Multi-Analytical Study of Damage to Marine Ballast Tank Coatings After Cyclic Corrosion Testing
Abstract
:1. Introduction
2. Experimental Methods
3. Results
3.1. Visual Appearance
3.2. Thermal Analysis
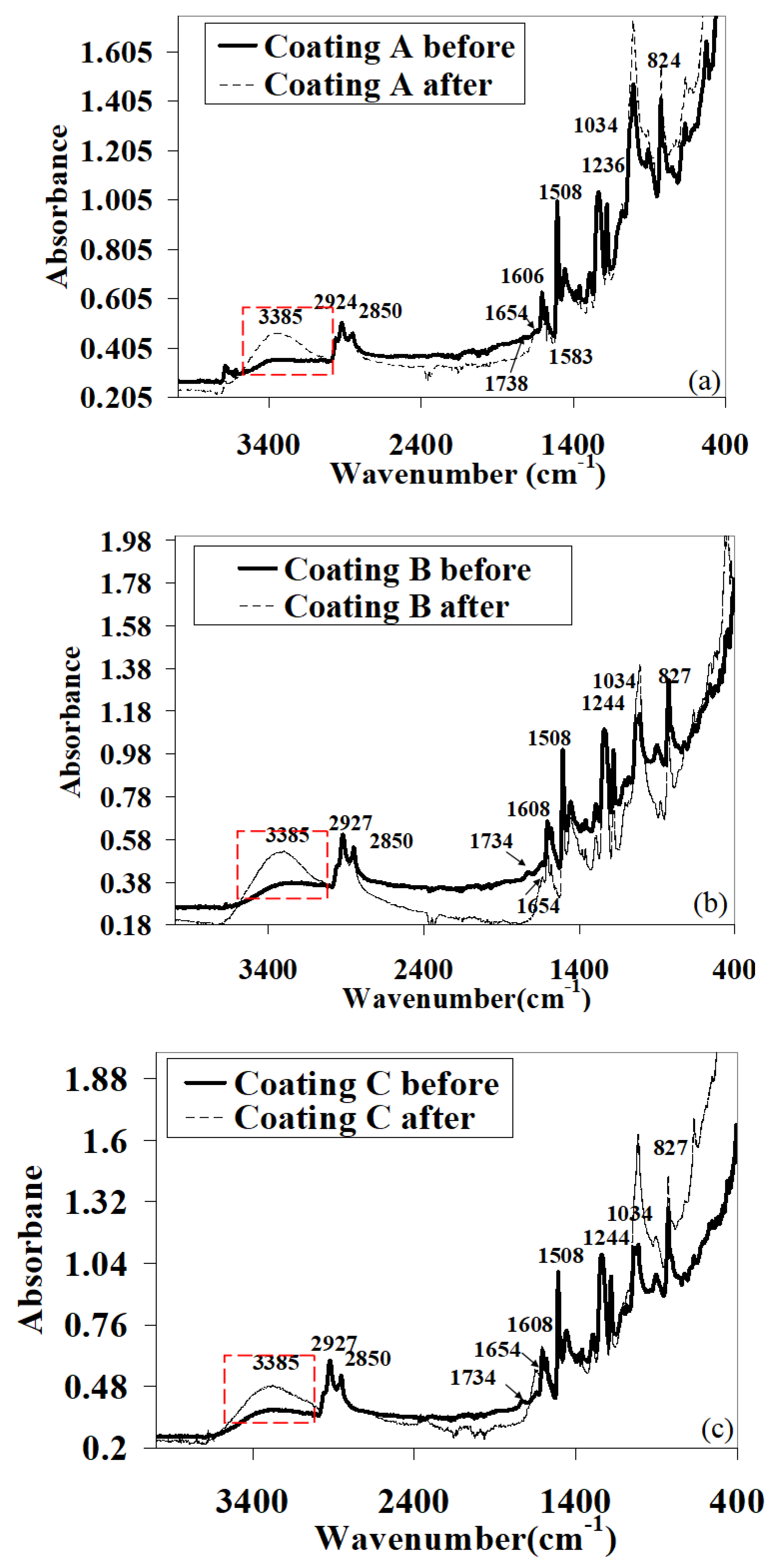
3.3. Vibrational Spectroscopy
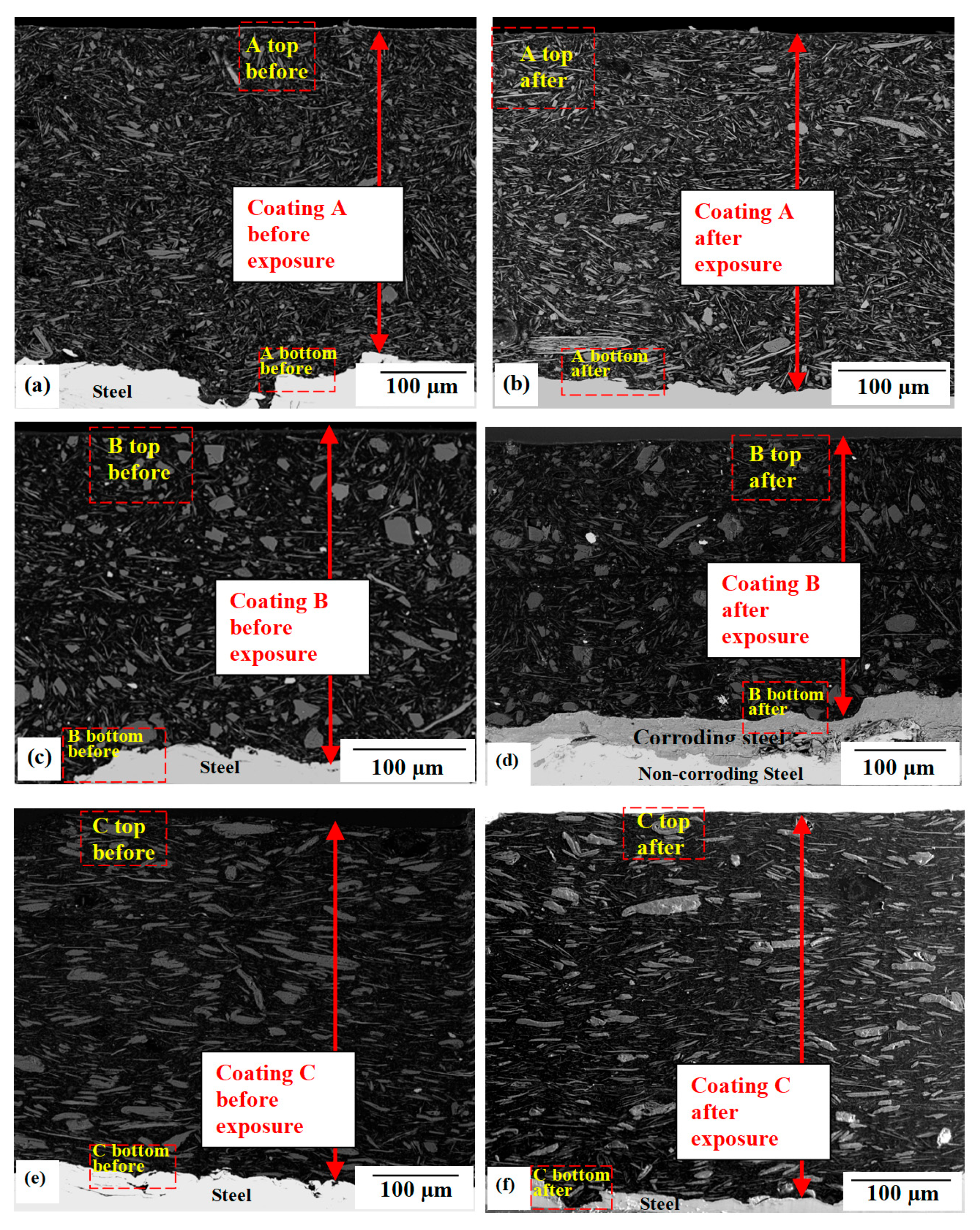
3.4. Coating Cross-Section
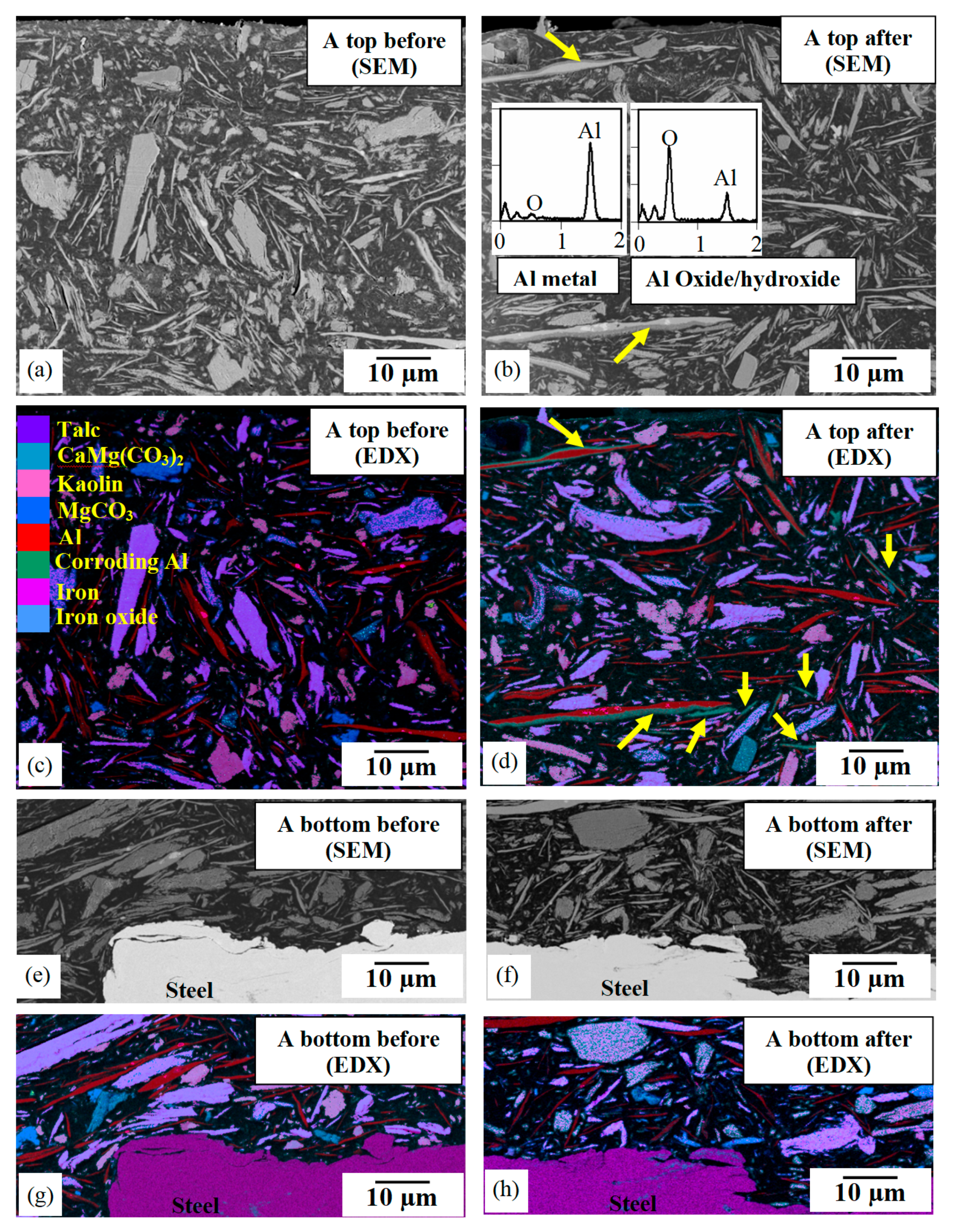
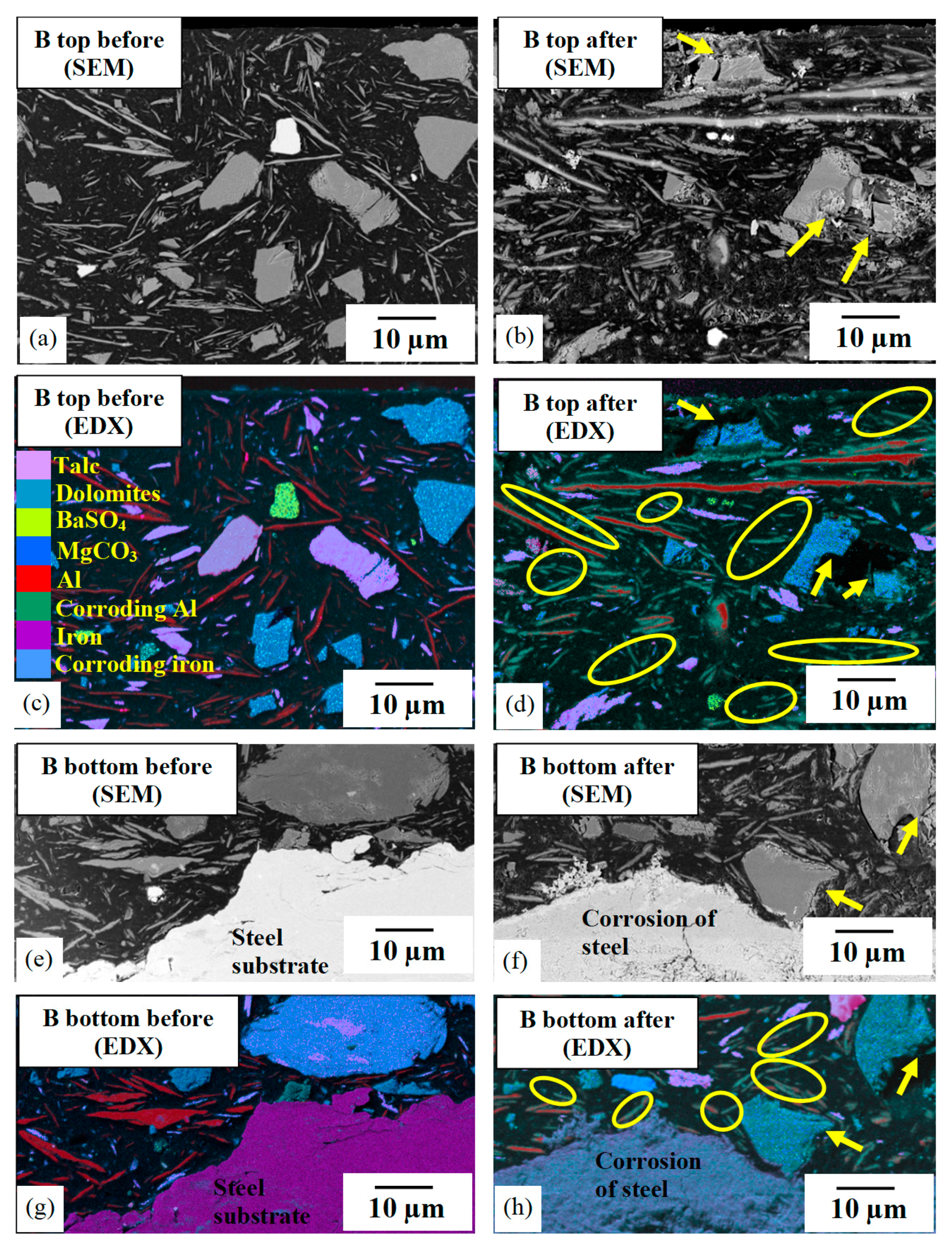
3.5. High-Resolution Analytical Microscopy
3.5.1. Pigment Analysis
3.5.2. Microstructural Damage
3.6. Nanoscale X-Ray Tomography
4. Discussion
4.1. Coating Performance and Ranking
4.2. Alignment and Orientation of Pigments
4.3. Aluminium Flake Pigments
4.4. Phenalkamine Cross-Linker
4.5. Inconsistency in Extender Pigments
5. Conclusions
- Three similar barrier-type marine ballast tank coatings, differing in phenalkamine curing agent, method of application, and extender pigments, were evaluated by analytical microscopy and spectroscopy before and after 6 months of hygro-thermal cyclic corrosion testing (CCT). Significant differences were observed in anti-corrosion performance, which can be related to the properties of the binder and pigments.
- Based on the evidence provided in this study, there was little difference in flake pigment orientation, whether the coating was applied by airless spray or by draw-down bar. This suggests, for these coatings at least, that draw-down bar-applied laboratory coatings may be used to reflect the performance of spray-applied coatings, but this needs to be assessed on a case-by-case basis.
- Where the environment had penetrated into the coating, varying amounts of corrosion damage to the steel substrate and of the aluminium flake pigments were revealed by cross-sectional microscopy. As aluminium flakes were isolated from each other and from the substrate, galvanic protection was absent in this system. High-resolution studies of individual flakes failed to detect any localised attack, and aluminium therefore appeared to corrode relatively uniformly. Volume expansion by the generation of an aluminium corrosion product did not appear to disrupt the binder/pigment interface.
- It is confirmed that the polymer binder cross-link density and its environmental resistance is influenced by the stability of the phenalkamine curing agent. Thus, the more stable cross-linker had an increased Tg with no corrosion evident after CCT. Conversely, the less stable cross-linker had a decreased Tg with substrate and flake aluminium corrosion observed.
- Where present, sparingly soluble contaminants (e.g., dolomite and barium sulphate) in the extender pigment increased water uptake into the coating and diminished its barrier protection.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reed, C.; Eliasson, J. Coatings Cracking in Water Ballast Tanks: A Different Look. In NACE—International Corrosion Conference Series, Proceedings of the Corrosion Conference and Expo 2017, New Orleans, LA, USA, 26–30 March 2017; Volume 3, pp. 1928–1935.
- Iannarelli, P.; Beaumont, D.; Liu, Y.; Zhou, X.; Burnett, T.L.; Curioni, M.; Lyon, S.B.; Gibbon, S.R.; Morsh, S.; Emad, S.; et al. The degradation mechanism of a marine coating under service conditions of water ballast tank. Prog. Org. Coat. 2022, 162, 106588. [Google Scholar] [CrossRef]
- Song, E.; Lee, S.; Lee, H.; Kim, D. Effect of Coating Formulation on Crack Resistance of Epoxy Coatings. In NACE—International Corrosion Conference Series; NACE: Philadelphia, PA, USA, 2011; p. 11033. [Google Scholar]
- Oriaifo, E.; Perera, N.; Guy, A.; Leung, P.S.; Tan, K. A review of test protocols for assessing coating performance of water ballast tank coatings. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2014, 8, 1691–1696. [Google Scholar]
- 87 IACS Recommendation, Guidelines for Coating Maintenance & Repairs for Ballast Tanks and Combined Cargo/Ballast Tanks on Oil Tankers. Available online: https://www.iacs.org.uk/resolutions/recommendations/81-100/rec-87-rev2-cln/ (accessed on 20 December 2024).
- The Maritime Safety Committee (MSC); International Maritime Organization (IMO). RESOLUTION MSC.215(82): Performance Standard for Protective Coatings for Dedicated Seawater Ballast Tanks in All Types of Ships and Double-Side Skin Spaces of Bulk Carriers. 2006. Available online: https://www.imo.org/en/OurWork/Safety/Pages/ProtectiveCoatings.aspx (accessed on 20 December 2024).
- Eliasson, J.; Rauta, D.; Gunner, T. Ballast water tank coatings for the future. In Corrosion 2005; OnePetro: Richardson, TX, USA, 2005. [Google Scholar]
- EU Regulation (EC) No 1907/2006 “Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)”. Available online: https://echa.europa.eu/regulations/reach/legislation (accessed on 20 December 2024).
- Sakashita, S.; Shiotani, K.; Kashima, K.; Murakoshi, S.; Fukunaga, K.; Baba, T.; Takai, A.; Takada, A.; Osawa, N. Onboard study on deterioration of coated steel in water ballast tank. In Proceedings of the Twenty-Fifth International Ocean and Polar Engineering Conference, Kona, HI, USA, 21–26 June 2015; OnePetro: Richardson, TX, USA, 2015. [Google Scholar]
- Morsch, S.; Liu, Y.; Lyon, S.; Gibbon, S.; Gabriele, B.; Malanin, M.; Eichhorn, K.-J. Examining the early stages of thermal oxidative degradation in epoxy amine resins. Polym. Degrad. Stab. 2020, 176, 109147. [Google Scholar] [CrossRef]
- Morsch, S.; Emad, S.; Liu, Y.; Lyon, S.; Gibbon, S. Local oxidation of the buried epoxy-amine/iron oxide interphase. Prog. Org. Coat. 2021, 160, 106516. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Echtermeyer, A.T. Echtermeyer, Mechanism of Yellowing: Carbonyl Formation during hygro-thermal ageing in a Common Amine Epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Meiser, A.; Willstrand, K.; Possart, W. Possart, Influence of Composition, Humidity, and Temperature on Chemical ageing in Epoxies: A Local Study of the Interphase with Air. J. Adhes. 2010, 86, 222–243. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, X.; Zhang, H.J.; Wang, Z.P. Effects of hygro-thermal ageing on epoxy-based anisotropic conductive film. Mater. Lett. 2006, 60, 2958–2963. [Google Scholar] [CrossRef]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal-oxidation of epoxy/amine followed by glass transition temperature changes. Polym. Degrad. Stab. 2017, 138, 82–90. [Google Scholar] [CrossRef]
- Rocha, I.B.C.M.; Raijmaekers, S.; Nijssen, R.P.L.; van der Meer, F.P.; Sluys, L.J. Hygro-thermal ageing behaviour of a glass/epoxy composite used in wind turbine blades. Compos. Struct. 2017, 174, 110–122. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Shanahan, M.E.R. Irreversible Effects of hygro-thermal ageing on DGEBA/DDA Epoxy Resin. J. Appl. Polym. Sci. 1988, 69, 363–369. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Delamar, M.A.; Shanahan, M.E.R. Irreversible Interactions Between Water and DGEBA/DDA Epoxy Resin During hygro-thermal ageing. J. Appl. Polym. Sci. 1997, 65, 449–458. [Google Scholar] [CrossRef]
- Reed, C. Underfilm Corrosion Creep and Cathodic Delamination: Under the Microscope. In Corrosion 2014; NACE International: Philadelphia, PA, USA, 2014; p. 4271. [Google Scholar]
- Knudsen, O.Ø.; Bardal, E.; Steinsmo, U. Effect of Barrier Pigments on Cathodic Disbonding Part 1: Aluminium and Glass Pigments. J. Corros. Sci. Eng. 1999, 2, 13. [Google Scholar]
- Knudsen, O.Ø.; Steinsmo, U. Effect of Barrier Pigments on Cathodic Disbonding Part 2: Mechanism of the Effect of Aluminium Pigments. J. Corros. Sci. Eng. 1999, 2, 37. [Google Scholar]
- Ramezanzadeh, B.; Khazaei, M.; Rajabi, A.; Heidari, G.; Khazaei, D. Corrosion Resistance and Cathodic Delamination of an Epoxy/Polyamide Coating on Milled Steel. Corrosion 2014, 70, 56–65. [Google Scholar] [CrossRef]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Sato, K. The Internal Stress of Coating Films. Prog. Org. Coat. 1980, 8, 143–160. [Google Scholar] [CrossRef]
- Abil, E.; Arefinia, R. The influence of talc particles on corrosion protecting properties of polyurethane coating on carbon steel in 3.5% NaCl solution. Prog. Org. Coat. 2022, 172, 107067. [Google Scholar] [CrossRef]
- Katiyar, J.K.; Sinha, S.K.; Kumar, A. Friction and wear durability study of epoxy-based polymer(SU-8) composite coatings with talc and graphite as fillers. Wear 2016, 362–363, 199–208. [Google Scholar] [CrossRef]
- Dzulkafli, H.H.; Ahmad, F.; Ullah, S.; Hussain, P.; Mamat, O.; Megat-Yusoff, P.S. Effects of talc on fire retarding, thermal degradation and water resistance of intumescent coating. Appl. Clay Sci. 2017, 146, 350–361. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Nabey, B.A.-E.; Khamis, E.; Abdelattef, O.; Aglan, H.; Ludwick, A. Influence of natural inhibitor, pigment and extender on corrosion of polymer coated steel. Prog. Org. Coat. 2010, 69, 402–409. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Abdel-Fatah, H.T.M.; Youssef, E.A. Corrosion studies on tailored Zn·Co aluminate/kaolin core–shell pigments in alkyd based paints. Prog. Org. Coat. 2012, 73, 76–87. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, X.; Jin, Z.; Wang, P.; Fan, L.; Deng, J.; Yuan, S.; Sun, Y.; Duan, J. Mechanism of an organic-inorganic composite coating with anticorrosive and versatile superhydrophobic properties: A combined electrochemical and molecular dynamics exploration. Prog. Org. Coat. 2023, 182, 107610. [Google Scholar] [CrossRef]
- Abid, M.; Khan, S.M.; Butt, M.T.Z. Investigation of Cathodic Protection, Morphological, Rheological, and Mechanical Properties of Graphene/Iron Oxide Nanoparticle-Embedded Cold Galvanizing Compounds at Reduced Pigment Volume Concentration. ACS Omega 2022, 7, 20556–20568. [Google Scholar] [CrossRef] [PubMed]
- ISO11357:2; Plastics—Differential Scanning Calorimetry (DSC); Part 2: Determination of Glass Transition Temperature and Step Height. ISO: Geneva, Switzerland, 2020.
- Bukalo, N.N.; Ekosse, G.I.E.; Odiyo, J.O.; Ogola, J.S. Fourier Transform Infrared Spectroscopy of Clay Size Fraction of Cretaceous-Tertiary Kaolins in the Douala Sub-Basin, Cameroon. Open Geosci. 2017, 9, 407–418. [Google Scholar] [CrossRef]
- Ngally Sabouang, C.J.; Mbey, J.A.; Liboum Thomas, F.; Njopwouo, D. Talc as raw material for cementitious products formulation. J. Asian Ceram. Soc. 2014, 2, 263–267. [Google Scholar] [CrossRef]
- Shameli, K.; Darroudi, M.; Bin Ahmad, M.; Yunis, W.M.Z.W.; Ibrahim, N.A. Synthesis and characterization of silver/talc nanocomposites using the wet chemical reduction method. Int. J. Nanomed. 2010, 5, 743–751. [Google Scholar] [CrossRef]
- Gokulakumar, B.; Nedunchezhian, G.; Vijayaraj, C.; Balamurugan, A. Fourier transform infrared spectroscopy studies on talcum powders. Int. J. Recent. Sci. Res. 2016, 7, 14505–14507. [Google Scholar]
- Odegard, G.M.; Bandyopadhyay, A. Physical ageing of Epoxy Polymers and Their Composites. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1695–1716. [Google Scholar] [CrossRef]




| Application | Cross-Linker | “Active” Pigment | Extenders | Pigment Fraction | |
|---|---|---|---|---|---|
| Coating A | spray | phenalkamine #1 | aluminium flake | kaolin, talc | 28 ± 2% |
| Coating B | spray | phenalkamine #2 | aluminium flake | kaolin, talc | 28 ± 2% |
| Coating C | draw-down | phenalkamine #2 | aluminium flake | kaolin, talc | 28 ± 2% |
| CCT Exposure Time | Tg | |
|---|---|---|
| Coating A | T = 0 T = 6 months | 57 ± 1.5 °C 67 ± 1.5 °C |
| Coating B | T = 0 T = 6 months | 63 ± 1.5 °C 56 ± 1.5 °C |
| Coating C | T = 0 T = 6 months | 63 ± 1.5 °C 52 ± 1.5 °C |
| Coating A (Phenalkamine #1, Spray) | Coating B (Phenalkamine #2, Spray) | Coating C (Phenalkamine #2, Draw Bar) | |
|---|---|---|---|
| Binder stability | (a) Tg increase; (b) binder cross-linking increase; (c) binder hydration | (a) Tg decrease; (b) binder chain scission; (c) binder hydration and oxidation | (a) Tg decrease; (b) binder chain scission; (c) binder hydration and oxidation |
| Al flake pigments | very minor corrosion | significant corrosion | some corrosion |
| Pigment composition and stability | kaolin + talc: stable | kaolin + talc: stable | talc: stable |
| Impurity composition and stability | none identified | dolomite + barium sulphate: unstable | dolomite: unstable |
| Substrate steel corrosion | no corrosion | ~20 µm corrosion depth | ~10 µm corrosion depth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Beaumont, D.; Zhou, X.; Burnett, T.; Morsch, S.; Lyon, S.; Iannarelli, P.; Di Lullo, C.; Hijnen, N.; Emad, R.; et al. Multi-Analytical Study of Damage to Marine Ballast Tank Coatings After Cyclic Corrosion Testing. Corros. Mater. Degrad. 2025, 6, 1. https://doi.org/10.3390/cmd6010001
Liu Y, Beaumont D, Zhou X, Burnett T, Morsch S, Lyon S, Iannarelli P, Di Lullo C, Hijnen N, Emad R, et al. Multi-Analytical Study of Damage to Marine Ballast Tank Coatings After Cyclic Corrosion Testing. Corrosion and Materials Degradation. 2025; 6(1):1. https://doi.org/10.3390/cmd6010001
Chicago/Turabian StyleLiu, Yanwen, Douglas Beaumont, Xiaorong Zhou, Timothy Burnett, Suzanne Morsch, Stuart Lyon, Paul Iannarelli, Claudio Di Lullo, Niek Hijnen, Reza Emad, and et al. 2025. "Multi-Analytical Study of Damage to Marine Ballast Tank Coatings After Cyclic Corrosion Testing" Corrosion and Materials Degradation 6, no. 1: 1. https://doi.org/10.3390/cmd6010001
APA StyleLiu, Y., Beaumont, D., Zhou, X., Burnett, T., Morsch, S., Lyon, S., Iannarelli, P., Di Lullo, C., Hijnen, N., Emad, R., Coghlan, L., & Hashimoto, T. (2025). Multi-Analytical Study of Damage to Marine Ballast Tank Coatings After Cyclic Corrosion Testing. Corrosion and Materials Degradation, 6(1), 1. https://doi.org/10.3390/cmd6010001







