Rehabilitation for Chronic Constipation: Integrative Approaches to Diagnosis and Treatment
Abstract
1. Introduction
1.1. Incidence and Risk Factors of Constipation
1.2. Age and Gender Distribution
1.3. Causes of Constipation
1.4. Nutrition
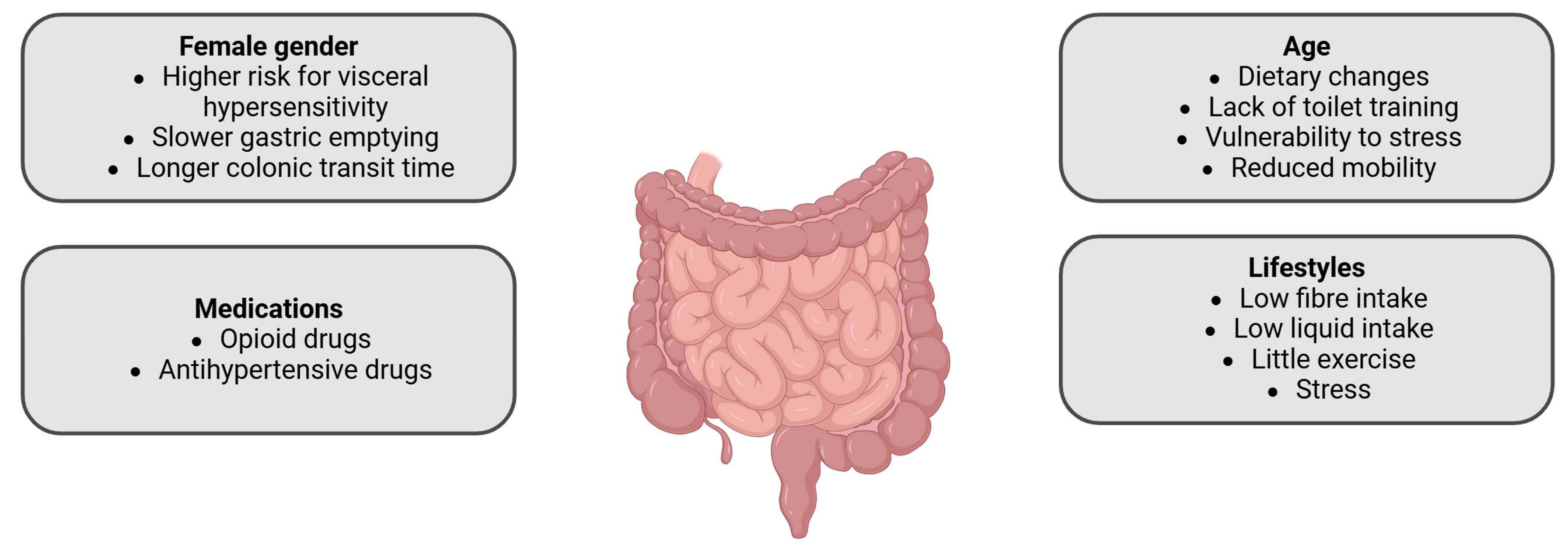
2. Predisposing Factors for Constipation
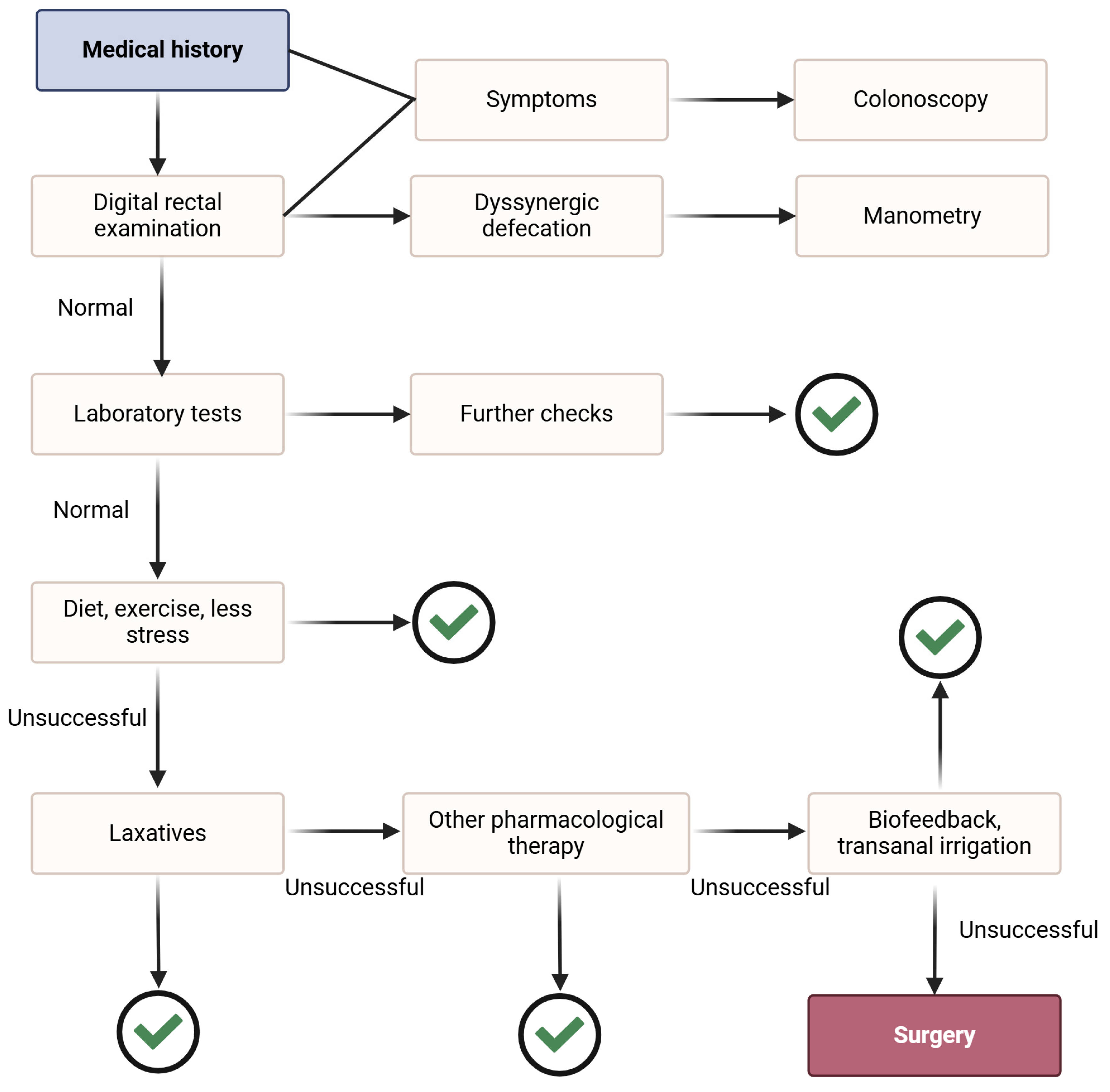
3. Rehabilitation in Chronic Constipation
3.1. Nonpharmacologic Rehabilitation
3.2. Pharmacological Rehabilitation
3.2.1. Laxatives
3.2.2. Prosecretory Agents
3.2.3. Serotoninergic Agonists
3.2.4. Probiotics and Prebiotics
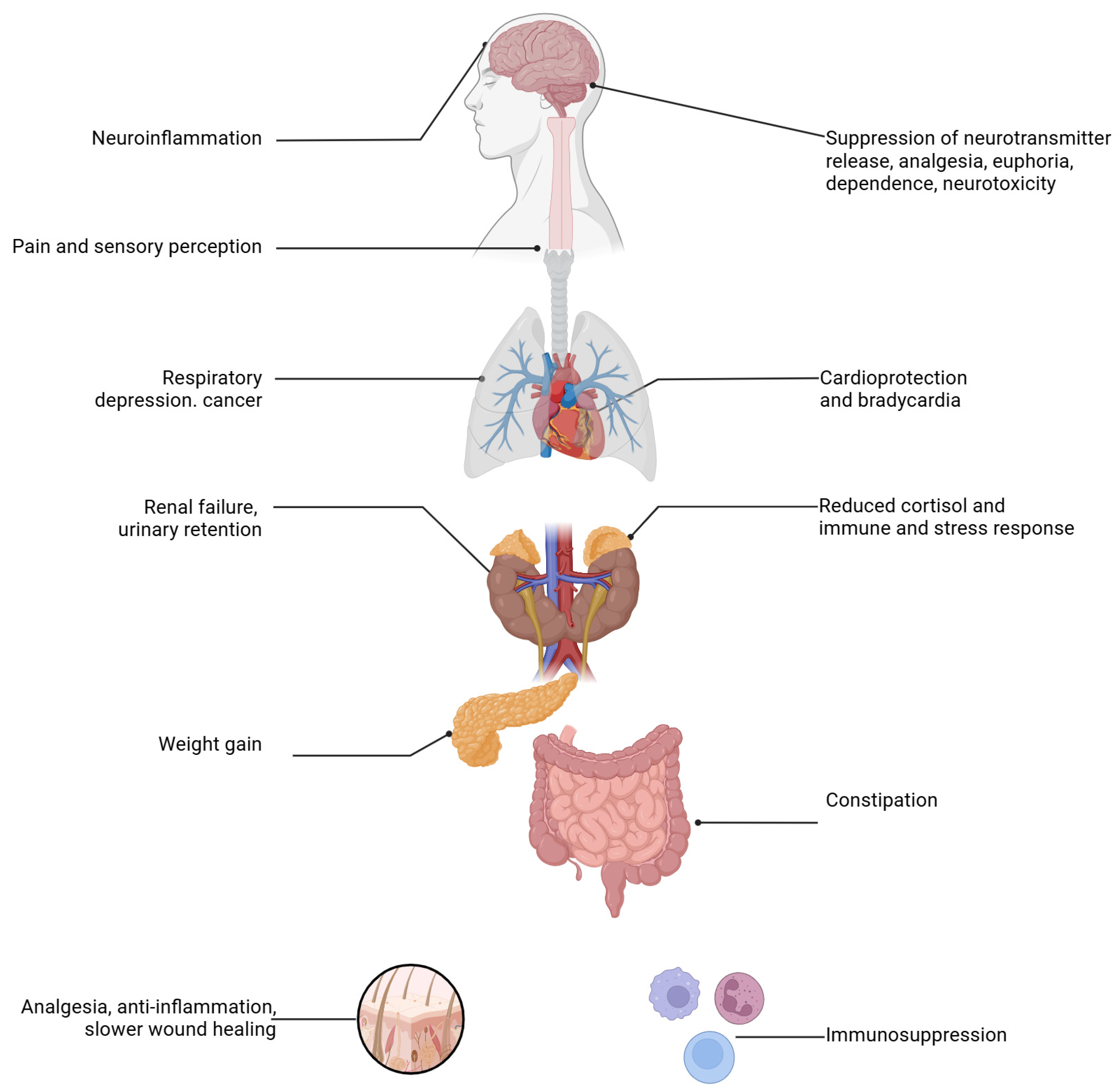
3.2.5. Other Interventions
3.2.6. Differentiation and Appropriate Use of Pharmacological Agents
3.3. Surgical Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suceveanu, A.I.; Mazilu, L.; Nitipir, C.; Stoian, A.P.; Parepa, I.; Voinea, C.; Suceveanu, A.P. Diabetes Mellitus raise the Risk for Interval Colorectal Cancer and Advanced Colorectal Adenomas. Rev. Chim. 2019, 70, 1808–1811. [Google Scholar] [CrossRef]
- De Giorgio, R.; Ruggeri, E.; Stanghellini, V.; Eusebi, L.H.; Bazzoli, F.; Chiarioni, G. Chronic constipation in the elderly: A primer for the gastroenterologist. BMC Gastroenterol. 2015, 15, 130. [Google Scholar] [CrossRef]
- Zhang, T.; Zullo, A.R.; James, H.O.; Lee, Y.; Taylor, D.C.A.; Daiello, L.A. The Burden and Treatment of Chronic Constipation Among US Nursing Home Residents. J. Am. Med. Dir. Assoc. 2023, 24, 1247–1252.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, W.F.; Liberman, J.N.; Sandler, R.S.; Woods, M.S.; Stemhagen, A.; Chee, E.; Lipton, R.B.; Farup, C.E. Epidemiology of constipation (EPOC) study in the United States: Relation of clinical subtypes to sociodemographic features. Am. J. Gastroenterol. 1999, 94, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, Y.; Hoshide, S.; Mizuno, H.; Kario, K. Constipation-induced pressor effects as triggers for cardiovascular events. J. Clin. Hypertens. 2019, 21, 421–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Z.; Guo, M.; Bai, X.; Ruan, G.; Sun, Y.; Han, W.; Yang, H. Exploring the association between cardiovascular health and bowel health. Sci. Rep. 2024, 14, 11819. [Google Scholar] [CrossRef]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Morales-Brown, L. What to Know About the Bristol Stool Form Scale. 2024. Available online: https://www.medicalnewstoday.com/articles/bristol-stool-scale (accessed on 12 November 2024).
- Gray, J.R. What is chronic constipation? Definition and diagnosis. Can. J. Gastroenterol. 2011, 25 (Suppl. B), 7B–10B. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yabunaka, K.; Nakagami, G.; Komagata, K.; Sanada, H. Ultrasonographic follow-up of functional chronic constipation in adults: A report of two cases. SAGE Open Med. Case Rep. 2017, 5, 2050313X17694234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Najjar, R. Radiology’s Ionising Radiation Paradox: Weighing the Indispensable Against the Detrimental in Medical Imaging. Cureus 2023, 15, e41623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomita, T.; Kazumori, K.; Baba, K.; Zhao, X.; Chen, Y.; Miwa, H. Impact of chronic constipation on health-related quality of life and work productivity in Japan. J. Gastroenterol. Hepatol. 2021, 36, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Sommers, T.; Corban, C.; Sengupta, N.; Jones, M.; Cheng, V.; Bollom, A.; Nurko, S.; Kelley, J.; Lembo, A. Emergency department burden of constipation in the United States from 2006 to 2011. Am. J. Gastroenterol. 2015, 110, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Șerbănescu, L.; Badiu Diana Popescu, S.; Busu, D. Costea Andreea-Cristina. The management of tubo-ovarian abscesses associated with appendicitis. J. Mind Med. Sci. 2021, 8, 280–285. [Google Scholar] [CrossRef]
- Iuga, I.C.; Nerişanu, R.A.; Iuga, H. The impact of healthcare system quality and economic factors on the older adult population: A health economics perspective. Front. Public Health 2024, 12, 1454699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, L.; Chey, W.D.; Imdad, A.; Almario, C.V.; Bharucha, A.E.; Diem, S.; Greer, K.B.; Hanson, B.; Harris, L.A.; Ko, C.; et al. American Gastroenterological Association-American College of Gastroenterology Clinical Practice Guideline: Pharmacological Management of Chronic Idiopathic Constipation. Am. J. Gastroenterol. 2023, 118, 936–954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiang, C.Y.; Lo, S.C.; Beckstead, J.W.; Yang, C.Y. Associations between constipation risk and lifestyle, medication use, and affective symptoms in patients with schizophrenia: A multicenter cross-sectional study. Soc. Psychiatry Psychiatr. Epidemiol. 2024; epub ahead of print. Erratum in Soc. Psychiatry Psychiatr. Epidemiol. 2024, online ahead of print. https://doi.org/10.1007/s00127-024-02743-w. [Google Scholar] [CrossRef] [PubMed]
- Scientific Image and Illustration Software|BioRender. Available online: https://www.biorender.com/ (accessed on 30 November 2024).
- Schuster, B.G.; Kosar, L.; Kamrul, R. Constipation in older adults: Stepwise approach to keep things moving. Can. Fam. Physician. 2015, 61, 152–158. [Google Scholar] [PubMed] [PubMed Central]
- Mari, A.; Mahamid, M.; Amara, H.; Baker, F.A.; Yaccob, A. Chronic Constipation in the Elderly Patient: Updates in Evaluation and Management. Korean J. Fam. Med. 2020, 41, 139–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.; Ravi Kiran, S.; Shankar, R. Rehabilitation of Flabby Ridges for Denture Fabrication: An Enigma for Dental Professionals. Cureus 2024, 16, e62345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quigley, E.M. Disorders of the pelvic floor and anal sphincters; a gastroenterologist’s perspective. Rev. Médica Clínica Las Condes 2013, 24, 293–298. [Google Scholar] [CrossRef]
- Verkuijl, S.J.; Meinds, R.J.; Trzpis, M.; Broens, P.M.A. The influence of demographic characteristics on constipation symptoms: A detailed overview. BMC Gastroenterol. 2020, 20, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernando, J.; Otero, M.D. The Link Between Constipation and Pelvic Organ Prolapse: Women’s Clinic of the Rio Grande Valley: Gynecology. Available online: https://www.wcrgv.com/blog/the-link-between-constipation-and-pelvic-organ-prolapse (accessed on 14 January 2025).
- Costea, D.O.; Șerbănescu, L.; Badiu Diana Ardeleanu, V.; Branescu, C.M.; Zgura, A.; Costea, A.C. Pain Managemnent in the Right Iliac Fossa During the COVID-19 Pandemic. J. Mind Med. Sci. 2022, 9, 162–167. [Google Scholar] [CrossRef]
- Callan, N.G.L.; Mitchell, E.S.; Heitkemper, M.M.; Woods, N.F. Constipation and diarrhea during the menopause transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause 2018, 25, 615–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muskiet, F.A.J. Chapter 2—Pathophysiology and Evolutionary Aspects of Dietary Fats and Long-Chain Polyunsaturated Fatty Acids across the Life Cycle. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., le Coutre, J., Eds.; CRC Press/Taylor&Francis: Boca Raton, FL, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53557/ (accessed on 2 December 2024).
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L. Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients 2023, 15, 3662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constipation. Cleveland Clinic. 2024. Available online: https://my.clevelandclinic.org/health/diseases/4059-constipation (accessed on 2 December 2024).
- Ho, K.S.; Tan, C.Y.; Mohd Daud, M.A.; Seow-Choen, F. Stopping or reducing dietary fiber intake reduces constipation and its associated symptoms. World J. Gastroenterol. 2012, 18, 4593–4596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meldrum, O.W.; Yakubov, G.E. Journey of dietary fiber along the gastrointestinal tract: Role of physical interactions, mucus, and biochemical transformations. Crit. Rev. Food Sci. Nutr. 2024, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Chuy, D.S.; Wi, R.S.; Tadros, M. Irritable Bowel Syndrome: Current Landscape of Diagnostic Guidelines and Therapeutic Strategies. Gastroenterol. Insights 2024, 15, 786–809. [Google Scholar] [CrossRef]
- Włodarczyk, J.; Waśniewska, A.; Fichna, J.; Dziki, A.; Dziki, Ł.; Włodarczyk, M. Current Overview on Clinical Management of Chronic Constipation. J. Clin. Med. 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diaz, S.; Bittar, K.; Hashmi, M.F.; Mendez, M.D. Constipation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513291/ (accessed on 2 December 2024).
- Miller, L.E.; Ibarra, A.; Ouwehand, A.C.; Zimmermann, A.K. Normative values for stool frequency and form using Rome III diagnostic criteria for functional constipation in adults: Systematic review with meta-analysis. Ann. Gastroenterol. 2017, 30, 161–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadeghi, A.; Akbarpour, E.; Majidirad, F.; Bor, S.; Forootan, M.; Hadian, M.R.; Adibi, P. Dyssynergic Defecation: A Comprehensive Review on Diagnosis and Management. Turk. J. Gastroenterol. 2023, 34, 182–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McFarlane, M.J. Chapter 97—The Rectal Examination. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. Available online: https://www.ncbi.nlm.nih.gov/books/NBK424/ (accessed on 2 December 2024).
- Higuero, T. Update on the management of anal fissure. J. Visc. Surg. 2015, 152 (Suppl. S2), S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Lee, Y.J.; Shin, J.E.; Jung, H.K.; Park, S.Y.; Kang, S.J.; Song, K.H.; Kim, J.W.; Lim, H.C.; Park, H.S.; et al. 2022 Seoul Consensus on Clinical Practice Guidelines for Functional Constipation. J. Neurogastroenterol. Motil. 2023, 29, 271–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Gastroenterological Association; Bharucha, A.E.; Dorn, S.D.; Lembo, A.; Pressman, A. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013, 144, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, S.A.; Kamel, D.M.; Abdelbasset, W.K.; Elgohary, H.M. Effects of a proposed physical activity and diet control to manage constipation in middle-aged obese women. Diabetes Metab. Syndr. Obes. 2017, 10, 513–519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garg, P. Inflammation in Irritable Bowel Syndrome (IBS): Role of Psyllium Fiber Supplementation in Decreasing Inflammation and Physiological Management of IBS. Turk. J. Gastroenterol. 2021, 32, 108–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- UCSF Health. Increasing Fiber Intake. ucsfhealth.org. 2024. Available online: https://www.ucsfhealth.org/education/increasing-fiber-intake (accessed on 2 December 2024).
- Li, C.; Li, J.; Zhou, Q.; Wang, C.; Hu, J.; Liu, C. Effects of Physical Exercise on the Microbiota in Irritable Bowel Syndrome. Nutrients 2024, 16, 2657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson-Martínez, J.P.; Diener, C.; Levine, A.E.; Wilmanski, T.; Suskind, D.L.; Ralevski, A.; Hadlock, J.; Magis, A.T.; Hood, L.; Rappaport, N.; et al. Aberrant bowel movement frequencies coincide with increased microbe-derived blood metabolites associated with reduced organ function. Cell Rep. Med. 2024, 5, 101646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.; Ouyang, J.; Tian, M.; Yang, J.; Gao, J.; Yang, M.; Zhang, M.; Yuan, H.; Zheng, Y.; Wang, Y.; et al. The associations between modifiable risk factors and constipation: A comprehensive mendelian randomization study. BMC Gastroenterol. 2024, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Özkütük, N.; Eşer, İ.; Bor, S. Effectiveness of Biofeedback Therapy on Quality of Life in Patients with Dyssynergic Defecation Disorder. Turk. J. Gastroenterol. 2021, 32, 22–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bashir, A.; Sizar, O. Laxatives. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537246/ (accessed on 2 December 2024).
- Zhao, Q.; Chen, Y.Y.; Xu, D.Q.; Yue, S.J.; Fu, R.J.; Yang, J.; Xing, L.M.; Tang, Y.P. Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation. Front. Pharmacol. 2021, 12, 630249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dabaja, A.; Abbas, M. Polyethylene Glycol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557652/ (accessed on 2 December 2024).
- Gremse, D.A.; Hixon, J.; Crutchfield, A. Comparison of Polyethylene Glycol 3350 and Lactulose for Treatment of Chronic Constipation in Children. Clin. Pediatr. 2002, 41, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.; Ling, J.; Jie, H.; Leung, K.; Poon, E. The potential role of lactulose pharmacotherapy in the treatment and prevention of diabetes. Front. Endocrinol. 2022, 13, 956203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mori, H.; Tack, J.; Suzuki, H. Magnesium Oxide in Constipation. Nutrients 2021, 13, 421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupont, C.; Hébert, G. Magnesium Sulfate-Rich Natural Mineral Waters in the Treatment of Functional Constipation—A Review. Nutrients 2020, 12, 2052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farinde, A. Laxatives, Stool Softeners, and Prokinetic Agents: Laxatives, Stool Softeners, and Prokinetic Agents. Available online: https://emedicine.medscape.com/article/2172208-overview (accessed on 2 December 2024).
- Alsalimy, N.; Madi, L.; Awaisu, A. Efficacy and safety of laxatives for chronic constipation in long-term care settings: A systematic review. J. Clin. Pharm. Ther. 2018, 43, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Lawrensia, S.; Patel, P.; Raja, A. Bisacodyl. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547733/ (accessed on 2 December 2024).
- Krans, B. Natural Ways and Tips to Soften Your Stool. Healthline. 2024. Available online: https://www.healthline.com/health/digestive-health/natural-stool-softeners (accessed on 2 December 2024).
- Rao, S.S.; Manabe, N.; Karasawa, Y.; Hasebe, Y.; Nozawa, K.; Nakajima, A.; Fukudo, S. Comparative profiles of lubiprostone, linaclotide, and elobixibat for chronic constipation: A systematic literature review with meta-analysis and number needed to treat/harm. BMC Gastroenterol. 2024, 24, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hillemeier, C. An overview of the effects of dietary fiber on gastrointestinal transit. Pediatrics 1995, 96 Pt 2, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.M.; Sharma, A.; Rao, S.S.C.; Laitman, A.P.; Heimanson, Z.; Allen, C.; Sayuk, G.S. Plecanatide Improves Abdominal Bloating and Bowel Symptoms of Irritable Bowel Syndrome with Constipation. Dig. Dis. Sci. 2024, 69, 1731–1738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miner, P.B., Jr. Elobixibat, the first-in-class Ileal Bile Acid Transporter inhibitor, for the treatment of Chronic Idiopathic Constipation. Expert Opin. Pharmacother. 2018, 19, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Camilleri, M. Elobixibat and its potential role in chronic idiopathic constipation. Therap. Adv. Gastroenterol. 2014, 7, 167–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordon, M.; MacDonald, J.K.; Parker, C.E.; Akobeng, A.K.; Thomas, A.G. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst. Rev. 2016, 2016, CD009118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corsetti, M.; Landes, S.; Lange, R. Bisacodyl: A review of pharmacology and clinical evidence to guide use in clinical practice in patients with constipation. Neurogastroenterol. Motil. 2021, 33, e14123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.H.; Suh, W.S.; Jeong, J.S.; Kim, D.S.; Kim, S.W.; Kwak, D.M.; Hwang, J.S.; Kim, H.J.; Park, M.W.; Shim, M.C.; et al. Effectiveness of Sodium Picosulfate/Magnesium Citrate (PICO) for Colonoscopy Preparation. Ann. Coloproctol. 2014, 30, 222–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Omer, A.; Quigley, E.M.M. An update on prucalopride in the treatment of chronic constipation. Therap. Adv. Gastroenterol. 2017, 10, 877–887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greenwood-Van Meerveld, B.; Standifer, K.M. Methylnaltrexone in the treatment of opioid-induced constipation. Clin. Exp. Gastroenterol. 2008, 1, 49–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braun, U.K.; Jackson, L.K.; Garcia, M.A.; Imam, S.N. A Systematic Review of Naldemedine and Naloxegol for the Treatment of Opioid-Induced Constipation in Cancer Patients. Pharmacy 2024, 12, 48. [Google Scholar] [CrossRef]
- Lacy, B.E.; Yu, S. Tegaserod: A new 5-HT4 agonist. J. Clin. Gastroenterol. 2002, 34, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V.; Corsetti, M. Exploring Pharmacological Treatments for Chronic Idiopathic Constipation in Adults: A Look Back to the Future. J. Clin. Med. 2023, 12, 1702. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Goldstein, S.; Witkowski, T.; Andonakakis, A.; Jan, R.; Gabriel, K.; Du, W.; Techner, L.; Wallin, B. Alvimopan, a peripherally acting mu-opioid receptor antagonist, compared with placebo in postoperative ileus after major abdominal surgery: Results of a randomized, double-blind, controlled study. Surg. Endosc. 2006, 20, 64–70, Erratum in Surg. Endosc. 2006, 20, 537. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, T. Luminally Acting Agents for Constipation Treatment: A Review Based on Literatures and Patents. Front. Pharmacol. 2017, 8, 418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahsan, M.K.; Tchernychev, B.; Kessler, M.M.; Solinga, R.M.; Arthur, D.; Linde, C.I.; Silos-Santiago, I.; Hannig, G.; Ameen, N.A. Linaclotide activates guanylate cyclase-C/cGMP/protein kinase-II-dependent trafficking of CFTR in the intestine. Physiol. Rep. 2017, 5, e13299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, Y.S.; Kang, S.B.; Marchelletta, R.R.; Penrose, H.M.; Ruiter-Visser, R.; Jung, B.; Docherty, M.J.; Boland, B.S.; Sandborn, W.J.; McCole, D.F. The ClC-2 Chloride Channel Activator, Lubiprostone, Improves Intestinal Barrier Function in Biopsies from Crohn’s Disease but Not Ulcerative Colitis Patients. Pharmaceutics 2023, 15, 811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Busby, R.W.; Bryant, A.P.; Bartolini, W.P.; Cordero, E.A.; Hannig, G.; Kessler, M.M.; Mahajan-Miklos, S.; Pierce, C.M.; Solinga, R.M.; Sun, L.J.; et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur. J. Pharmacol. 2010, 649, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Videlock, E.J.; Cheng, V.; Cremonini, F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: A meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1084–1092.e3, quiz e68. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C. Plecanatide: A new guanylate cyclase agonist for the treatment of chronic idiopathic constipation. Therap. Adv. Gastroenterol. 2018, 11, 1756284818777945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. Serotonin 5-HT4 Receptor Agonists. 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548948/ (accessed on 2 December 2024).
- Ke, M.; Zou, D.; Yuan, Y.; Li, Y.; Lin, L.; Hao, J.; Hou, X.; Kim, H.J. Prucalopride in the treatment of chronic constipation in patients from the Asia-Pacific region: A randomized, double-blind, placebo-controlled study. Neurogastroenterol. Motil. 2012, 24, 999-e541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manini, M.L.; Camilleri, M.; Goldberg, M.; Sweetser, S.; McKinzie, S.; Burton, D.; Wong, S.; Kitt, M.M.; Li, Y.P.; Zinsmeister, A.R. Effects of Velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol. Motil. 2010, 22, 42-e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ihara, E.; Manabe, N.; Ohkubo, H.; Ogasawara, N.; Ogino, H.; Kakimoto, K.; Kanazawa, M.; Kawahara, H.; Kusano, C.; Kuribayashi, S.; et al. Evidence-Based Clinical Guidelines for Chronic Constipation 2023. Digestion, 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Ren, D.; Tao, Y.; Mai, F.; Zhu, J.; Li, X.; Colla, E.; Grimaldi, M.; Giovannini, R.; et al. The 5HT4R agonist velusetrag efficacy on neuropathic chronic intestinal pseudo-obstruction in PrP-SCA7-92Q transgenic mice. Front. Pharmacol. 2024, 15, 1411642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grigoletto, J.; Miraglia, F.; Benvenuti, L.; Pellegrini, C.; Soldi, S.; Galletti, S.; Cattaneo, A.; Pich, E.M.; Grimaldi, M.; Colla, E.; et al. Velusetrag rescues GI dysfunction, gut inflammation and dysbiosis in a mouse model of Parkinson’s disease. npj Park. Dis. 2023, 9, 140. [Google Scholar] [CrossRef]
- Belu, A.M.; Nicoara, A.D.; Belu, D.M.; Circo, E. Evaluation of MELD Scores and Thyroid Hormones as Prognostic Factors of Liver Cirrhosis. Medicina 2024, 60, 1474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of Probiotic Compounds in Relieving Constipation and Their Colonization in Gut Microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Umeano, L.; Iftikhar, S.; Alhaddad, S.F.; Paulsingh, C.N.; Riaz, M.F.; Garg, G.; Mohammed, L. Effectiveness of Probiotic Use in Alleviating Symptoms of Irritable Bowel Syndrome: A Systematic Review. Cureus 2024, 16, e58306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Sánchez, C.; Escudero-López, B.; Fernández-Pachón, M.S. Evaluation of the efficacy of probiotics as treatment in irritable bowel syndrome. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2024, 71, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic significance of Lactobacillus strains: A comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terrén Lora, A.; Penadés, B.F.; López Oliva, S.; Arponen, S.; Okutan, G.; Sánchez Niño, G.M.; San Mauro Martín, I. Supplementation with probiotics, prebiotics, and synbiotics in patients with chronic functional constipation: A randomized, double-blind, placebo-controlled pilot clinical trial. Gastroenterol. Rep. 2024, 12, goae101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Squeo, F.; Celiberto, F.; Ierardi, E.; Russo, F.; Riezzo, G.; D’Attoma, B.; Di Leo, A.; Losurdo, G. Opioid-induced Constipation: Old and New Concepts in Diagnosis and Treatment. J. Neurogastroenterol. Motil. 2024, 30, 131–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Go, J.T. Update on the management of constipation in the elderly: New treatment options. Clin. Interv. Aging 2010, 5, 163–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voskuijl, W.; de Lorijn, F.; Verwijs, W.; Hogeman, P.; Heijmans, J.; Mäkel, W.; Taminiau, J.; Benninga, M. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: A double blind, randomised, controlled, multicentre trial. Gut 2004, 53, 1590–1594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quigley, E.M. Prucalopride: Safety, efficacy and potential applications. Therap. Adv. Gastroenterol. 2012, 5, 23–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, R.R.; Park, S.Y.; Camilleri, M. Constipation Research Group of Korean Society of Neurogastroenterology and Motility. Constipation in Patients With Chronic Kidney Disease. J. Neurogastroenterol. Motil. 2023, 29, 428–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee-Robichaud, H.; Thomas, K.; Morgan, J.; Nelson, R.L. Lactulose versus Polyethylene Glycol for Chronic Constipation. Cochrane Database Syst. Rev. 2010, 7, CD007570. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.; Richter, E.; Eberlin, M.; Weigmann, H.; Mück, T.; Kamm, M.A. Bisacodyl and Sodium Picosulfate Improve Bowel Function and Quality of Life in Patients with Chronic Constipation—Analysis of Pooled Data from Two Randomized Controlled Trials. Open J. Gastroenterol. 2017, 07, 32–43. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Christo, P.J.; LeQuang, J.A.; Magnusson, P. The Use of Peripheral μ-Opioid Receptor Antagonists (PAMORA) in the Management of Opioid-Induced Constipation: An Update on Their Efficacy and Safety. Drug Des. Devel. Ther. 2020, 14, 1009–1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andronache, I.T.; Şuţa, V.C.; Şuţa, M.; Ciocodei, S.L.; Vladareanu, L.; Nicoara, A.D.; Arghir, O.C. Better Safe than Sorry: Rheumatoid Arthritis, Interstitial Lung Disease, and Medication-A Narrative Review. Biomedicines 2023, 11, 1755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chamie, K.; Golla, V.; Lenis, A.T.; Lec, P.M.; Rahman, S.; Viscusi, E.R. Peripherally Acting μ-Opioid Receptor Antagonists in the Management of Postoperative Ileus: A Clinical Review. J. Gastrointest. Surg. 2021, 25, 293–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.Y.; Zhou, R.; Gu, W.J. Efficacy and Safety of Methylnaltrexone for the Treatment of Opioid-Induced Constipation: A Meta-analysis of Randomized Controlled Trials. Pain Ther. 2021, 10, 165–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakajima, A.; Fujimaki, M.; Arai, Y.; Emori, K. Safety and Efficacy of Elobixibat, an Ileal Bile Acid Transporter Inhibitor, in Elderly Patients With Chronic Idiopathic Constipation According to Administration Time: Interim Analysis of Post-marketing Surveillance. J. Neurogastroenterol. Motil. 2022, 28, 431–441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomie, A.; Yoshida, N.; Kugai, M.; Hirose, R.; Dohi, O.; Inoue, K.; Okuda, K.; Motoyoshi, T.; Fukumoto, K.; Inagaki, Y.; et al. The Efficacy and Safety of Elobixibat for the Elderly with Chronic Constipation: A Multicenter Retrospective Cohort Study. Gastroenterol. Res. Pract. 2020, 2020, 9656040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verne, G.N.; Eaker, E.Y.; Davis, R.H.; Sninsky, C.A. Colchicine is an effective treatment for patients with chronic constipation: An open-label trial. Dig. Dis. Sci. 1997, 42, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Roarty, T.P.; Weber, F.; Soykan, I.; McCallum, R.W. Misoprostol in the treatment of chronic refractory constipation: Results of a long-term open label trial. Aliment. Pharmacol. Ther. 1997, 11, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Soffer, E.E.; Metcalf, A.; Launspach, J. Misoprostol is effective treatment for patients with severe chronic constipation. Dig. Dis. Sci. 1994, 39, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Sohn, G.; Yu, C.S.; Kim, C.W.; Kwak, J.Y.; Jang, T.Y.; Kim, K.H.; Yang, S.S.; Yoon, Y.S.; Lim, S.B.; Kim, J.C. Surgical outcomes after total colectomy with ileorectal anastomosis in patients with medically intractable slow transit constipation. J. Korean Soc. Coloproctol. 2011, 27, 180–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bharucha, A.E. Constipation. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.E. Surgical management of constipation. Clin. Colon. Rectal. Surg. 2005, 18, 81–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Mazilu, L.; Ciufu, N.; Gălan, M.; Suceveanu, A.I.; Suceveanu, A.P.; Parepa, I.R.; Tofolean, D. Postherapeutic follow-up of colorectal cancer patients treated with curative intent. Chirurgia 2012, 107, 55–58. [Google Scholar] [PubMed]
- Patton, V.; Stewart, P.; Lubowski, D.Z.; Cook, I.J.; Dinning, P.G. Sacral Nerve Stimulation Fails to Offer Long-term Benefit in Patients With Slow-Transit Constipation. Dis. Colon. Rectum. 2016, 59, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Suceveanu, A.P.; Serban, D.; Caloian, A.D.; Cozaru, G.C.; Chisoi, A.; Nicolau, A.A.; Ioan Sergiu, M.; Andra Iulia, S. Correlation between molecular prognostic factors and bevacizumab therapeutic resistance in patients with metastatic colorectal cancer; the AVAMET study. J. Mind Med. Sci. 2024, 11, 22. [Google Scholar] [CrossRef]
| Category | Criteria |
|---|---|
| Functional constipation |
|
| IBS-C |
|
| OIC |
|
| Defecatory disorders |
|
| Agent | Mechanism of Action | Clinical Effects |
|---|---|---|
| Lubiprostone | ClC-2 activator, EP4 receptor agonist | Improves transit, relieves discomfort and bloating, and lessens the intensity of constipation [60]. |
| Misoprostol | Synthetic prostaglandin E2 analogue | Increases frequency of bowel movements, transit time, and releases fluids and bicarbonate [61]. |
| Linaclotide | Guanylate cyclase-C (GC-C) agonist | Increases the frequency of bowel movements and transit [62]. |
| Plecanatide | Guanylate cyclase-C (GC-C) agonist | Taken once a day, it has localised intestinal activity and extremely powerful activity [63]. |
| Sodium chenodeoxycholate | Bile acid analogue | Increases mucus secretion, improves mucosal permeability and intestinal motility [64]. |
| Elobixibat | Ileal bile acid transporter (IBAT) inhibitor | Alters the circulation of bile acids and promotes the motility and production of colonic fluid [65,66]. |
| Polyethylene glycol (PEG) | Osmotic laxative | Retains water in the intestine, softens stools, and improves stool frequency [67]. |
| Lactulose | Osmotic laxative | Fermented in the colon to increase osmotic pressure, soften stool, and reduce strain [68]. |
| Bisacodyl | Stimulant laxative | Stimulates intestinal motility and alters electrolyte secretion, effective for acute constipation [69]. |
| Sodium picosulfate | Stimulant laxative | Stimulates peristalsis and promotes bowel emptying, commonly used in bowel preparation [70]. |
| Prucalopride | 5-HT4 receptor agonist | Enhances colonic motility, promotes spontaneous bowel movements, and is effective in refractory constipation [71]. |
| Methylnaltrexone | Peripheral μ-opioid receptor antagonist | Relieves opioid-induced constipation without affecting central analgesia [72]. |
| Naloxegol | PEGylated μ-opioid receptor antagonist | Effective for opioid-induced constipation in non-cancer patients [73]. |
| Tegaserod | Partial 5-HT4 receptor agonist | Enhances intestinal motility and reduces visceral hypersensitivity, approved for IBS-C [74]. |
| Colchicine | Anti-inflammatory | Increases intestinal motility as a secondary effect, useful in refractory constipation [75]. |
| Alvimopan | Peripheral μ-opioid receptor antagonist | Accelerates recovery of bowel function after surgery, particularly postoperative ileus [76]. |
| Glycerin suppositories | Hyperosmotic laxative | Rapidly softens stool and provides relief for rectal constipation [72]. |
| Mineral oils | Lubricant | Lubricates the intestinal lining and softens stool, reducing water absorption [55]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrescu, L.; Iordache, I.E.; Stanigut, A.M.; Condur, L.M.; Tofolean, D.E.; Popescu, R.C.; Nelson Twakor, A.; Dumitru, E.; Dumitru, A.; Tocia, C.; et al. Rehabilitation for Chronic Constipation: Integrative Approaches to Diagnosis and Treatment. Gastrointest. Disord. 2025, 7, 11. https://doi.org/10.3390/gidisord7010011
Alexandrescu L, Iordache IE, Stanigut AM, Condur LM, Tofolean DE, Popescu RC, Nelson Twakor A, Dumitru E, Dumitru A, Tocia C, et al. Rehabilitation for Chronic Constipation: Integrative Approaches to Diagnosis and Treatment. Gastrointestinal Disorders. 2025; 7(1):11. https://doi.org/10.3390/gidisord7010011
Chicago/Turabian StyleAlexandrescu, Luana, Ionut Eduard Iordache, Alina Mihaela Stanigut, Laura Maria Condur, Doina Ecaterina Tofolean, Razvan Catalin Popescu, Andreea Nelson Twakor, Eugen Dumitru, Andrei Dumitru, Cristina Tocia, and et al. 2025. "Rehabilitation for Chronic Constipation: Integrative Approaches to Diagnosis and Treatment" Gastrointestinal Disorders 7, no. 1: 11. https://doi.org/10.3390/gidisord7010011
APA StyleAlexandrescu, L., Iordache, I. E., Stanigut, A. M., Condur, L. M., Tofolean, D. E., Popescu, R. C., Nelson Twakor, A., Dumitru, E., Dumitru, A., Tocia, C., Herlo, A., & Tofolean, I. T. (2025). Rehabilitation for Chronic Constipation: Integrative Approaches to Diagnosis and Treatment. Gastrointestinal Disorders, 7(1), 11. https://doi.org/10.3390/gidisord7010011







