Percutaneous Biliary Rendez-Vous to Treat Complete Hepatic-Jejunal Anastomosis Dehiscence after Duodeno-Cephalo-Pancreasectomy
Abstract
1. Introduction
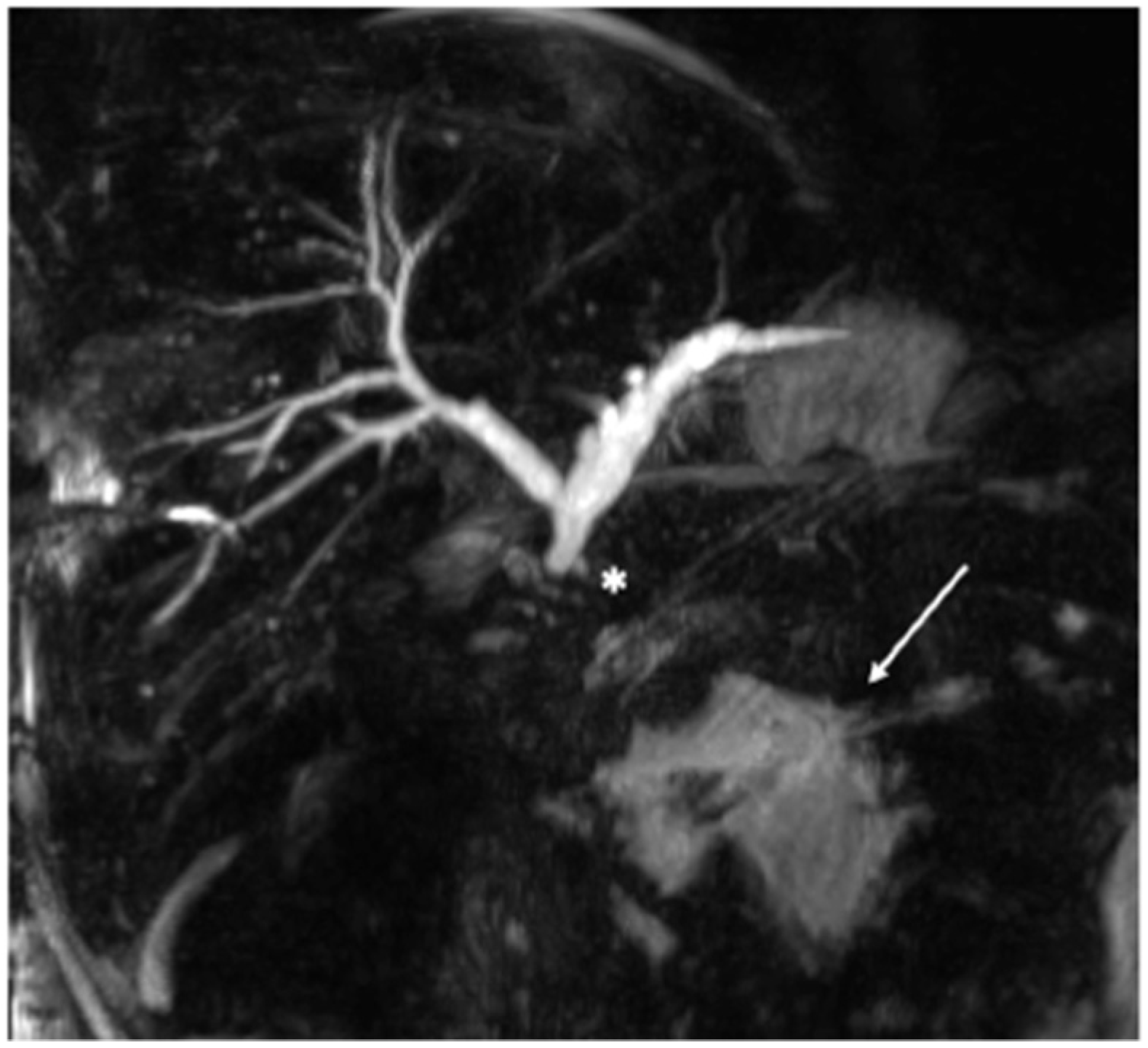
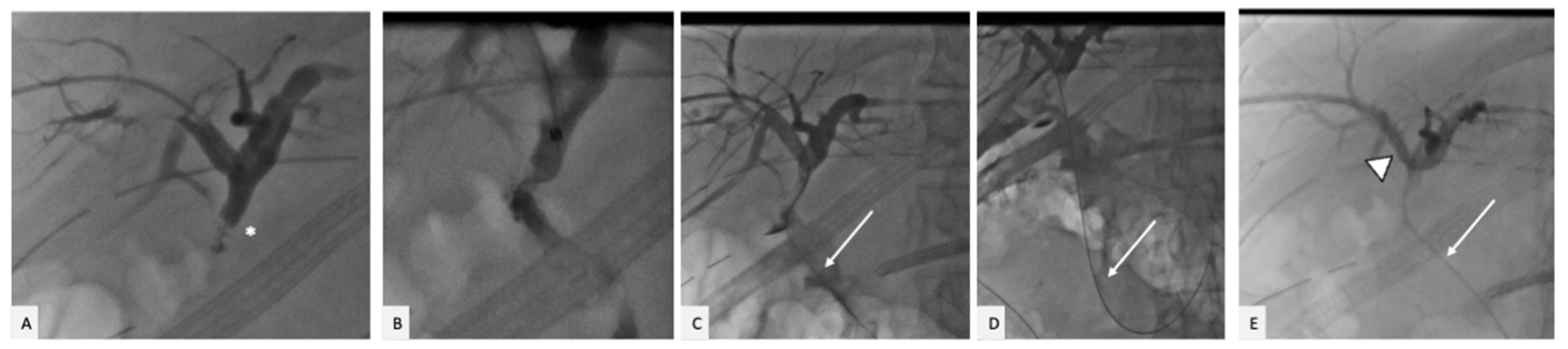
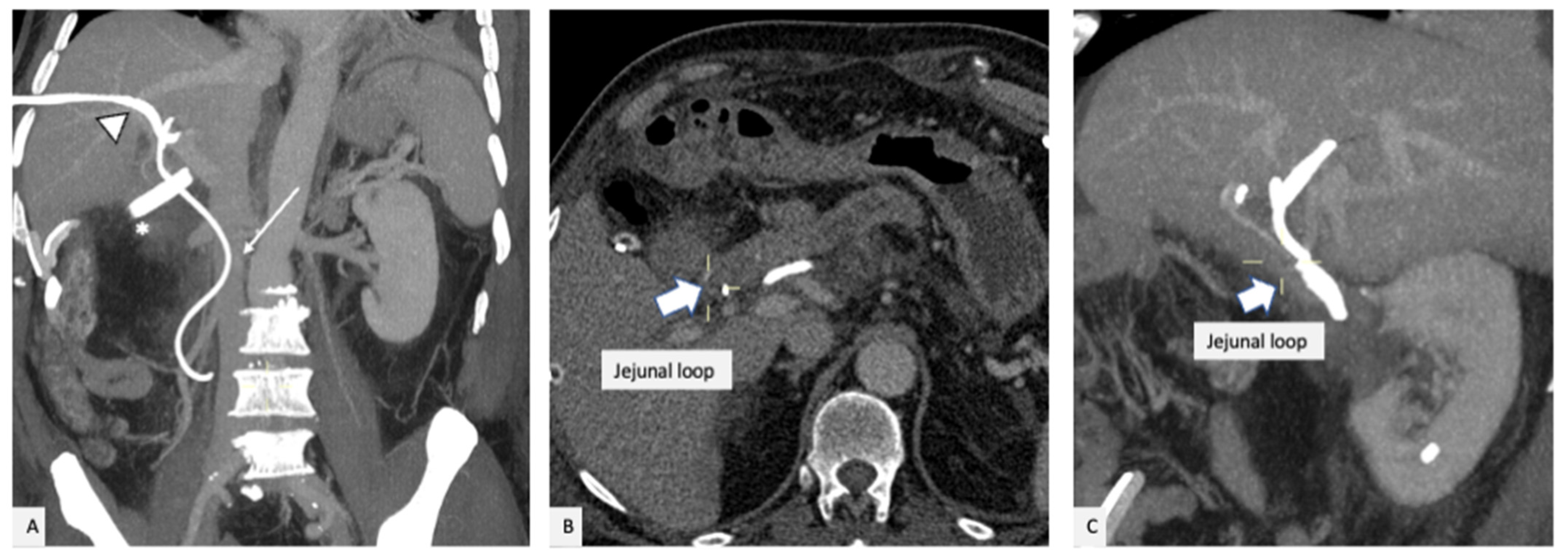
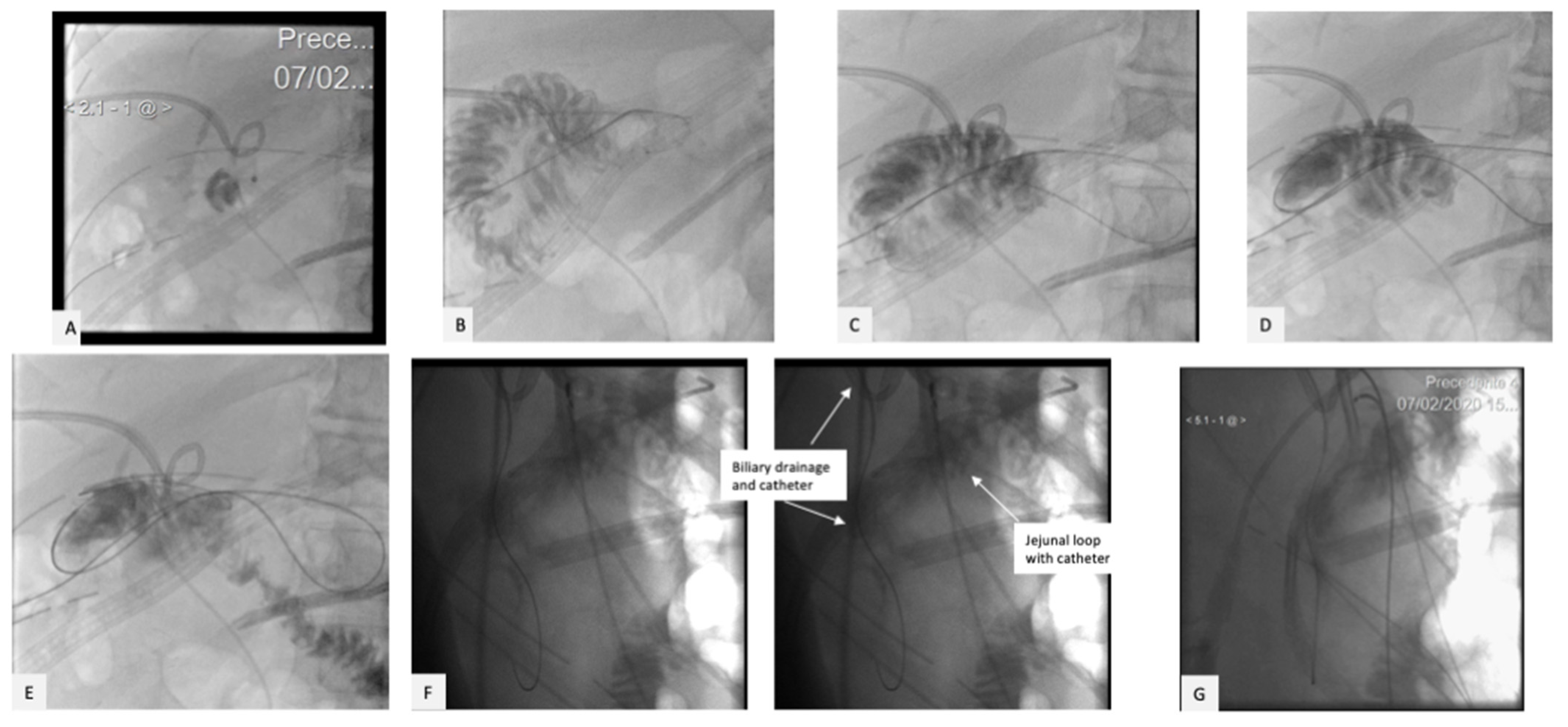
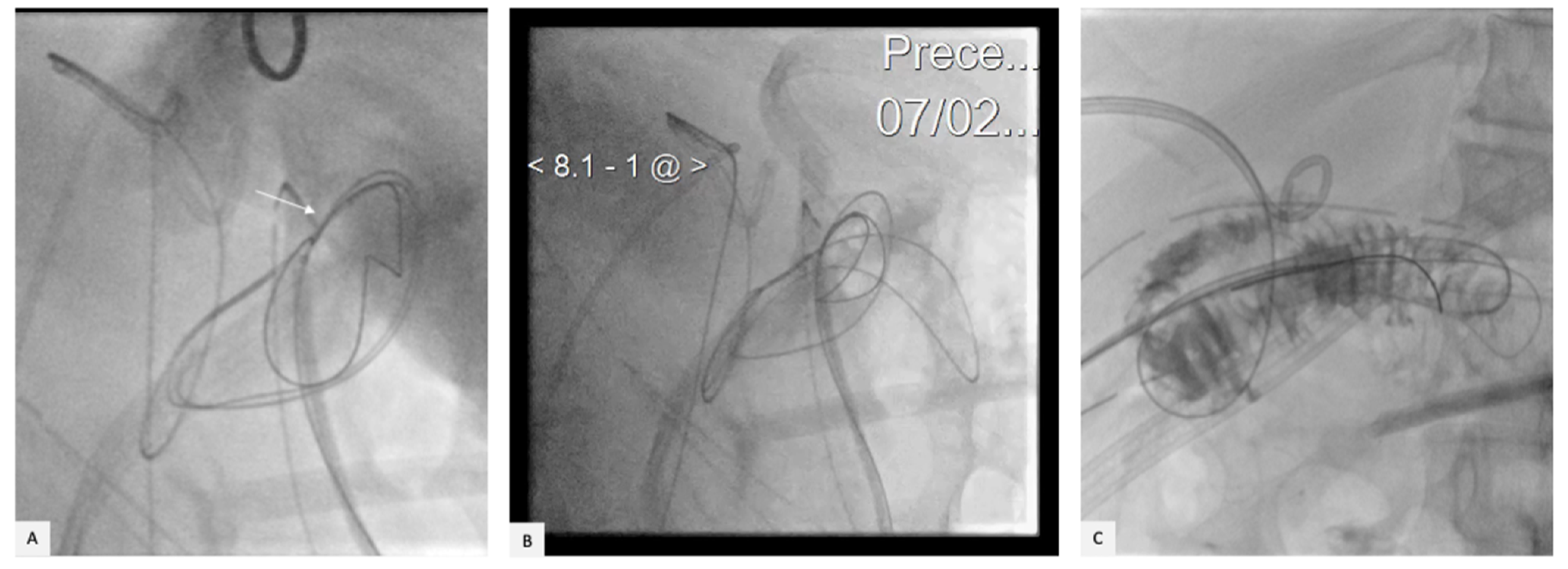
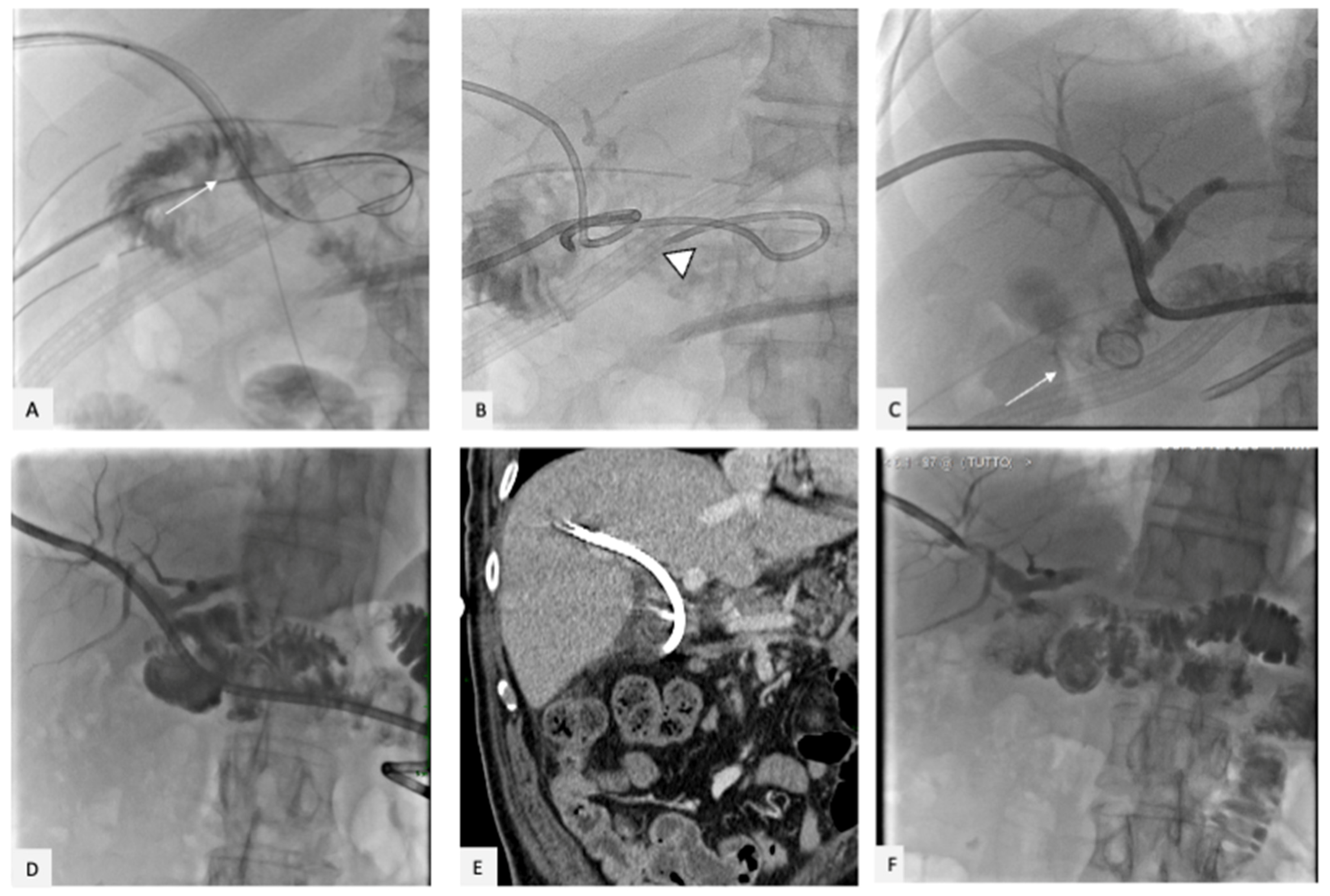
2. Case Presentation
3. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Castro, S.; Kuhlmann, K.; Busch, O.; van Delden, O.; Lameris, J.; van Gulik, T.; Obertop, H.; Gouma, D. Incidence and management of biliary leakage after hepaticojejunostomy. J. Gastrointest. Surg. 2005, 9, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, W.; Penninga, L.; Burgdorf, S.K.; Storkholm, J.H.; Hansen, C.P. Biliary Leakage Following Pancreatoduodenectomy: Experience from a High-Volume Center. J. Pancreat. Cancer 2021, 7, 80–85. [Google Scholar] [CrossRef] [PubMed]
- El Nakeeb, A.; El Sorogy, M.; Hamed, H.; Said, R.; Elrefai, M.; Ezzat, H.; Askar, W.; Elsabbagh, A. Biliary leakage following pancreaticoduodenectomy: Prevalence, risk factors and management. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Righi, D.; Franchello, A.; Ricchiuti, A.; Breatta, A.D.; Versace, K.; Calvo, A.; Romagnoli, R.; Fonio, P.; Gandini, G.; Salizzoni, M. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl. 2008, 14, 611–615. [Google Scholar] [CrossRef]
- Booij, K.A.; Coelen, R.J.; de Reuver, P.R.; Besselink, M.G.; van Delden, O.M.; Rauws, E.A.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Long-term follow-up and risk factors for strictures after hepaticojejunostomy for bile duct injury: An analysis of surgical and percutaneous treatment in a tertiary center. Surgery 2018, 163, 1121–1127. [Google Scholar] [CrossRef]
- de Reuver, P.R.; Sprangers, M.A.G.; Rauws, E.A.J.; Lameris, J.S.; Busch, O.R.; Van Gulik, T.M.; Gouma, D.J. Impact of bile duct injury after laparoscopic cholecystectomy on quality of life: A longitudinal study after multidisciplinary treatment. Endoscopy 2008, 40, 637–643. [Google Scholar] [CrossRef]
- de Reuver, P.R.; Rauws, E.A.; Bruno, M.J.; Lameris, J.S.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Survival in bile duct injury patients after laparoscopic cholecystectomy: A multidisciplinary approach of gastroenterologists, radiologists, and surgeons. Surgery 2007, 142, 1–9. [Google Scholar] [CrossRef]
- Henry, A.C.; Smits, F.J.; van Lienden, K.; Heuvel, D.A.V.D.; Hofman, L.; Busch, O.R.; van Delden, O.M.; Zijlstra, I.A.; Schreuder, S.M.; Lamers, A.B.; et al. Biliopancreatic and biliary leak after pancreatoduodenectomy treated by percutaneous transhepatic biliary drainage. HPB (Oxf.) 2022, 24, 489–497. [Google Scholar] [CrossRef]
- May, K.; Hunold, P. Leakage of Hepaticojejunal Anastomosis: Radiological Interventional Therapy. Visc. Med. 2017, 33, 192–196. [Google Scholar] [CrossRef]
- Lau, W.Y.; Lai, E.C.; Lau, S.H. Management of bile duct injury after laparoscopic cholecystectomy: A review. ANZ J. Surg 2010, 80, 75–81. [Google Scholar] [CrossRef]
- Kaffes, A.J.; Hourigan, L.; De Luca, N.; Byth, K.; Williams, S.J.; Bourke, M.J. Impact of endoscopic intervention in 100 patients with suspected postcholecystectomy bile leak. Gastrointest. Endosc. 2005, 61, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sandha, G.S.; Bourke, M.J.; Haber, G.B.; Kortan, P.P. Endoscopic therapy for bile leak based on a new classification: Results in 207 patients. Gastrointest. Endosc. 2004, 60, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Kim, A.R.; Hwang, J.C.; Yoo, B.M.; Kim, J.H. Long-type double-balloon enteroscopy-assisted ERCP using hand-made accessories in Roux-en-Y hepaticojejunostomy (with video). Hepatobiliary Pancreat. Dis. Int. 2021, 20, 407–408. [Google Scholar] [CrossRef]
- Farina, E.; Cantù, P.; Cavallaro, F.; Iori, V.; Rosa-Rizzotto, E.; Cavina, M.; Tontini, G.E.; Nandi, N.; Scaramella, L.; Sassatelli, R.; et al. Effectiveness of double-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography (DBE-ERCP): A multicenter real-world study. Dig. Liver Dis. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sandha, G.S.; Bourke, M.J.; Haber, G.B.; Kortan, P.P. Percutaneous transhepatic biliary drainage in the management of postsurgical biliary leaks in patients with nondilated intrahepatic bile ducts. Cardiovasc. Intervent. Radiol. 2006, 29, 380–388. [Google Scholar]
- Mosconi, C.; Cocozza, M.A.; Piacentino, F.; Fontana, F.; Cappelli, A.; Modestino, F.; Coppola, A.; Palumbo, D.; Marra, P.; Maffi, P.; et al. Interventional Radiological Management and Prevention of Complications after Pancreatic Surgery: Drainage, Embolization and Islet Auto-Transplantation. J. Clin. Med. 2022, 11, 6005. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Geschwind, J.F.; Mitchell, S.E.; Venbrux, A.C.; Lillemoe, K.D. Pancreaticoduodenectomy: Role of interventional radiologists in managing patients and complications. J. Gastrointest. Surg. 2003, 7, 209–219. [Google Scholar] [CrossRef]
- Mauri, G.; Mattiuz, C.; Sconfienza, L.M.; Pedicini, V.; Poretti, D.; Melchiorre, F.; Rossi, U.; Lutman, F.R.; Montorsi, M. Role of interventional radiology in the management of complications after pancreatic surgery: A pictorial review. Insights Imaging 2015, 6, 231–239. [Google Scholar] [CrossRef]
- Büchler, M.W.; Wagner, M.; Schmied, B.M.; Uhl, W.; Friess, H.; Z’Graggen, K. Changes in the morbidity after pancreatic resection. Arch. Surg. 2003, 138, 1310–1314. [Google Scholar] [CrossRef]
- House, M.G.; Cameron, J.L.; Schulick, R.D.; Campbell, K.A.; Sauter, P.K.; Coleman, J.; Lillemoe, K.D.; Yeo, C.J. Incidence and outcome of biliary strictures after pancreaticoduodenectomy. Ann. Surg. 2006, 243, 571–578. [Google Scholar] [CrossRef]
- De Robertis, R.; Contro, A.; Zamboni, G.; Mansueto, G. Totally percutaneous rendezvous techniques for the treatment of bile strictures and leakages. J. Vasc. Interv. Radiol. 2014, 25, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.; Fletcher, S.; Crumley, K.; Culp, W.C.; Meek, M. Percutaneous rendezvous technique for the management of a bile duct injury. Radiol. Case Rep. 2018, 13, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Huespe, P.E.; Oggero, S.; de Santibañes, M.; Boldrini, G.; D’agostino, D.; Pekolj, J.; de Santibañes, E.; Ciardullo, M.; Hyon, S.H. Percutaneous patency recovery and biodegradable stent placement in a totally occluded hepaticojejunostomy after paediatric living donor liver transplantation. Cardiovasc. Intervent. Radiol. 2019, 42, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.F. Combined percutaneous and endoscopic procedures for bile duct obstruction. Gut 1994, 35, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.P.; Ferrari, A.P. Cholangioscopy-assisted guidewire placement in post-liver transplant anastomotic biliary stricture: Efficient and potentially also cost-effective. Endoscopy 2017, 49, E283–E284. [Google Scholar] [CrossRef]
- Woo, Y.S.; Lee, J.K.; Noh, D.H.; Park, J.K.; Lee, K.H.; Lee, K.T. SpyGlass cholangioscopy-assisted guidewire placement for post-LDLT biliary strictures: A case series. Surg. Endosc. 2016, 30, 3897–3903. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andresciani, F.; Pacella, G.; Faiella, E.; Buoso, A.; Altomare, C.; Grasso, R.F. Percutaneous Biliary Rendez-Vous to Treat Complete Hepatic-Jejunal Anastomosis Dehiscence after Duodeno-Cephalo-Pancreasectomy. Gastrointest. Disord. 2023, 5, 68-74. https://doi.org/10.3390/gidisord5010007
Andresciani F, Pacella G, Faiella E, Buoso A, Altomare C, Grasso RF. Percutaneous Biliary Rendez-Vous to Treat Complete Hepatic-Jejunal Anastomosis Dehiscence after Duodeno-Cephalo-Pancreasectomy. Gastrointestinal Disorders. 2023; 5(1):68-74. https://doi.org/10.3390/gidisord5010007
Chicago/Turabian StyleAndresciani, Flavio, Giuseppina Pacella, Eliodoro Faiella, Andrea Buoso, Carlo Altomare, and Rosario Francesco Grasso. 2023. "Percutaneous Biliary Rendez-Vous to Treat Complete Hepatic-Jejunal Anastomosis Dehiscence after Duodeno-Cephalo-Pancreasectomy" Gastrointestinal Disorders 5, no. 1: 68-74. https://doi.org/10.3390/gidisord5010007
APA StyleAndresciani, F., Pacella, G., Faiella, E., Buoso, A., Altomare, C., & Grasso, R. F. (2023). Percutaneous Biliary Rendez-Vous to Treat Complete Hepatic-Jejunal Anastomosis Dehiscence after Duodeno-Cephalo-Pancreasectomy. Gastrointestinal Disorders, 5(1), 68-74. https://doi.org/10.3390/gidisord5010007






