Coadjuvant Anti-VEGF A Therapy Improves Survival in Patients with Colorectal Cancer with Liver Metastasis: A Systematic Review
Abstract
:1. Introduction
2. Results
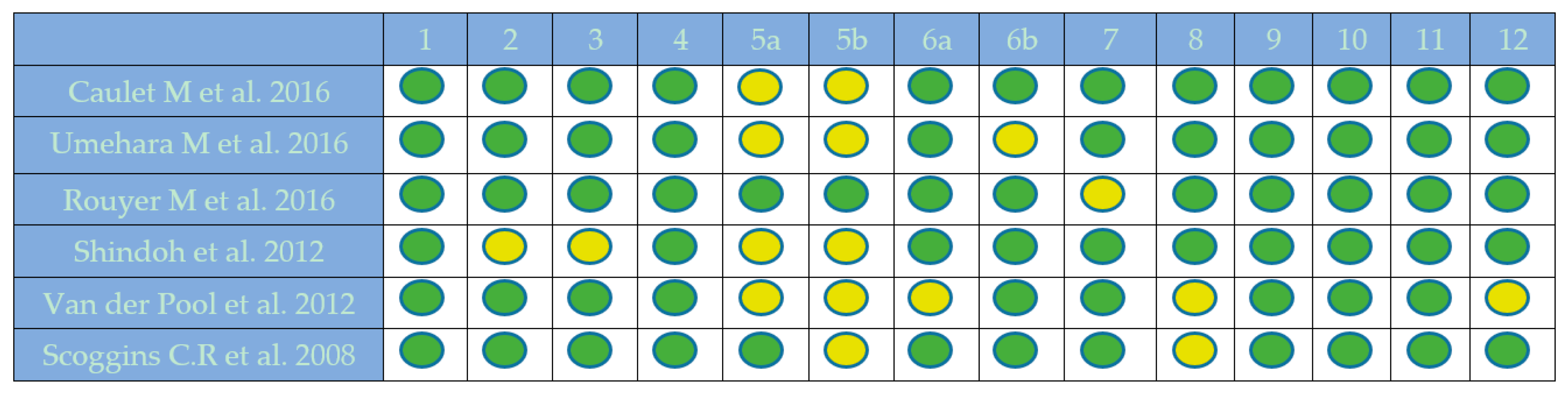
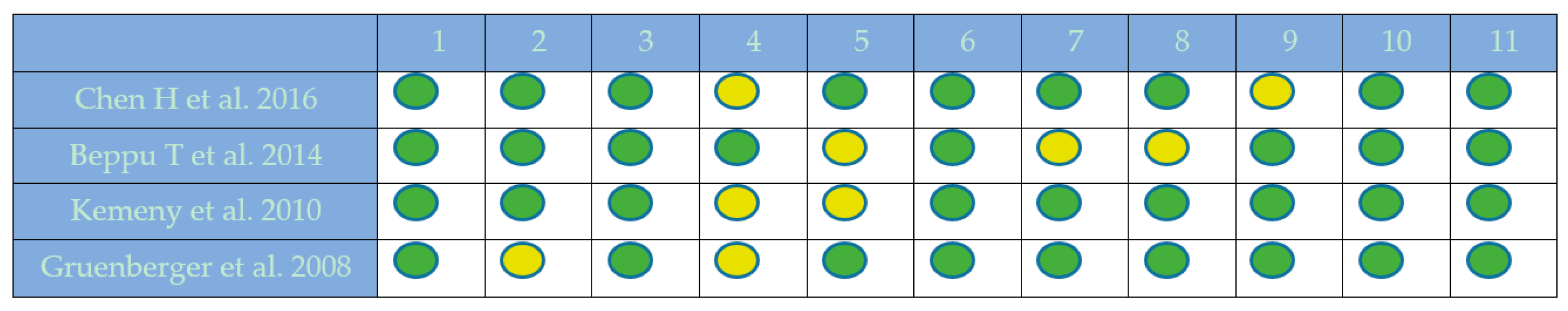
2.1. Quality Assessment
2.2. Population Description
2.3. Pharmacotherapy
2.4. Survival Outcome
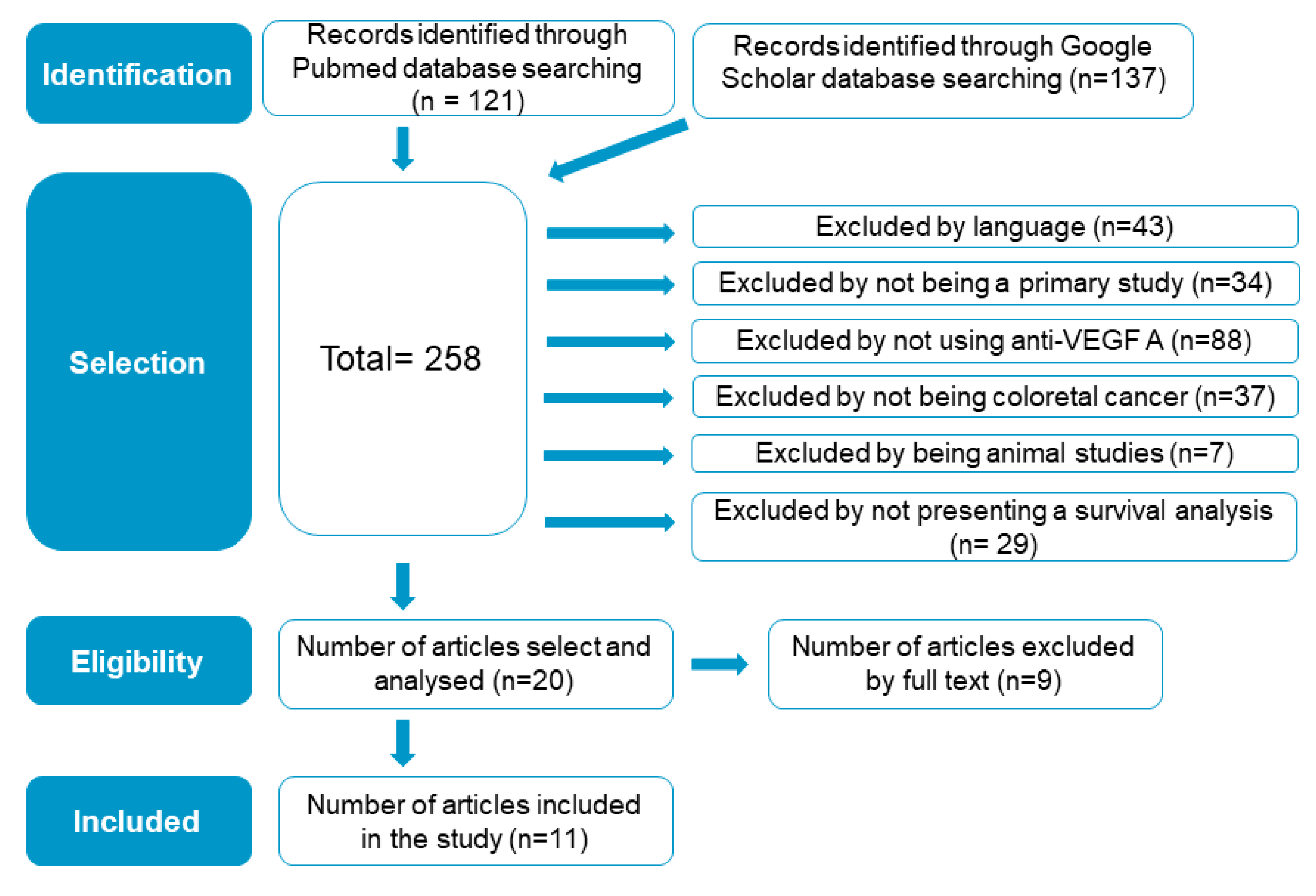
2.5. Included-Study Literature Search Results
3. Discussion
Recommendations
4. Materials and Methods
4.1. Criteria for Considering Studies for this Review
4.1.1. Types of Studies
4.1.2. Types of Participants
4.1.3. Types of Interventions
4.2. Search Methods for Identification of Studies
4.2.1. Data Sources and Searches
4.2.2. Selection of Studies, and Data Extraction and Management
4.2.3. Study-Eligibility Criteria
4.2.4. Quality Assessment of Included Studies
4.2.5. Data Synthesis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ilic, I.; Jankovic, S.; Ilic, M. Bevacizumab combined with chemotherapy improves survival for patients with metastatic colorectal cancer: Evidence from meta-analysis. PLoS ONE 2016, 11, 0161912. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, Y.L.; Chuang, J.P.; Lee, J.C. Differences in survival between colon and rectal cancer from SEER data. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Brouquet, A.; Cervantes, A. Primary colon cancer: ESMO clinical practice guidelines for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 2010, 21, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Umehara, Y.; Takahashi, K.; Murata, A.; Nishikawa, S.; Tokura, T.; Matsuzaka, M.; Tanaka, R.; Morita, T. Preoperative Chemotherapy with Bevacizumab Extends Disease-free Survival After Resection of Liver Metastases from Colorectal Cancer. Anticancer Res. 2016, 36, 1949–1954. [Google Scholar] [PubMed]
- Gruenberger, B.; Tamandl, D.; Schueller, J.; Scheithauer, W.; Zielinski, C.; Herbst, F.; Gruenberger, T. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Buchberger, B.; Pox, C.; Graeven, U.; Holch, J.W.; Schmiegel, W.; Heinemann, V. Efficacy of bevacizumab in first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Eur. J. Cancer 2019, 106, 37–44. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Lambrechts, D.; Prenen, H.; Jain, R.K.; Carmeliet, P. Lessons From the Adjuvant Bevacizumab Trial on Colon Cancer: What Next? J. Clin. Oncol. 2010, 29, 1–4. [Google Scholar] [CrossRef]
- Lambrechts, D.; Lenz, H.; De Haas, S.; Carmeliet, P.; Scherer, S.J. Markers of Response for the Antiangiogenic Agent Bevacizumab. J. Clin. Oncol. 2013, 31, 1219–1230. [Google Scholar] [CrossRef]
- Caulet, M.; Lecomte, T.; Bouché, O.; Rollin, J.; Gouilleux-Gruart, V.; Azzopardi, N.; Capitain, O.; Ferru, A.; Moussata, D.; Smith, D.; et al. Bevacizumab Pharmacokinetics Influence Overall and Progression-Free Survival in Metastatic Colorectal Cancer Patients. Clin. Pharmacokinet. 2016, 55, 1381–1394. [Google Scholar] [CrossRef]
- Rouyer, M.; Smith, D.; Laurent, C.; Becouarn, Y.; Guimbaud, R.; Michel, P.; Moore, N.; Ravaud, A.; Robinson, P.; Noize, P.; et al. Secondary Metastases Resection After Bevacizumab Plus Irinotecan-Based Chemotherapy in First-Line Therapy of Metastatic Colorectal Cancer in a Real-Life Setting: Results of the ETNA Cohort. Target Oncol. 2016, 11, 83–92. [Google Scholar] [CrossRef]
- Kemeny, N.E.; Jarnagin, W.R.; Capanu, M.; Fong, Y.; Gewirtz, A.N.; DeMatteo, R.P.; D’Angelica, M.I. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J. Clin. Oncol. 2011, 29, 884–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindoh, J.; Loyer, E.M.; Kopetz, S.; Boonsirikamchai, P.; Maru, D.M.; Chun, Y.S.; Zimmitti, G.; Curley, S.A.; Charnsangavej, C.; Aloia, T.A.; et al. Optimal morphologic response to preoperative chemotherapy: An alternate outcome end point before resection of hepatic colorectal metastases. J. Clin. Oncol. 2012, 30, 4566–4572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhang, J.; Cao, G.; Liu, P.; Xu, H.; Wang, X.; Zhu, X.; Gao, S.; Guo, J.; Zhang, P.; et al. Target hepatic artery regional chemotherapy and Bevacizumab perfusion in liver metastatic Colorectal cancer after failure of first-line or second-line systemic chemotherapy. Anticancer Drugs 2016, 27, 118–126. [Google Scholar] [CrossRef]
- Beppu, T.; Emi, Y.; Tokunaga, S.; Oki, E.; Shirabe, K.; Ueno, S.; Samura, H.; Akagi, Y.; Ogata, Y.; Eguchi, S.; et al. Liver resectability of advanced liver-limited colorectal liver metastases following mFOLFOX6 with bevacizumab (KSCC0802 study). Anticancer Res. 2014, 34, 6655–6662. [Google Scholar] [PubMed]
- Mahfud, M.; Breitenstein, S.; El-Badry, A.M.; Puhan, M.; Rickenbacher, A.; Samaras, P.; Pessaux, P.; Lopez-Ben, S.; Jaeck, D.; Alain-Clavien, P.; et al. Impact of preoperative bevacizumab on complications after resection of colorectal liver metastases: Case-matched control study. World J. Surg. 2010, 34, 92–100. [Google Scholar] [CrossRef]
- Van der Pool, A.E.; Marsman, H.A.; Verheij, J.; Ten Kate, F.J.; Eggermont, A.M.; IJzermans, J.N.; Verhoef, C. Effect of bevacizumab added preoperatively to oxaliplatin on liver injury and complications after resection of colorectal liver metastases. J. Surg. Oncol. 2012, 106, 892–897. [Google Scholar] [CrossRef]
- Scoggins, C.R.; Campbell, M.L.; Landry, C.S.; Slomiany, B.A.; Woodall, C.E.; McMasters, K.M.; Martin, R.C. Preoperative chemotherapy does not increase morbidity or mortality of hepatic resection for colorectal cancer metastases. Ann. Surg. Oncol. 2009, 16, 35–41. [Google Scholar] [CrossRef]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, Y.; Brennan, M.Y.; Blumgart, L.H.; D’Angelica, M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef]
- House, M.G.; Kemeny, N.E.; Gönen, M.; Fong, Y.; Allen, P.J.; Paty, P.B.; DeMatteo, R.P.; Blumgart, L.H.; Jarnagin, W.R.; D’Angelica, M.I. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann. Surg. 2011, 254, 851–856. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–321. [Google Scholar] [CrossRef]
- Pearce-Smith, N. Critical Appraisal Skills Programme (CASP) Checklist: 10 Questions to Help You Make Sense of a Systematic Review. Available online: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Systematic-Review-Checklist_2018.pdf (accessed on 12 May 2019).
- CASP. Critical appraisal skills programme. J. Pharmacol. Pharmacother. 2013, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- CASP. Critical Appraisal Skills Programme: CASP Case Control Study Checklist. Available online: https://www.casp-uk.net (accessed on 12 May 2019).

| Authors | Year | Study Type | Exclusion Criteria | Inclusion Criteria | No. of Patients | Age | No. Men | No. Women |
|---|---|---|---|---|---|---|---|---|
| Caulet et al. [9] | 2016 | Cohort | No info. | Eligible patients (18–80 years old) had histologically confirmed colorectal cancer (CRC) with at least one instance of hepatic metastasis detected by ultrasonography, life expectancy of more than two months, a World Health Organization (WHO) performance status of two or less, and were treated with first-line treatment by a bevacizumab-based chemotherapy. | 137 | 58–72 | 79 | 58 |
| Umehara et al. [4] | 2016 | Cohort | No info. | Patients were required to be between the ages of 18–80 years and to have histologically proven colorectal cancer with a World Health Organization performance status of two or less, potentially resectable liver metastases, and no detectable extrahepatic tumors. | 27 | 56–67 | 13 | 14 |
| Chen et al. [13] | 2016 | Clinical trial | Brain metastasis; ileus; inextricable obstructive jaundice; abdominal and pelvic effusion; and breastfeeding or pregnant women. | Patients diagnosed with colorectal cancer with liver metastasis; number of instances of liver metastasis of at least two or one, but difficult to resect by conventional surgery; after failure of first- or second-line or more systemic chemotherapy; expected lifetime of at least three months; no contraindication to treatment with chemotherapy; and age of at least 18 years old. | 63 | 40–80 | 41 | 22 |
| Rouyer et al. [10] | 2016 | Cohort | They were opposed to the collection of data regarding themselves or if they participated in a clinical trial, unless they were receiving a standard treatment (control arm) in an open-label Phase III study. | Proven diagnosis of CRC with nonresectable metastases, and treated with bevacizumab as first-line palliative therapy (nonresectability was documented in the medical files according to the multidisciplinary staff); when applicable, ≥6-month interval between adjuvant chemotherapy for primary tumor and initiation of bevacizumab; no chemotherapy for metastatic disease before the initiation of bevacizumab; and no prior history of treatment with bevacizumab, including as part of a clinical trial and during the period when bevacizumab was available under temporary use authorization (Autorisation Temporaire d’Utilisation). | 360 | 61–64 | 221 | 149 |
| Beppu et al. [14] | 2014 | Clinical trial | No info. | Patients with histologically proven colorectal cancer and at least one measurable lesion in the liver (with no nonhepatic distal metastasis/relapse) were eligible for this study if they met all of the following criteria: H2 or H3 CRC liver-metastasis (CRLM; either synchronous or metachronous); age ≥20 and ≤75 years; no prior chemotherapy except adjuvant chemotherapy if ended ≥6 months before study entry; no prior radiotherapy for advanced/recurrent colorectal cancer; Eastern Cooperative Oncology Group performance status (PS) 0 to 1; life expectancy estimated ≥ 3 months; adequate bone marrow and renal function. | 40 | 37–74 | 29 | 11 |
| Shindoh et al. [12] | 2012 | Cohort | No info. | No info. | 209 | 58 | 124 | 85 |
| Van der et al. [16] | 2012 | Cohort | Patients who had undergone portal-vein embolization (PVE) or portal-vein ligation (PVL), and patients who had been treated with other chemotherapeutics besides oxaliplatin-based chemotherapy. | Macroscopic radical resection of the liver metastases and the use of oxaliplatin-based CTx in neoadjuvant setting. | 104 | 41–79 | 62 | 42 |
| Kemeny et al. [11] | 2010 | Clinical trial | Extrahepatic disease, prior hepatic radiation, infection, history of stroke or transient ischemic attack, history of serious systemic illness, Karnofsky performance score <60, other malignancy (within five years before study entry), WBC count 3000/µL, absolute neutrophil count <1500/µL, platelet count ≤75,000/µL, and total bilirubin >2.0 mg/dL | Histologically confirmed colorectal adenocarcinoma with fully resected liver metastases. | 73 | 29 persons > or = to 60 years | 25 | 48 |
| Mahfud et al. [15] | 2009 | Case Control Study | No info. | Patients included were retrospectively assessed for eligibility using well-established databases in each respective center. | 90 | 54–65 | 50 | 40 |
| Scoggins et al. [17] | 2008 | Cohort | Patients treated with RFA only were not included. | Only patients who underwent hepatic resection or combined resection/radiofrequency ablation (RFA) for metastatic colorectal cancer between 1996 and 2006 were included in this analysis. | 186 | Preop.C group median age: 59 years versus 68.5 years in the NC group | 105 | 81 |
| Gruenberger et al. [5] | 2008 | Clinical trial | Prior chemotherapy for metastatic disease; prior history of bleeding diathesis or coagulopathy; clinical evidence of CNS metastases; history of thromboembolic or hemorrhagic events within 6 months before treatment; clinically significant cardiovascular disease. | Patients with histologically confirmed resectable CRC liver metastases who were at high risk of early recurrence defined as one or more risk factors according to Fong et al. These risk factors included: synchronous liver metastases; metastatic disease developed within one year after primary resection; lymph-node-positive primary tumors; more than one instance of liver metastasis; liver metastasis larger than 5 cm; and a positive carcinoembryonic antigen level. Patients were also required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, adequate bone-marrow reserve, and adequate renal and hepatic function. | 56 | 61.5 | 32 | 24 |
| Authors | Year | Metastasis Tipe | Treatment | No. Chemocycles | Survival Outcome |
|---|---|---|---|---|---|
| Caulet M et al. [9] | 2016 | 75% liver metastasis only. | Bevacizumab + FOLFIRI (irinotecan, fluorouracil, leucovorin). | 4 | There were significant differences in overall survival (OS; P < 0.001) and in PFS (P = 0.001) between the two groups. OS was 17.3 months for patients with bevacizumab (Bev) lower than 15.5 mg/L, and 33.9 months for patients with Bev higher 15.5 mg/L. Median PFS (95% CI) was 8.7 months for patients with Bev lower than 15.5 mg/L, and 13.2 months for patients with Bev higher 15.5 mg/L. Increased drug exposure was shown to be associated with better clinical response and/or survival. |
| Umehara et al. [4] | 2016 | Liver metastasis only. | mFolfox6 (5-FU, leucovorin and oxaliplatin) + bevacizumab. | 6 | Three-year OS rate was 73.9%, and five-year OS rate was 62.5%. Disease-free survival (DFS) was significantly longer after mFOLFOX6 + Bev therapy compared to mFOLFOX6 therapy alone (p = 0.015). Optimal morphological response was associated with high five-year OS and DFS rates of 74% and 47%, respectively, and was observed in 47% of patients treated with bevacizumab and 12% of patients treated without bevacizumab. In the current study, we found no significant improvement in OS in patients treated with mFOLFOX6 + Bev compared to those treated with mFOLFOX6 alone (P = 0.23). Nevertheless, the observed increase in DFS was encouraging and significant (p = 0.015). |
| Chen et al. [13] | 2016 | Liver metastasis and extrahepatic metastasis. | Group 1: mFolfox6 + bevacizumab 100 mg. Group 2: mFolfox6 + bevacizumab 400 mg. | 6 | Two groups: in Group 1, target vessel regional chemotherapy (TVRC) treatment was without bevacizumab; in Group 2, TVRC was associated with bevacizumab. Time to progression of intrahepatic metastases (TTPIHM) was 3.53 and 6.23 (P = 0.018), and time to progression of extrahepatic metastases was 4.17 and 5.63 (P = 0.049) months in Groups 1 and 2, respectively. This indicated that bevacizumab could not only inhibit intrahepatic but also extrahepatic tumor vessels. and there was significant difference between groups in terms of survival. |
| Rouyer et al. [9] | 2016 | 52% liver metastasis only. | Irinotecan and bevacizumab. | Maximum of 8. | They observed that complete remission after surgery was high, in particular for patients with exclusively hepatic metastases, in line with induction chemotherapy associated with targeted therapy such as bevacizumab. These results support the idea that patients with exclusive hepatic metastases are better candidates to benefit from this type of curative treatment. |
| Beppu et al. [14] | 2014 | Liver metastasis only. | mFolfox6 (5-FU, leucovorin and oxaliplatin) + bevacizumab. | 5 | Stable disease was achieved in 55.0% of patients, and tumor-control rate was 85.0%. Median PFS of all patients (n = 40) was 9.7 months (95% CI = 6.2–11.8 months). Estimated one-, two- and three-year PFS was 35.0%, 22.5%, and 12.5%, respectively. Median survival time (MST) was 33.0 months (95%CI = 22.8 months—not reached). Estimated one-, two- and three-year OS was 87.5%, 62.3%, and 49.3%, respectively. |
| Shindoh et al. [12] | 2012 | Hepatic metastasis and extrahepatic lesions. | Oxaliplatin and bevacizumab. Irinotecan and bevacizumab. | 6 | This analysis determined that CT morphologic response to preoperative chemotherapy is a strong predictor of long-term outcomes after surgery in patients treated with or without bevacizumab. Optimal morphologic response was associated with high five-year OS and DFS rates of 74% and 47%, respectively. (95% CI: 1.46 to 4.49)On multivariate analysis, bevacizumab was strongly associated with optimal morphologic response (odds ratio, 6.71) |
| Van der et al. [16] | 2012 | Liver metastasis only. | Oxaliplatin and bevacizumab. | 4–6 | Estimated three-year disease-free survival in Groups 1 (non-bevacizumab) and 2 (bevacizumab) was 32% and 23%, respectively (P = 0.35). Bevacizumab added to oxaliplatin-based CTx may protect against moderate sinusoidal dilatation without significantly influencing morbidity. |
| Scoggins et al. [17] | 2010 | Liver metastasis only. | FOLFOXIRI (Irinotecan, oxaplatin, 5-FU and leucovorin) plus systemic therapy with or without bevacizumab | 6 | Addition of Bev to adjuvant hepatic arterial infusion (HAI) plus systemic therapy after liver resection did not seem to increase RFS or survival but appeared to increase biliary toxicity. One-year RFS was 83% (95% CI, 66% to 92%) and 71% (95% CI, 52% to 83%), and four-year RFS was 46% and 37% (P=.4) for non-bev and bev arms. Four-year survival was 85% (95% CI, 60% to 95%) in the non-bev arm, and 81% (95% CI,56%to 93%) in the bev arm (log-rank test P = 0.5) |
| Mahfud et al. [15] | 2009 | Liver metastasis only. | FOLFOXIRI (Irinotecan, Oxaplatin, 5-FU and Leucovorin) plus systemic therapy with or without Bevacizumab | 8 | Overall morbidity rate was 56% (Bev) versus 40% (control); adjusted OR 1.74, 95% CI 0.71–4.28; p = 0.23. Mortality was 0 versus 2 in Bev and control groups, respectively. Causes of death entailed hepatic insufficiency with multiorgan failure. |
| Scoggins C.R et al. [17] | 2008 | Liver metastasis only. | FOLFOXIRI (irinotecan, oxaplatin, 5-FU, and leucovorin) plus systemic therapy with or without bevacizumab. | 8 | DFS and OS rates of preoperative-chemotherapy group were lower than in the nonpreoperative-chemotherapy group, although this was not statistically significant. DFS of the preoperative-chemotherapy group was 40 months, while the nonpreoperative-chemotherapy group had a DFS of 56 months. OS was 56 months for the preoperative-chemotherapy group compared with 65 months for the nonpreoperative-chemotherapy group. Five-year DFS and OS for the entire cohort were 48% and 53%, respectively. |
| Gruenberger et al. [5] | 2008 | Liver metastasis only. | Oxaplatin + bevacuzimab. | 6 | A total of 41 patients responded (objective response rate, 73.2%); five patients had a complete pathologic response (8.9%), and 36 had a partial response. Twelve additional patients (21.4%) had stable disease, and overall disease-control rate was 94.6%. This trial showed that perioperative chemotherapy followed by surgery and postoperative chemotherapy significantly improved three-year PFS in all eligible patients (36.2% v 28.1%; P 0.041). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novo, I.; Campos, B.; Pinto-Ribeiro, F.; Martins, S.F. Coadjuvant Anti-VEGF A Therapy Improves Survival in Patients with Colorectal Cancer with Liver Metastasis: A Systematic Review. Gastrointest. Disord. 2020, 2, 71-85. https://doi.org/10.3390/gidisord2020007
Novo I, Campos B, Pinto-Ribeiro F, Martins SF. Coadjuvant Anti-VEGF A Therapy Improves Survival in Patients with Colorectal Cancer with Liver Metastasis: A Systematic Review. Gastrointestinal Disorders. 2020; 2(2):71-85. https://doi.org/10.3390/gidisord2020007
Chicago/Turabian StyleNovo, Isabel, Bárbara Campos, Filipa Pinto-Ribeiro, and Sandra F. Martins. 2020. "Coadjuvant Anti-VEGF A Therapy Improves Survival in Patients with Colorectal Cancer with Liver Metastasis: A Systematic Review" Gastrointestinal Disorders 2, no. 2: 71-85. https://doi.org/10.3390/gidisord2020007





