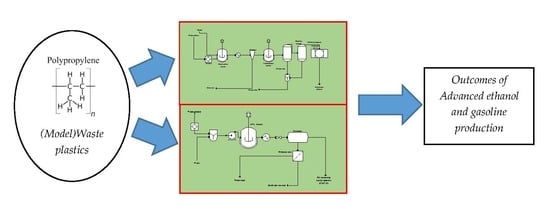
Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies
Abstract
1. Introduction
2. Methodology
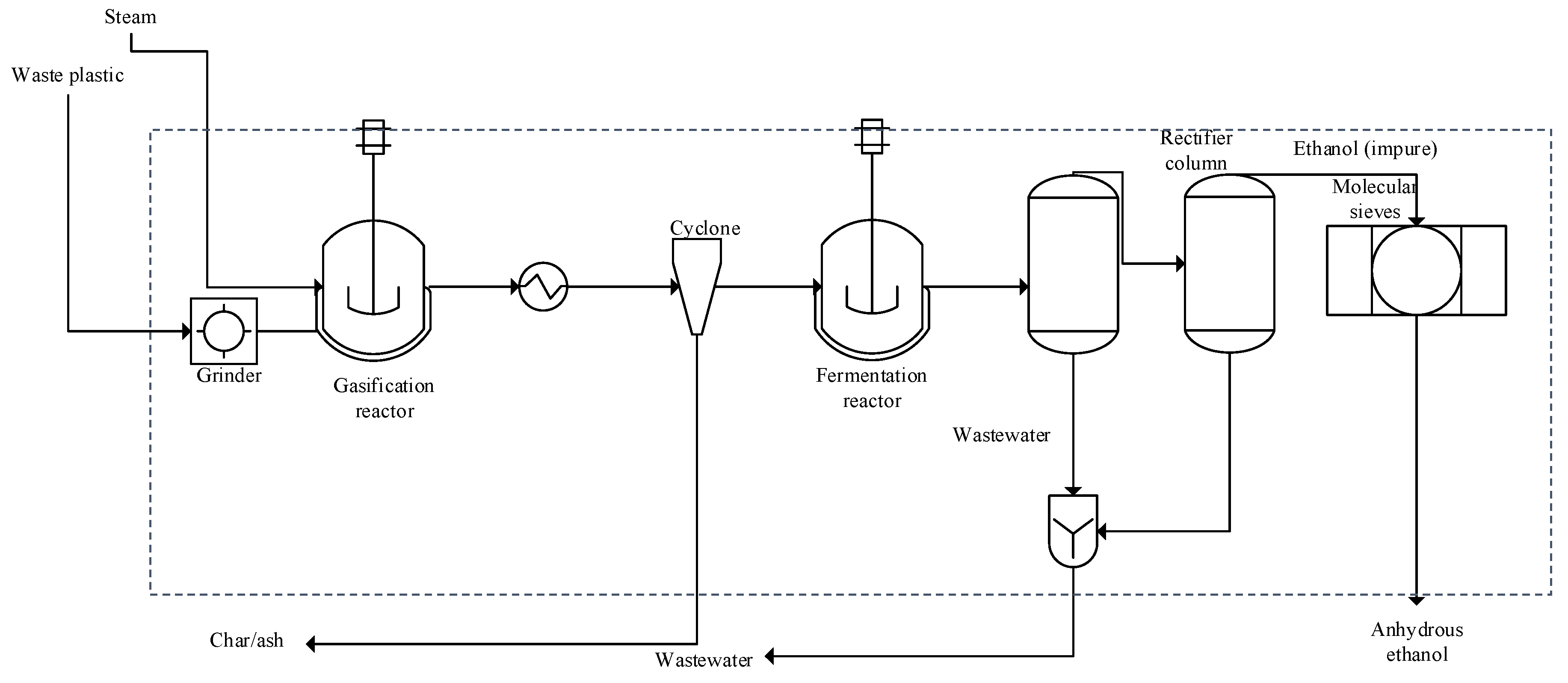
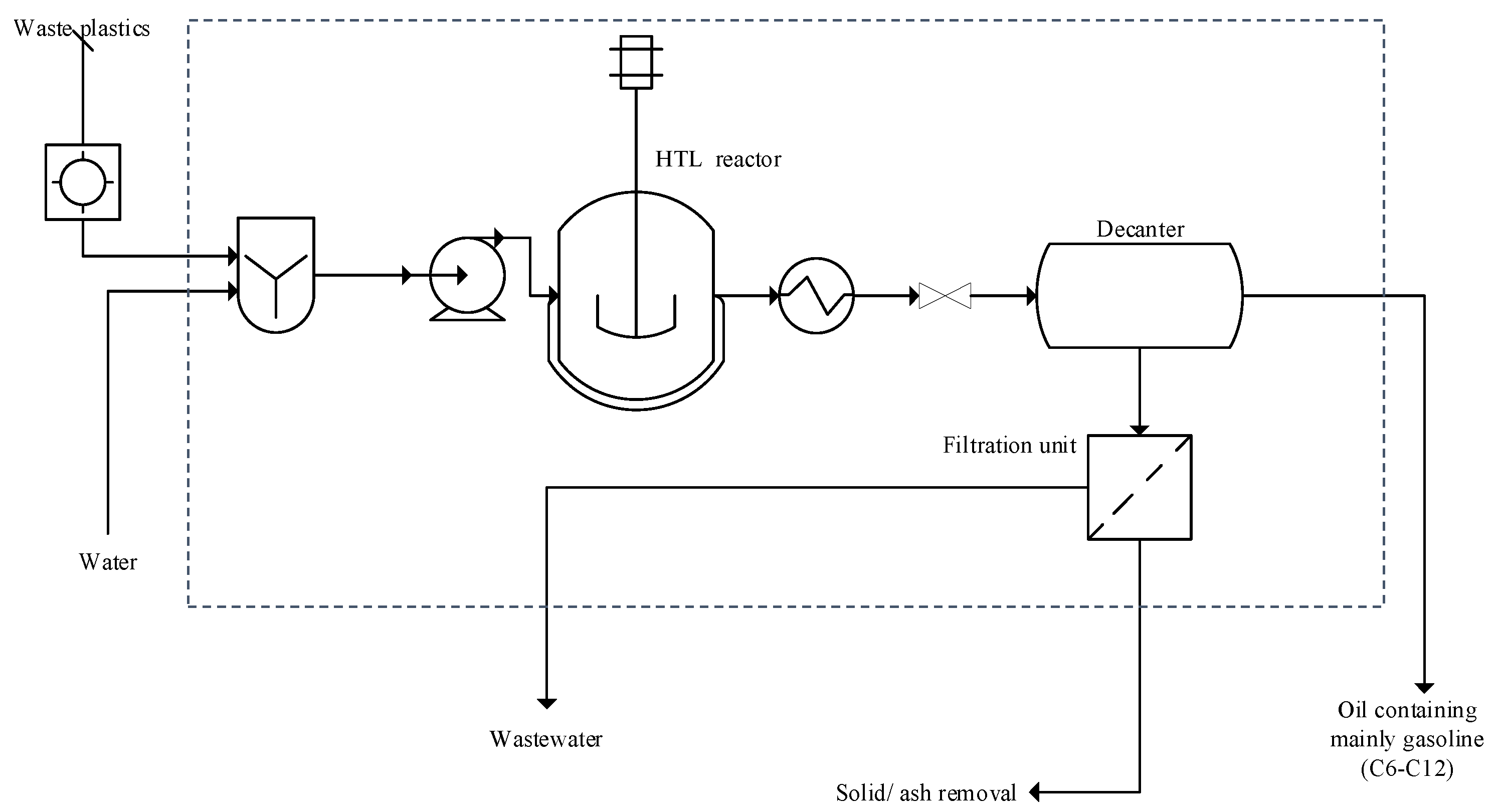
2.1. Model and Process Description
2.2. Economic Assessment Methods
3. Results and Discussion
3.1. ASPEN Plus Simulation
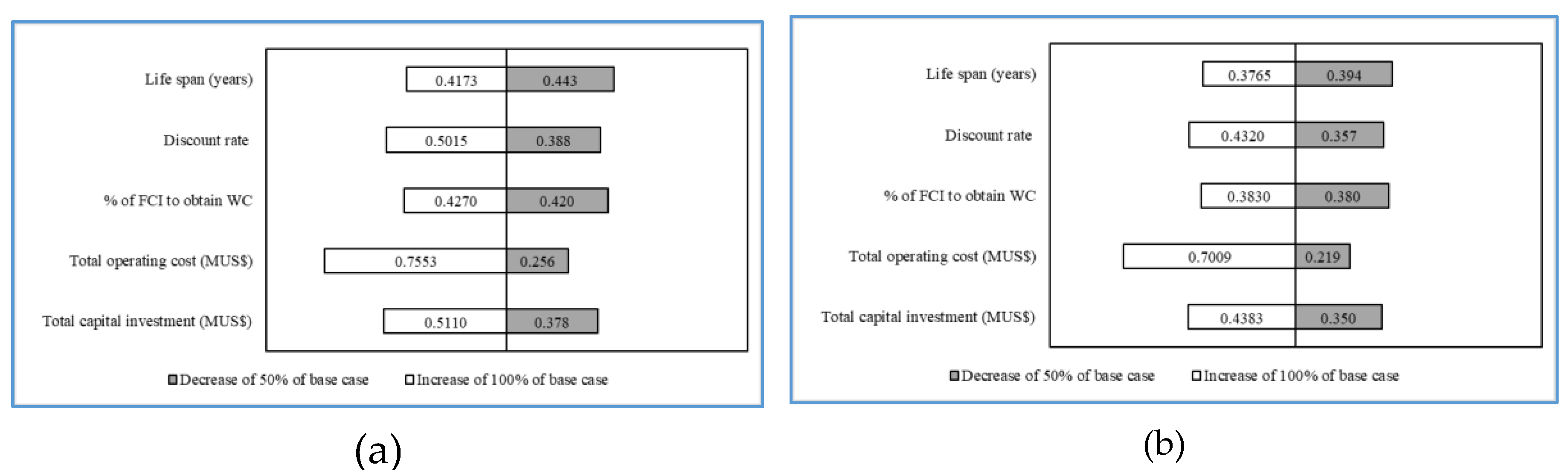
3.2. Economic Assessments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Navas-Anguita, Z.; García-Gusano, D.; Iribarren, D. A review of techno-economic data for road transportation fuels. Renew. Sustain. Energy Rev. 2019, 112, 11–26. [Google Scholar] [CrossRef]
- International Energy Agency. Railway Handbook. Energy Consumption and CO2 Emissions; OECD/International Energy Agency: Paris, France, 2016. [Google Scholar]
- BP. Statistical Review of World Energy; BP PLC: London, UK, 2016. [Google Scholar]
- Jensen, C.U.; Guerrero, J.K.R.; Karatzos, S.; Olofsson, G.; Iversen, S.B. Hydrofaction of forestry residues to drop-in renewable transportation fuels. Direct Thermochem. Liq. Energy Appl. 2018, 319–345. [Google Scholar] [CrossRef]
- Phanisankar, B.S.S.; Vasudeva, R.N.; Manikanta, J.E. Conversion of waste plastics to fuel products. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Fineberg, H. Reinveinting Plastics; Veolia Institute: Paris, France, 2019. [Google Scholar]
- Plastics-Euro. Plastics the Facts; Plastics Europe: Brussels, Belgium, 2018. [Google Scholar]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorisation of waste polyolefinic plastics to produce fuels and chemicals. Areview. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid awste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Zhao, X.; Zhan, L.; Xie, B.; Gao, B. Products derived from waste plastics (PC, HIPS, ABS, PP and PA6) via hydrothermal treatment: Charcaterisation and potential applications. Chemosphere 2018, 207, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Artexe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent adavances in the gasification of wasteplastics. Acritical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Huang, J. Polymer waste management -biodegradation, incineration, and recycling. J. Macromol. Sci. 1995, 32, 593–597. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degard. Stabil. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, H.; Peng, J.; Wange, Y.; Xiong, X.; Wu, C.; Lam, P.K.S. Microplastics pollution in China’s inland water systems: A review of findings, methods, characteristics, effects and management. Sci. Total Env. 2018, 630, 1641–1653. [Google Scholar] [CrossRef]
- Munir, M.T.; Mansouri, I.A.; Udugama, S.; Baroutian, K.V.; Gernaey, B.; Young, B.R. Resource recovery from organic solid waste using hydrothermal processing: Opportunities and challemges. Renew. Sustain. Energy Rev. 2018, 96, 64–75. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Banu, R.J.; Shaarmila, G.V.; Urshani, U.; Amhdha, V.; Kumar, G. Impervious and Influence in the liquid fuel production from municipal plastic waste through thermochemical biomass conversion technologies-A review. Sci. Total Environ. Sci. 2020, 718, 137287. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste-A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Synergistic effects insteam gasification of combined biomass and plastic waste mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Wierckx, N.; Prieto, M.A.; Pomposiello, P.; de Lorenzo, V.; O’Connor, K.; Blank, L.M. Plastic waste as a novel substrate for industrial biotechnology. Microb. Biotechnol. 2015, 8, 900–903. [Google Scholar] [CrossRef]
- Bian, G.-Z.; Fan, L.; Fu, Y.-L.; Fujimoto, K. Mixed Alcohol Synthesis from Syngas on Sulfided K–Mo-Based Catalysts: Influence of Support Acidity. Ind. Eng. Chem. Res. 1998, 37, 1736–1743. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Shivashankar, M.; Majumder, S. Plastic solid waste utilization technologies: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022024. [Google Scholar] [CrossRef]
- Li, H.; Lu, J.; Zhang, Y.; Liu, Z. Hydrothermal liquefaction of typical livestock manures in China: Biocrudeoil production and migration of heavy metals. J. Anal. Appl. Pyrol. 2018, 135, 133–140. [Google Scholar] [CrossRef]
- Tian, C.; Li, B.; Liu, Z.; Zhang, Y.; Lu, H. Hydrothermal liquefaction for algal biorefinery: A critical review. Renew. Sust. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.; Rosendahl, L. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Zhang, Y.; Li, B.; Lu, H.; Duan, N. Conversion efficiency and oil quality of low lipid low protein microalgae via hydrothermal liquefacttion. Bioresour. Technol. 2014, 154, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Okoro, O.V.; Sun, Z. The characterisation of biochar and biocrude products of the hydrothermal liquefaction of raw digestate biomass. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, D.S. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jin, K.; Linda Wang, N.-H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]
- Li, D.-T.; Li, W.; Li, B.-Q. CO-Carbonization of Coking-Coal with Different Waste Plastics. J. Fuel Chem. Technol. 2001, 29, 19–23. [Google Scholar]
- Nakayama, T.; Sagara, H.; Arai, K.; Saito, S. High pressure liquid-liquid equilibria for the system of water, ethanol and 1,1-difluoroethane at 323.2 K. Fluid Ph. Equilib. 1987, 38, 109–127. [Google Scholar] [CrossRef]
- Geanã, D.; Feroiu, V. Prediction of Vapor–Liquid Equilibria at Low and High Pressures from UNIFAC Activity Coefficients at Infinite Dilution. Ind. Eng. Chem. Res. 1998, 37, 1173–1180. [Google Scholar] [CrossRef]
- Totsch, W.; Gaensslen, H. Polyvinylchloride: Environmental Aspects of a Common Plastic; Elsevier: London, UK, 2012. [Google Scholar]
- Bhaskar, T.; Balagurumurthy, B.; Singh, R.; Poddar, M.K. Thermochemical route for biohydrogen production. In Biohydrogen; Pandey, A., Chang, J.-S., Hallenbecka, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 12; pp. 285–316. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Fidalgo, B. 15—Production of bio-syngas and bio-hydrogen via gasification. In Handbook of Biofuels Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2016; pp. 431–494. [Google Scholar] [CrossRef]
- Basu, P. (Ed.) Production of synthetic fuels and chemicals from biomass. In Biomass Gasification, Pyrolysis and Torrefaction, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; Chapter 12; pp. 415–443. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from steam gasification of polyethylene in a conical spouted bed reactor. Fuel 2013, 109, 461–469. [Google Scholar] [CrossRef]
- Cempa-Balewicz, M.; Jacek Łączny, M.; Smoliński, A.; Iwaszenko, S. Equilibrium Model of Steam Gasification of Coal. J. Sustain. Min. 2013, 12, 21–28. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Techno-Economic Assessment of a Scaled-Up Meat Waste Biorefinery System: A Simulation Study. Materials 2019, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Thermo-Chemical Analysis of Biomass Gasification by Gibbs Free Energy Minimization Model-Part: II (Optimization of Biomass Feed and Steam to Biomass Ratio). Int. J. Green Energy 2013, 10, 610–639. [Google Scholar] [CrossRef]
- Ukpong, M.N.; Atiyeh, H.K.; De Lorme, M.J.; Liu, K.; Zhu, X.; Tanner, R.S.; Wilkins, M.R.; Stevenson, B.S. Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol. Bioeng. 2012, 109, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int. J. Syst. Evol. Microbiol. 1993, 43, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.R.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. Biological production of ethanol from coal synthesis gas. Appl. Biochem. Biotechnol. 1993, 39, 559–571. [Google Scholar] [CrossRef]
- Petersen, A.M.; Haigh, K.; Görgens, J.F. Techno-economics of Integrating Bio-Ethanol Production from Spent Sulphite Liquor for Reduction of Greenhouse Gas Emissions from Sulphite Pulping Mills. Biotechnol. Biofuels 2014, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Amigun, B.; Petrie, D.; Görgens, J. Feedstock and Technology Options for Bioethanol Production in South Africa: Technoeconomic Prefeasibility Study. Energy Fuels 2012, 26, 5887–5896. [Google Scholar] [CrossRef]
- Valdez, P.J.; Tocco, V.J.; Savage, P.E. A general kinetic model for the hydrothermal liquefaction of microalgae. Bioresour. Technol. 2014, 163, 123–127. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels—A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Sinnot, R.; Towler, G. Chemical Engineering Design, 5th ed.; Elsevier: Burlington, NJ, USA, 2009. [Google Scholar]
- Okoro, O.V.; Sun, Z.; Birch, J. Catalyst-Free Biodiesel Production Methods: A Comparative Technical and Environmental Evaluation. Sustainability 2018, 10, 127. [Google Scholar] [CrossRef]
- Násner, A.M.L.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Restrepo, J.C.; Venturini, O.J.; Ratner, A. Refuse Derived Fuel (RDF) production and gasification in a pilot plant integrated with an Otto cycle ICE through Aspen plus™ modelling: Thermodynamic and economic viability. Waste Manag. 2017, 69, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, K. IRR Performs Better than NPV: A Critical Analysis of Cases of Multiple IRR and Mutually Exclusive and Independent Investments. Soc. Sci. Res. Netw. 2017, 23, 31. [Google Scholar] [CrossRef]
- Global Petrol Prices. Retail Energy Price Data. 2020. Available online: https://www.globalpetrolprices.com/USA/ (accessed on 20 February 2020).
- Environment. The Potential of Recycled Plastics; Plastics Industry Association: Washington, DC, USA, 2020. [Google Scholar]
- Dutta, A.; Talmadge, M.; Hensley, J.; Worley, M.; Dudgeon, D.; Barton, D.; Groendijk, P.; Ferrari, D.; Stears, B.; Searcy, E.M. Process. Design and Economics for Conversion of Lignocellulosic Biomass to Ethanol: Thermochemical Pathway by Indirect Gasification and Mixed Alcohol Synthesis; National Renewable Energy Lab.: Golden, CO, USA, 2011. [Google Scholar]
- Rickman, W.S. Handbook of Incineration of Hazardous Wastes (1991); CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Alibaba. Plastic Machinery Hdpe Polycarbonate. 2020. Available online: https://www.alibaba.com/product-detail/Plastic-Machinery-hdpe-polycarbonate-7-5_62538362812.html?spm=a2700.galleryofferlist.0.0.48fd258aHoj76o (accessed on 20 February 2020).
- Towler, G.; Sinnott, R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Okoro, O.V.; Sun, Z.; Birch, J. Thermal depolymerization of biogas digestate as a viable digestate processing and resource recovery strategy. In Advances in Eco-Fuels for a Sustainable Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–308. [Google Scholar]
- Okoro, V.O.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. Chem. Eng. 2019, 3, 76. [Google Scholar] [CrossRef]
- Chemengonline. 2019 Chemical Engineering Plant Cost Index Annual Average. 2020. Available online: https://www.chemengonline.com/2019-chemical-engineering-plant-cost-index-annual-average/ (accessed on 2 July 2020).
- Magnehi. Chemical Engineering Plant Cost Index (Averaged over Year). 2011. Available online: http://folk.ntnu.no/magnehi/cepci_2011_py.pdf (accessed on 12 February 2020).
- Peters, M.; Timmerhaus, K.; West, R. Plant Design and Economics for Chemical Engineers; McGraw-Hill Education: New York, NY, USA, 2003. [Google Scholar]
- Deloitte. Corporate Tax Rates 2019; Deloitte: London, UK, 2019. [Google Scholar]
- Shen, Y.; Brown, R.; Wen, Z. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Appl. Energy 2014, 136, 68–76. [Google Scholar] [CrossRef]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M.; Qian, L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediments 2012, 12, 1350–1359. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Midgett, J.S. Assessing a Hydrothermal Liquefaction Process Using Biomass Feedstocks; Louisiana State University: Baton Rouge, LA, USA, 2008. [Google Scholar]
- Remón, J.; Randall, J.; Budarin, V.L.; Clark, J.H. Production of bio-fuels and chemicals by microwave-assisted, catalytic, hydrothermal liquefaction (MAC-HTL) of a mixture of pine and spruce biomass. Green Chem. 2019, 21, 284–299. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- EIA. Petroleum & Other Liquids; USA Energy Information Administration: Washington, DC, USA, 2020.

| Proximate Analysis | Ultimate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Ash a (wt.%) | Volatiles a (wt.%) | FC a (wt.%) | Carbon b (wt.%) | Hydrogen b (wt.%) | Oxygen b (wt.%) | Nitrogen b (wt.%) | Sulphur b (wt.%) |
| 0.82 | 99.18 | 0 | 83.75 | 13.98 | 2.27 | 0 | 0 |
| Stream Property | Waste Plastics | Syngas | Fermentation Broth | Wastewater | Anhydrous Ethanol |
|---|---|---|---|---|---|
| Temperature (°C) | 25 | 37 | 37 | 29.3 | 25 |
| Pressure (atm) | 1 | 1 | 1 | 1 | 1 |
| Mass Fractions (x) | |||||
| Water | 0 | 0 | 0.289 | 0.480 | 0 |
| Waste plastics | 1 | 0 | 0 | 0 | 0 |
| Hydrogen | 0 | 0.117 | 0.035 | 0 | 0 |
| Carbon monoxide | 0 | 0.267 | 0.027 | 0 | 0 |
| Carbon (iv) oxide | 0 | 0.616 | 0.128 | 0 | 0 |
| Ethanol | 0 | 0 | 0.226 | 0.031 | 1 |
| Acetic acid | 0 | 0 | 0.295 | 0.489 | 0 |
| Mass Flows (kg/h) | 1000 | 2992.6 | 2997.4 | 1808.7 | 621.5 |
| Stream Property | Waste Plastics | Gas-Product | Post-HTL Product | Char | Oil Product |
|---|---|---|---|---|---|
| Temperature (°C) | 25 | 25 | 25 | 25 | 25 |
| Pressure (atm) | 1 | 1 | 227 | 1 | 1 |
| Mass Fractions (x) | |||||
| Water | 0 | 0 | Trace | Trace | 0 |
| Waste plastic | 1 | 0 | 0 | 0 | 0 |
| CO | 0 | 0 | 0.229 | 0 | 0 |
| CO2 | 0 | 0 | 0.027 | 0 | 0 |
| Char | 0 | 0 | 0.006 | 1 | 0 |
| C5H12 | 0 | 0 | 0.072 | 0 | 0.123 |
| C6-C12 | 0 | 0 | 0.511 | 0 | 0.877 |
| C13-C23 | 0 | 0 | 0 | 0 | 0 |
| CH4 | 0 | 0 | 0.051 | 0 | 0 |
| C2H6 | 0 | 0.172 | 0.018 | 0 | 0 |
| C3H8 | 0 | 0.318 | 0.043 | 0 | 0 |
| C4H10 | 0 | 0.510 | 0.045 | 0 | 0 |
| Mass Flows (kg/h) | 1000 | 123.1 | 1175.4 | 7.4 | 685.3 |
| Process Result | Scenario i | Scenario ii |
|---|---|---|
| Externally required cooling utility (kW) | 784.4 | 1836.4 |
| Externally required heating utility (kW) | 392.2 | - |
| Externally required electricity demand (kW) | 700 | 704 |
| Economic Parameter | Scenario i | Scenario ii |
|---|---|---|
| Fixed capital investment (MUSD) | 5.80 | 3.79 |
| Working capital investment (MUSD) | 0.29 | 0.19 |
| Total capital investment (MUSD) | 6.09 | 3.98 |
| Fixed operating cost (MUSD) | 1.64 | 1.47 |
| Variable operating cost (MUSD) | 0.76 | 0.84 |
| Total operating cost (MUSD) | 2.40 | 2.31 |
| Unit processing (USD/kg) | 0.42 | 0.38 |
| Payback period (y) a | 5 | 2 |
| IRR | 22.2% | 51.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, O.V.; Faloye, F.D. Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies. AgriEngineering 2020, 2, 378-392. https://doi.org/10.3390/agriengineering2030026
Okoro OV, Faloye FD. Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies. AgriEngineering. 2020; 2(3):378-392. https://doi.org/10.3390/agriengineering2030026
Chicago/Turabian StyleOkoro, Oseweuba Valentine, and Funmilayo D. Faloye. 2020. "Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies" AgriEngineering 2, no. 3: 378-392. https://doi.org/10.3390/agriengineering2030026
APA StyleOkoro, O. V., & Faloye, F. D. (2020). Comparative Assessment of Thermo-Syngas Fermentative and Liquefaction Technologies as Waste Plastics Repurposing Strategies. AgriEngineering, 2(3), 378-392. https://doi.org/10.3390/agriengineering2030026