In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Preparation of the Bioactive Conjugates
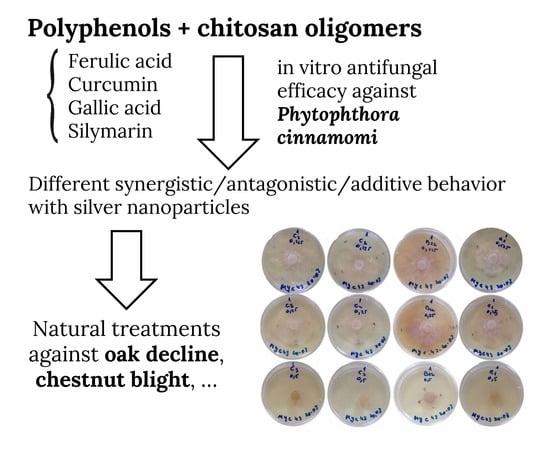
2.2. Fungal Isolate, Growth Conditions and In Vitro Tests of Mycelial Growth Inhibition
2.3. Statistical Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef]
- Ramírez Gil, J.G. Incidencia, diagnóstico, comportamiento y alternativas de manejo de la marchitez del aguacate con énfasis en Phytophthora cinnamomi Rands; Universidad Nacional de Colombia: Medellín, Colombia, 2013. [Google Scholar]
- Homet, P.; González, M.; Matías, L.; Godoy, O.; Pérez-Ramos, I.M.; García, L.V.; Gómez-Aparicio, L. Exploring interactive effects of climate change and exotic pathogens on Quercus suber performance: Damage caused by Phytophthora cinnamomi varies across contrasting scenarios of soil moisture. Agricultural and Forest Meteorology 2019, 276, 107605. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group Auckland: Auckland, NZ, USA, 2004; p. 12. [Google Scholar]
- Sánchez, M.; Caetano, P.; Romero, M.; Navarro, R.; Trapero, A. Phytophthora root rot as the main factor of oak decline in southern Spain. In Progress in Research on Phytophthora Diseases of Forest Trees; Brasier, C., Jung, T., Oßwald, W., Eds.; Forest Research: Farnham, Surrey, UK, 2004; pp. 149–154. [Google Scholar]
- Jackson, T.J.; Burgess, T.; Colquhoun, I.; Hardy, G.E.S. Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathol. 2000, 49, 147–154. [Google Scholar] [CrossRef]
- Fialova, S.; Rendekova, K.; Mucaji, P.; Slobodnikova, L. Plant natural agents: Polyphenols, alkaloids and essential oils as perspective solution of microbial resistance. Curr. Org. Chem. 2017, 21. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; Liberto, M.D.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef]
- Teplova, V.V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural polyphenols: Biological activity, pharmacological potential, means of metabolic engineering (review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Gupta, M.; Chandra, A.; Aggarwal, G. Curcumin: potential therapeutic moiety for fungal infections. Current Traditional Medicine 2019, 4, 249–262. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Si, J.; Kang, C.; Chung, B.; Chung, D.; Kim, D. Facile preparation of water soluble curcuminoids extracted from turmeric (Curcuma longa L.) powder by using steviol glucosides. Food Chem. 2017, 214, 366–373. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Matei, P.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.; Martín-Ramos, P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gil, J.; Matei Petruta, M.; Pérez Lebeña, E. Complejo de Inclusión para Mejorar la Biodisponibilidad de Compuestos BiolóGicamente Activos no Hidrosolubles. US patent p201731489, 28 December 2017. [Google Scholar]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. In vitro antifungal activity of some plant extracts against Fusarium oxysporum in blackcurrant (Ribes nigrum L.). Acta Sci. Pol. Hortorum 2017, 16, 167–176. [Google Scholar] [CrossRef]
- Wadley, F.M. The Evidence Required to Show Synergistic Action of Insecticides and a Short Cut in Analysis; U.S. Government Printing Office: Washington DC, USA, 1945.
- De Souza, A.; Mehta, D.; Leavitt, R. Bactericidal activity of combinations of silver-water dispersion with 19 antibiotics against seven microbial strains. Curr. Sci. 2006, 91, 1825–1851. [Google Scholar]

| Treatment | EC50 (µg·mL−1) | EC90 (µg·mL−1) | ||
|---|---|---|---|---|
| w/o AgNPs | w/AgNPs | w/o AgNPs | w/AgNPs | |
| Control | -- | 458.4 | -- | 1192.8 |
| Curcumin | 257.5 | 279.9 | 448.3 | 487.4 |
| Ferulic acid | 228.7 | 171.6 | 446.2 | 450.4 |
| Gallic acid | 373.6 | 261.3 | 795.3 | 455.6 |
| Silymarin | 195.5 | 261.8 | 453.1 | 963.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, P.M.; Buzón-Durán, L.; Pérez-Lebeña, E.; Martín-Gil, J.; Iacomi, B.M.; Ramos-Sánchez, M.C.; Martín-Ramos, P. In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi. AgriEngineering 2020, 2, 72-77. https://doi.org/10.3390/agriengineering2010005
Matei PM, Buzón-Durán L, Pérez-Lebeña E, Martín-Gil J, Iacomi BM, Ramos-Sánchez MC, Martín-Ramos P. In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi. AgriEngineering. 2020; 2(1):72-77. https://doi.org/10.3390/agriengineering2010005
Chicago/Turabian StyleMatei, Petruta Mihaela, Laura Buzón-Durán, Eduardo Pérez-Lebeña, Jesús Martín-Gil, Beatrice Michaela Iacomi, M. Carmen Ramos-Sánchez, and Pablo Martín-Ramos. 2020. "In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi" AgriEngineering 2, no. 1: 72-77. https://doi.org/10.3390/agriengineering2010005
APA StyleMatei, P. M., Buzón-Durán, L., Pérez-Lebeña, E., Martín-Gil, J., Iacomi, B. M., Ramos-Sánchez, M. C., & Martín-Ramos, P. (2020). In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi. AgriEngineering, 2(1), 72-77. https://doi.org/10.3390/agriengineering2010005