Measuring Microbiome Effectiveness: A Role for Ingestible Sensors
Abstract
:1. Introduction: The Microbiome in Health and Disease
2. Horizontal Gene Transfer
3. Microbiome Diversity: Probiotics and Sentinel Cells
4. Detecting Diversity and Overall Composition of the Microbiome
5. The Mutualistic Microbiome: Dysbiosis
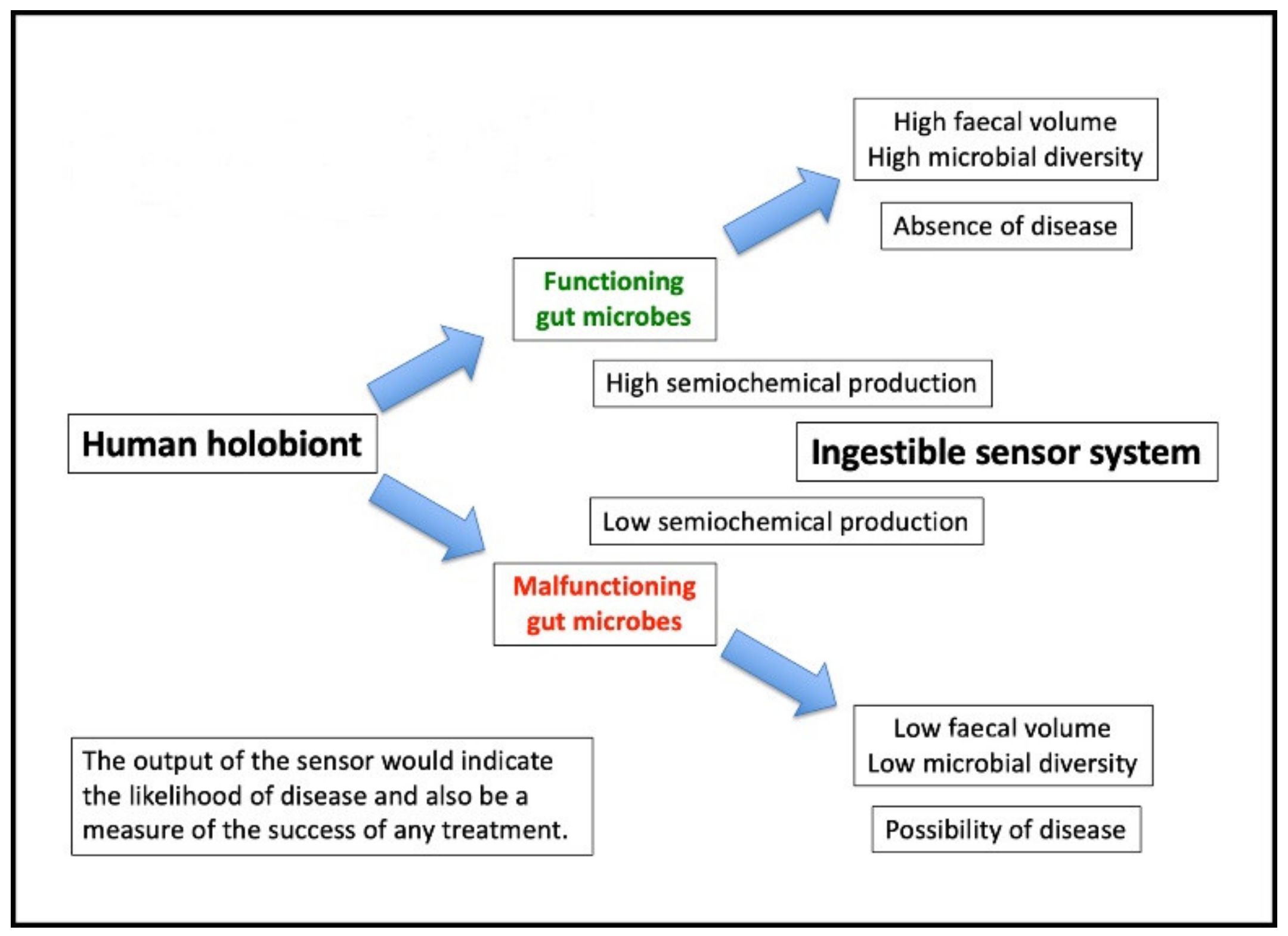
6. Ingestible Sensing Capsules in the Context of Microbiome Studies
7. Target Metabolites: A Surrogate Marker for Microbiome Effectiveness
8. What is the Story So Far…?
Conflicts of Interest
References
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the world-wide prevalence of obesity. Lancet 2018, 39, 1773–1774. [Google Scholar] [CrossRef]
- Steel, Z.; Mamane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Spergel, J.M. The atopic march: critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: an integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain-axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Lowry, C.A.; Smith, D.G.; Sielber, P.H.; Schmidt, D.; Stamper, C.E.; Hassell, J.E.; Yamashita, P.S.; Fox, J.H.; Reber, S.O.; Brenner, L.A.; et al. The Microbiota, Immunoregulation, and Mental Health: Implications for Human Health. Curr. Environ. Health Rep. 2016, 3, 270–286. [Google Scholar] [CrossRef] [Green Version]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Opazo, M.C.; Ortega-Rocha, E.M.; Coronado-Arrazola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.; Kalgeris, A.M.; Riedel, C.A. Intestinal microbiota influences non-intestinal related autoimmune diseases. Front. Microbiol. 2018, 9, 432. [Google Scholar] [CrossRef] [Green Version]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “perfect storm” for type 1 diabetes – the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Greer, J.B.; O’Keefe, S.J. Microbial induction of immunity, inflammation, and cancer. Front. Physiol. 2011, 1, 168. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Elson, C.A. Adaptive immune education by gut microbiota antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypoth. 2019, 125, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Cantarel, B.L.; Coutinho, P.M.; Henrissat, B.; Gordon, J.I.; Knight, R. The convergence of carbohydrate active gene repertoires in human gut microbes. Proc. Natl. Acad. Sci. USA 2008, 105, 15076–15081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and its discontents. mBio 2017, 8, e01492-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, F. The significance of pneumococcal types. J. Hyg. 1928, XXVII, 113–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederberg, J.; Tatum, E.L. Gene recombination in E. coli. Nature 1946, 158, 558. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Doudoroff, M.; Adelberg, E.A. General Microbiology; The MacMillan Press Ltd.: Basingstoke, UK, 1973; Chapter 14. [Google Scholar]
- Kellenberger, E. The genetic control of the shape of a virus. Sci. Am. 1966, 215, 32–39. [Google Scholar] [CrossRef]
- Koumandou, V.L.; Kossida, S. Evolution of the F0F1 ATP Synthase Complex in Light of the Patchy Distribution of Different Bioenergetic Pathways across Prokaryotes. PLOS Comput. Biol. 2014, 10, e1003821. [Google Scholar] [CrossRef] [Green Version]
- Agioutantis, P.; Koumandou, V.L. Bioenergetic diversity of the human gut microbiome. Meta Gene 2018, 16, 10–14. [Google Scholar] [CrossRef]
- Koumandou, V.L.; Kossida, S. Evolution of b-type cytochromes in prokaryotes. Peer J. PrePrints 2015, 3, e1564v1. [Google Scholar]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.A.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlFaleh, K.; Anabrees, J. Probiotics for the prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, 4, CD005496. [Google Scholar] [CrossRef]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.A.; Marcobal, A.; Tancredi, D.J.; Bevins, C.L.; Sherman, M.P. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Paediatr. Gastroenterol. Nutr. 2009, 48, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrof, E.O.; Khoruts, A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology 2014, 146, 1573–1582. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M.J.; Weingarden, A.R.; Unno, T.; Koruts, A.; Sadowsky, M.J. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 2013, 4, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Borody, T.J.; Campbell, J. Fecal microbiota transplantation: current status and future directions. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 653–655. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kleinewietfeld, M.; Smillie, C.; Levkovich, T.; Perrota, A.; Bhela, S.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Kearney, S.M.; et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS ONE 2013, 8, e68596. [Google Scholar] [CrossRef] [Green Version]
- Lekunberri, I.; Subirats, J.; Borrego, C.M.; Balcazar, J.L. Exploring the contribution of bacteriophage to antibiotic resistance. Envir. Pollut. 2017, 220, 981–984. [Google Scholar] [CrossRef]
- Jheeta, S. The Routes of Emergence of Life from LUCA during the RNA and Viral World: A Conspectus. Life 2015, 5, 1445–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jheeta, S. The Landscape of the Emergence of Life. Life 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L.; Groisman, E.A. Novel “superspreader” bacteriophages promote horizontal gene transfer by transformation. mBio 2017, 8, e02115-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietilä, M.K.; Demina, T.A.; Atanasova, N.S.; Oksanen, H.M.; Bamford, D.H. Archaeal viruses and bacteriophages: comparisons and contrasts. Trends Microbiol. 2014, 22, 334–344. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial eukaryotes: a missing link in gut microbiome studies. mSystems 2018, 3, e00201-17. [Google Scholar] [CrossRef] [Green Version]
- Margulis, L. Symbiogenesis and symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92. [Google Scholar]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: the holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl. Acad. Sci. USA 1974, 74, 5088–5090. [Google Scholar] [CrossRef] [Green Version]
- Yarus, M. Life from an RNA World; Harvard University Press: Cambridge, MA, USA, 2010; Chapter 3. [Google Scholar]
- Burkitt, D.P. Some diseases characteristic of modern western civilization. BMJ 1973, 1, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Semiochemicals of Forest and Shade Tree Insects in North America and Management Applications. Available online: https://iucat.iu.edu/iupui/5439806 (accessed on 11 September 2019).
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. AJP Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [Green Version]
- Xue, R.; Zhang, H.; Pan, J.; Du, Z.; Zhou, W.; Zhang, Z.; Tian, Z.; Zhou, R.; Bai, L. Peripheral dopamine controlled by gut microbes inhibits invariant natural killer T cell-mediated hepatitis. Front. Immunol. 2018, 9, 2398. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.-T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ha, N.; Ou, J.Z.; Berean, K.J. Ingestible sensors. ACS Sens. 2017, 2, 468–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, D.E.; Rondonotti, E.; Koulaouzidis, A. Review: capsule colonoscopy-a concise clinical overview of current status. Ann. Transl. Med. 2016, 4, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters-Strickland, T.; Pestreich, L.; Hatch, A.; Rohatagi, S.; Baker, R.A.; Docherty, J.P.; Markovtsova, L.; Raja, P.; Weiden, P.J.; Walling, D.P. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsychiatr. Dis. Treat. 2016, 12, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Batra, S.; Baker, R.A.; Wang, T.; Forma, F.; DiBiasi, F.; Peters-Strickland, T. Digital health technology for use in patients with serious mental illness: a systematic review of the literature. Med. Devices (Auckl) 2017, 10, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; McDonnell, S.; Swartwout, R.; Citorik, R.J.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018, 360, 915–918. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Berean, K.J.; Ha, N.; Chrimes, A.F.; Xu, K.; Grando, D.; Ou, J.Z.; Pillai, N.; Campbell, J.L.; Brkljača, R.; et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 2018, 1, 79–87. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Yao, C.K.; Berean, K.J.; Ha, N.; Ou, J.Z.; Ward, S.A.; Pillai, N.; Hill, J.; Cottrell, J.J.; Dunshea, F.R.; et al. Intestinal gas capsules: a proof-of-concept demonstration. Gastroenterology 2016, 150, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.Z.; Cottrell, J.J.; Ha, N.; Pillai, N.; Yao, C.K.; Berean, K.J.; Ward, S.A.; Grando, D.; Muir, J.; Harrison, C.J.; et al. Potential of in vivo real-time gastric gas profiling: a pilot evaluation of heat-stress and modulating dietary cinnamon effect in an animal model. Sci. Rep. 2016, 6, 33387. [Google Scholar] [CrossRef]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechn. 2002, 13, 7–27. [Google Scholar] [CrossRef]
- Davison, T.F.; Freeman, B.M. Physiological aspects of growth promotion in poultry. Vet. Res. Commun. 1983, 7, 59–68. [Google Scholar] [CrossRef]
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.-N.G.; El-Bouhy, Z.M. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egyptian J. Aquatic Res. 2013, 39, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, M.; Gray, J.A.; Roth, R.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Rainey III, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter Horst, K.W.; Lammers, N.M.; Trinko, R.; Opland, D.M.; Figee, M.; Ackermans, M.T.; Booij, J.; van den Munckhof, P.; Schuurman, P.R.; Fliers, E.; et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 2018, 10, eaar3752. [Google Scholar] [CrossRef] [Green Version]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef]
- Kaplan, H.; Thompson, R.C.; Trumble, B.C.; Wann, L.S.; Allam, A.H.; Beheim, B.; Frohlich, B.; Sutherland, M.L.; Sutherland, J.D.; Stieglitz, J.; et al. Coronary atherosclerosis in indigenous South American Tsimane: A cross sectional cohort study. Lancet 2017, 389, 1730–1739. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.; Jheeta, S. Measuring Microbiome Effectiveness: A Role for Ingestible Sensors. Gastrointest. Disord. 2020, 2, 3-11. https://doi.org/10.3390/gidisord2010002
Smith D, Jheeta S. Measuring Microbiome Effectiveness: A Role for Ingestible Sensors. Gastrointestinal Disorders. 2020; 2(1):3-11. https://doi.org/10.3390/gidisord2010002
Chicago/Turabian StyleSmith, David, and Sohan Jheeta. 2020. "Measuring Microbiome Effectiveness: A Role for Ingestible Sensors" Gastrointestinal Disorders 2, no. 1: 3-11. https://doi.org/10.3390/gidisord2010002
APA StyleSmith, D., & Jheeta, S. (2020). Measuring Microbiome Effectiveness: A Role for Ingestible Sensors. Gastrointestinal Disorders, 2(1), 3-11. https://doi.org/10.3390/gidisord2010002




