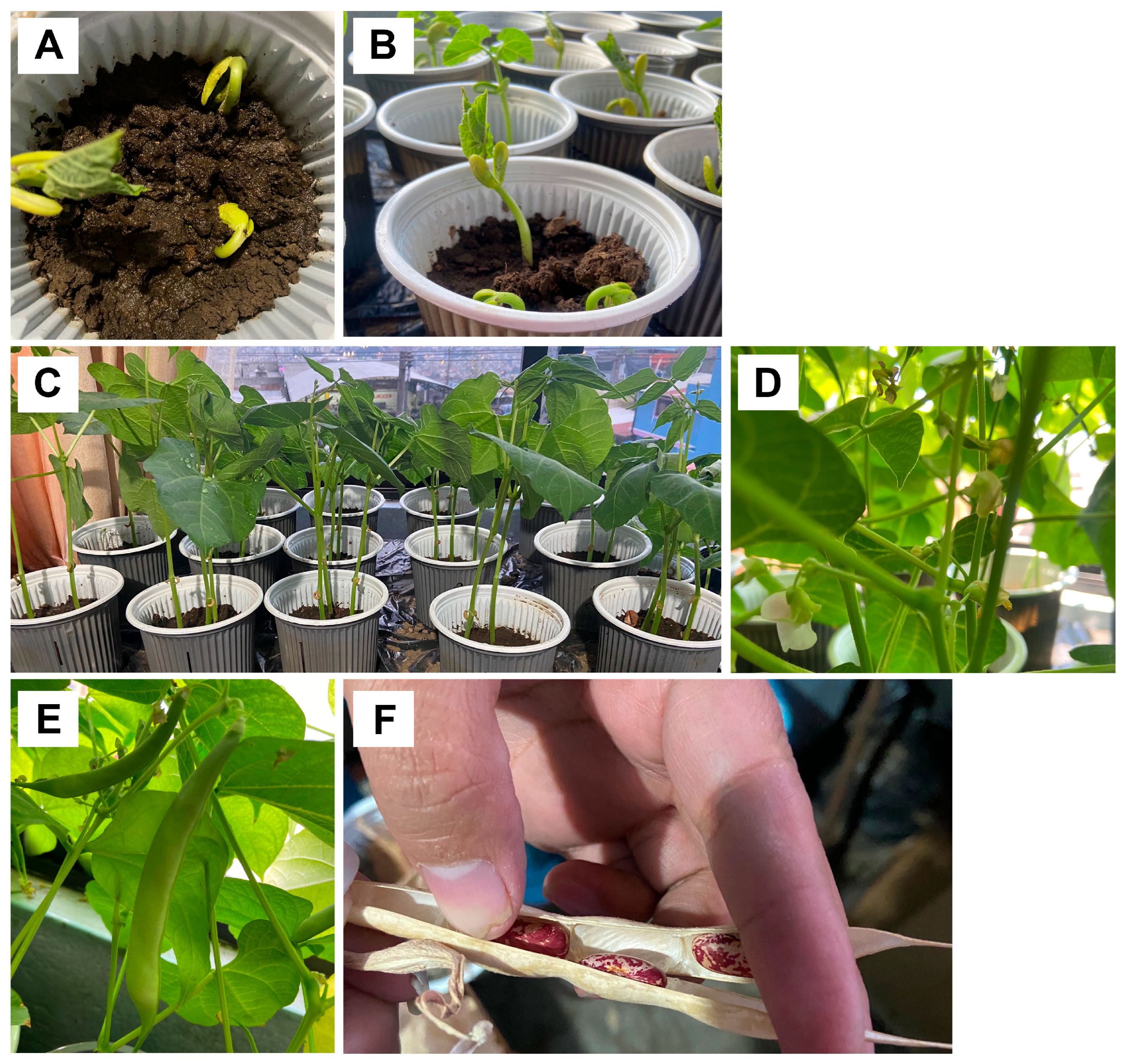
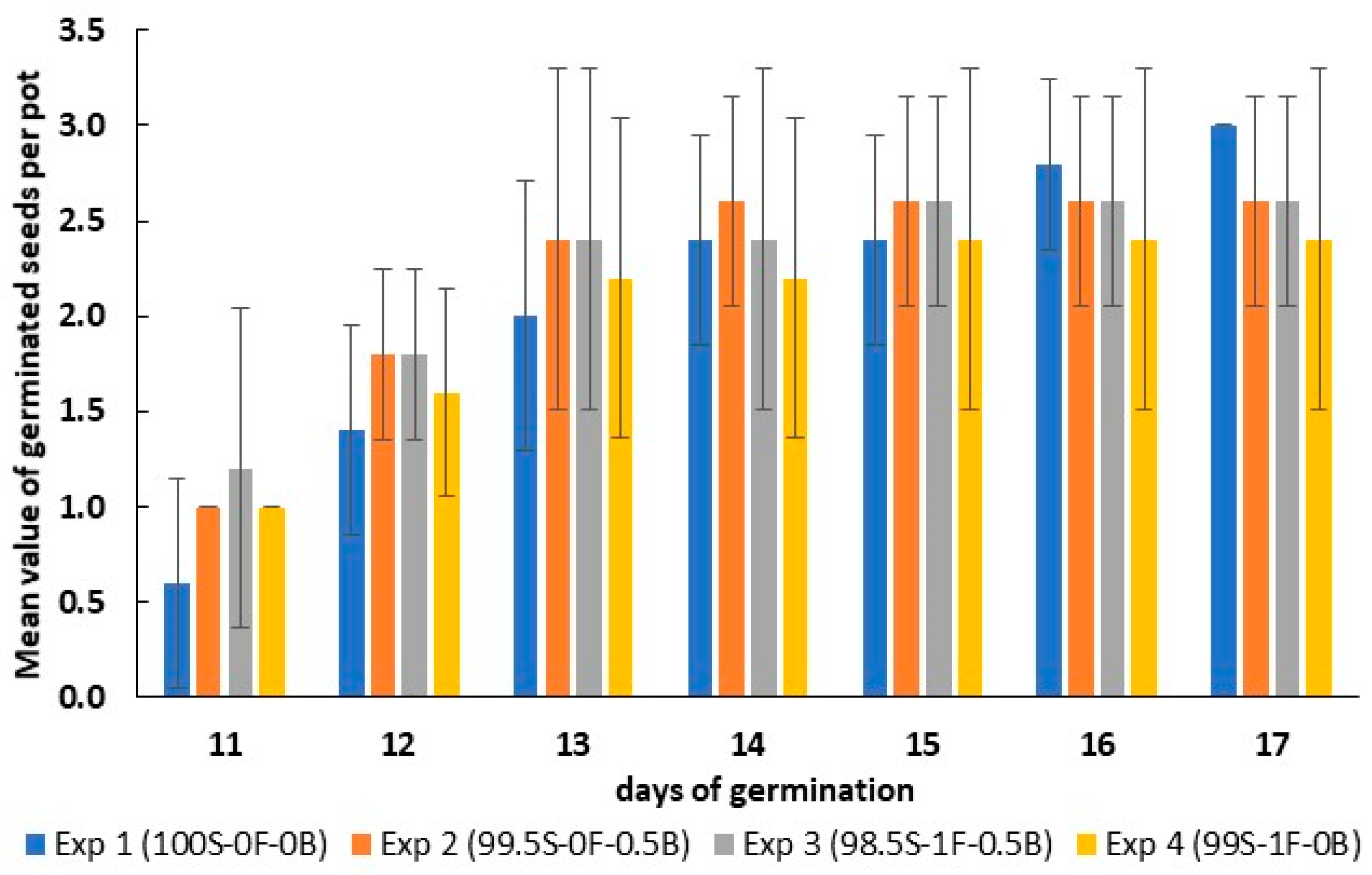
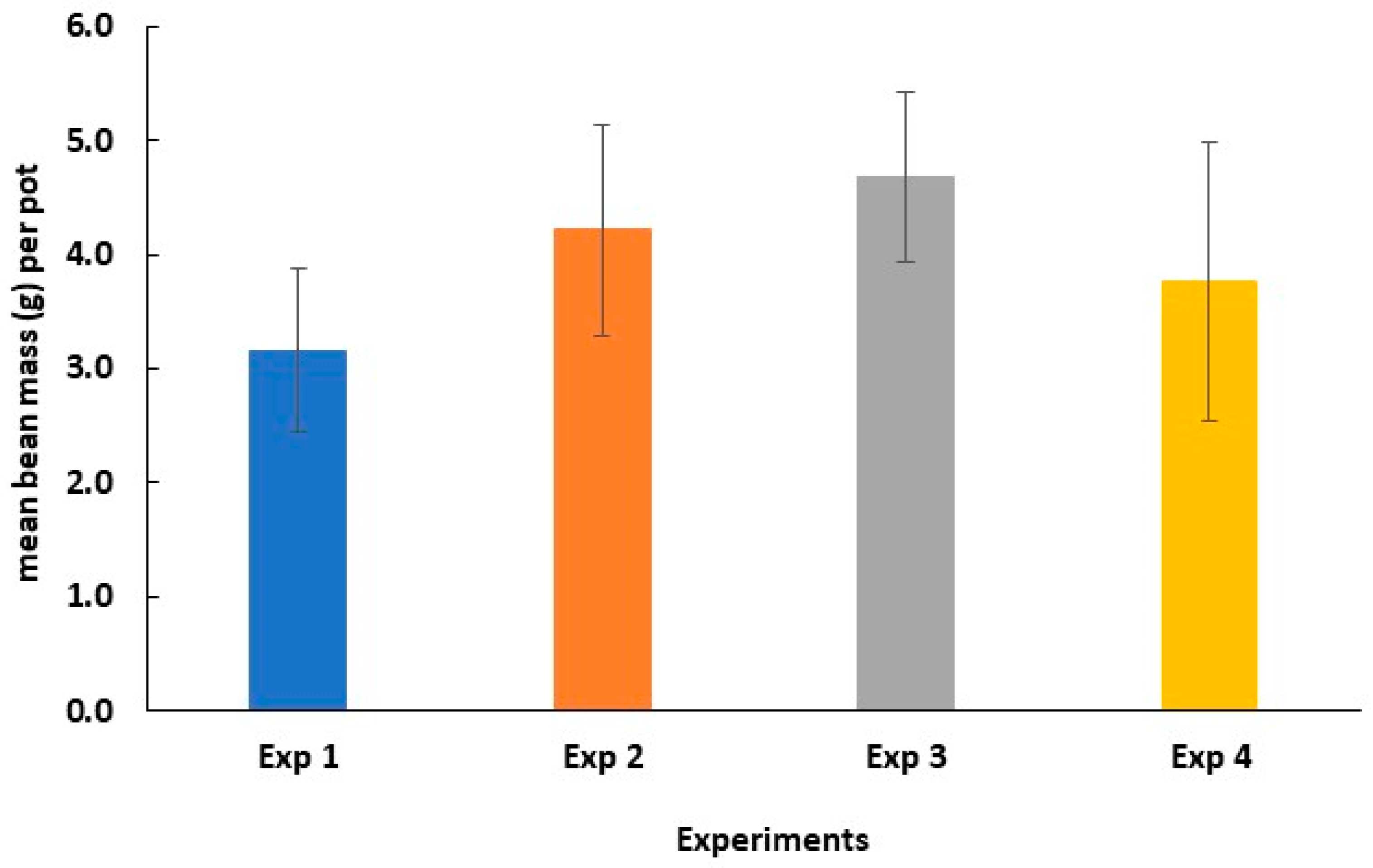
3.3. Application of Biochar to an Agricultural Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development; World Bank: Washington, DC, USA, 2018; Available online: http://hdl.handle.net/10986/30317 (accessed on 26 September 2023).
- Belhachemi, M.; Khiari, B.; Jeguirim, M.; Sepúlveda-Escribano, A. Characterization of biomass-derived chars. In Char and Carbon Materials Derived from Biomass: Production, Characterization and Applications; Jeguirim, M., Limousy, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 69–108. [Google Scholar]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Williams, P.; Besler, S. The influence of temperature and heating rate on the slow pyrolysis of biomass. Renew. Energy 1996, 7, 233–250. [Google Scholar] [CrossRef]
- Domínguez Domínguez, M.P. Pyrolysis and Gasification of Biomass: Optimization of the Energy Transformation Process Through Different Reaction Systems. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- IBI. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; Version 2.1; International Biochar Initiative: Washington, DC, USA, 2015; Available online: https://biochar-international.org/wp-content/uploads/2019/01/IBI_Biochar_Standards_V2.1_Final1.pdf (accessed on 20 February 2024).
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Glob. Change Biol. Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Mohawesh, O.; Coolong, T.; Aliedeh, M.; Qaraleh, S. Greenhouse evaluation of biochar to enhance soil properties and plant growth performance under arid environment. Bulg. J. Agric. Sci. 2018, 24, 1012–1019. [Google Scholar]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for soil amendment. In Char and Carbon Materials Derived from Biomass: Production, Characterization and Applications; Jeguirim, M., Limousy, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar]
- Chicaiza Ortiz, C.D.; Navarrete Villa, V.P.; Camacho López, C.O.; Chicaiza Ortiz, A.F. Evaluation of municipal solid waste management system of Quito—Ecuador through life cycle assessment approach. LALCA Rev. Lat. Am. Aval. Ciclo Vida 2020, 4, e45206. [Google Scholar] [CrossRef]
- Murillo, A.; Peralta, E.; Mazón, N.; Rodríguez, D.; Pinzón, J. INIAP 484. Centenario: Variedad de fréjol arbustivo con resistencia múltiple a enfermedades. Quito, Ecuador. INIAP Bol. Divulg. 2012, 421, 1–4. Available online: https://repositorio.iniap.gob.ec/handle/41000/384 (accessed on 7 February 2024).
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass proximate analysis using thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Le, P.T.T.; Boyd, C.E. Comparison of phenate and salicylate methods for determination of total ammonia nitrogen in freshwater and saline water. J. World Aquac. Soc. 2012, 43, 885–889. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Lo, S.-L. Predicting heating value of lignocellulosic biomass based on elemental analysis. Energy 2020, 191, 116501. [Google Scholar] [CrossRef]
- He, M.; Mourant, D.; Gunawan, R.; Lievens, C.; Wang, X.S.; Ling, K.; Bartle, J.; Li, C.Z. Yield and properties of bio-oil from the pyrolysis of mallee leaves in a fluidised-bed reactor. Fuel 2012, 102, 506–513. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Pütün, A.E. Preparation and characterization of pyrolytic chars from different biomass samples. J. Anal. Appl. Pyrolysis 2012, 98, 29–36. [Google Scholar] [CrossRef]
- Moreno, A.D.; Duque, A.; González, A.; Ballesteros, I.; Negro, M.J. Valorization of greenhouse horticulture waste from a biorefinery perspective. Foods 2021, 10, 814. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Lopez-Capel, E.; Peña, J.; Rieradevall, J.; Montero, J.I.; Puy, N. Technical feasibility and carbon footprint of biochar co-production with tomato plant residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef]
- Gary, C.; Bertin, N.; Frossard, J.S.; Lebot, J. High mineral contents explain the low construction cost of leaves, stems and fruits of tomato plants. J. Exp. Bot. 1998, 49, 49–57. [Google Scholar] [CrossRef]
- Callejón-Ferre, A.J.; Carreño-Sánchez, J.; Suárez-Medina, F.J.; Pérez-Alonso, J.; Velázquez-Martí, B. Prediction models for higher heating value based on the structural analysis of the biomass of plant remains from the greenhouses of Almería (Spain). Fuel 2014, 116, 377–387. [Google Scholar] [CrossRef]
- Boer, F.D.; Valette, J.; Commandré, J.; Fournier, M.; Thévenon, M. Slow Pyrolysis of Sugarcane Bagasse for the Production of Char and the Potential of Its By-Product for Wood Protection. J. Renew. Mater. 2021, 9, 97–117. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Martínez, G. Energetic use of the tomato plant waste. Fuel Process. Technol. 2008, 89, 1193–1200. [Google Scholar] [CrossRef]
- Luo, L.; Xu, C.; Chen, Z.; Zhang, S. Properties of biomass-derived biochars: Combined effects of operating conditions and biomass types. Bioresour. Technol. 2015, 192, 83–89. [Google Scholar] [CrossRef]
- Ronsse, F.; Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Abujabhah, I.; Bound, S.; Doyle, R.; Bowman, J. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2015, 98, 243–253. [Google Scholar] [CrossRef]
- Suvo, T.P.; Islam, S.; Khan, S.I.; Mehedi, N.H.; Ahamed, T. Effects of soil and closed soilless system on growth, productivity, fruit quality, and plant mineral composition of zucchini squash. Oct. J. Environ. Res. 2017, 5, 122–128. [Google Scholar]
- Martínez, S.; Losada, P.; Franco, I.; Carballo, J. Protein, amino acid, ash and mineral contents in Brassica spp. grown in Northwest Spain. Int. J. Food Sci. Technol 2011, 46, 146–153. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.; Hockaday, W.; Crowley, D. Biochar effects on soil biota-A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of biochar on composting of organic wastes and remediation of contaminated soils—A review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Tomato | Broccoli | Zucchini | Unified Biomass | Biochar |
|---|---|---|---|---|---|
| Elemental Analysis: | |||||
| C (%) | 37.7 ± 0.3 | 33.6 ± 0.3 | 34.6 ± 2.0 | nd * | 32.4 ± 0.2 |
| H (%) | 2.88 ± 0.36 | 1.97 ± 0.44 | 4.49 ± 0.51 | nd | 1.13 ± 0.07 |
| O 1 (%) | 26.3 ± 1.1 | 37.0 ± 0.9 | 27.1 ± 2.5 | nd | 8.14 ± 0.11 |
| N (%) | 3.95 ± 0.31 | 2.25 ± 0.10 | 4.41 ± 0.34 | nd | 2.30 ± 0.19 |
| S (%) | 1.70 ± 0.12 | 0.49 ± 0.05 | 0.35 ± 0.05 | nd | bd ** |
| H/C molar | 0.92 ± 0.11 | 0.70 ± 0.15 | 1.56 ± 0.21 | nd | 0.42 ± 0.03 |
| O/C molar | 0.52 ± 0.02 | 0.82 ± 0.01 | 0.59 ± 0.01 | nd | 0.188 ± 0.002 |
| Proximal Analysis: | |||||
| Equilibrium moisture (%) | 6.6 ± 0.1 | 7.8 ± 0.7 | 7.0 ± 0.2 | nd | 5.9 ± 0.9 |
| Volatiles (%) | 58.6 ± 0.4 | 60.0 ± 0.5 | 55.2 ± 1.5 | nd | 15.2 ± 0.2 |
| Fixed carbon (%) | 13.8 ± 1.0 | 15.0 ± 0.4 | 16.2 ± 1.0 | nd | 28.7 ± 3.0 |
| Ash (%) | 21.0 ± 0.7 | 17.2 ± 0.9 | 21.6 ± 1.5 | nd | 50.1 ± 3.4 |
| Properties: | |||||
| Density (g mL−1) | nd | nd | nd | nd | 1.73 ± 0.01 |
| Bulk Density (g mL−1) | nd | nd | nd | 0.35 ± 0.03 | 0.25 ± 0.02 |
| pH | 7.02 ± 0.01 | 5.68 ± 0.04 | 8.83 ± 0.03 | 7.08 ± 0.01 | 13.24 ± 0.03 |
| EC (mS cm−1) | 7.09 ± 0.05 | 4.67 ± 0.20 | 5.13 ± 0.09 | 6.03 ± 0.10 | 16.15 ± 0.05 |
| CEC (mmol kg−1) | nd | nd | nd | nd | 477 ± 27 |
| Surface area BET (m2 g−1) | nd | nd | nd | nd | 12.1 ± 0.1 |
| HHV (MJ kg−1) | 13.5 ± 0.3 | 9.7 ± 0.5 | 14.1 ± 0.2 | nd | 11.52 ± 0.06 |
| Concentration Range (ppm) | Metal |
|---|---|
| <0.1 | Be, Ag, Cd, Sb, Bi |
| 0.1–0.5 | As, Se, Pb |
| 0.5–1 | Li, Co, Tl |
| 1–5 | V, Sn |
| 5–10 | Ni |
| 10–20 | Cr |
| 40–50 | Cu, Zn, Mo |
| 90–100 | Ti |
| 100–200 | Mn, Ba |
| 400–500 | Sr |
| 1000–5000 | Na, Al, Fe |
| 20,000–30,000 | Mg |
| 100,000–110,000 | K, Ca |
| Experiment | Soil (%) | Fertilizer (%) | Biochar (%) | Coding |
|---|---|---|---|---|
| 1 | 100.0 | 0 | 0 | Exp 1 (100S-0F-0B) |
| 2 | 99.5 | 0 | 0.5 | Exp 2 (99.5S-0F-0.5B) |
| 3 | 98.5 | 1.0 | 0.5 | Exp 3 (98.5S-1F-0.5B) |
| 4 | 99.0 | 1.0 | 0 | Exp 4 (99S-1F-0B) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, S.; Stahl, U.; Ruiz, W.; García, A.N.; Marcilla, A. Characterization of Biochar Produced from Greenhouse Vegetable Waste and Its Application in Agricultural Soil Amendment. AgriEngineering 2025, 7, 348. https://doi.org/10.3390/agriengineering7100348
Medina S, Stahl U, Ruiz W, García AN, Marcilla A. Characterization of Biochar Produced from Greenhouse Vegetable Waste and Its Application in Agricultural Soil Amendment. AgriEngineering. 2025; 7(10):348. https://doi.org/10.3390/agriengineering7100348
Chicago/Turabian StyleMedina, Sergio, Ullrich Stahl, Washington Ruiz, Angela N. García, and Antonio Marcilla. 2025. "Characterization of Biochar Produced from Greenhouse Vegetable Waste and Its Application in Agricultural Soil Amendment" AgriEngineering 7, no. 10: 348. https://doi.org/10.3390/agriengineering7100348
APA StyleMedina, S., Stahl, U., Ruiz, W., García, A. N., & Marcilla, A. (2025). Characterization of Biochar Produced from Greenhouse Vegetable Waste and Its Application in Agricultural Soil Amendment. AgriEngineering, 7(10), 348. https://doi.org/10.3390/agriengineering7100348







