Development of a Reliable Accelerated Corrosion Test for Painted Aluminum Alloys Used in the Aerospace Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Field Exposures
2.3. Accelerated Corrosion Tests
2.3.1. Standardized Accelerated Tests
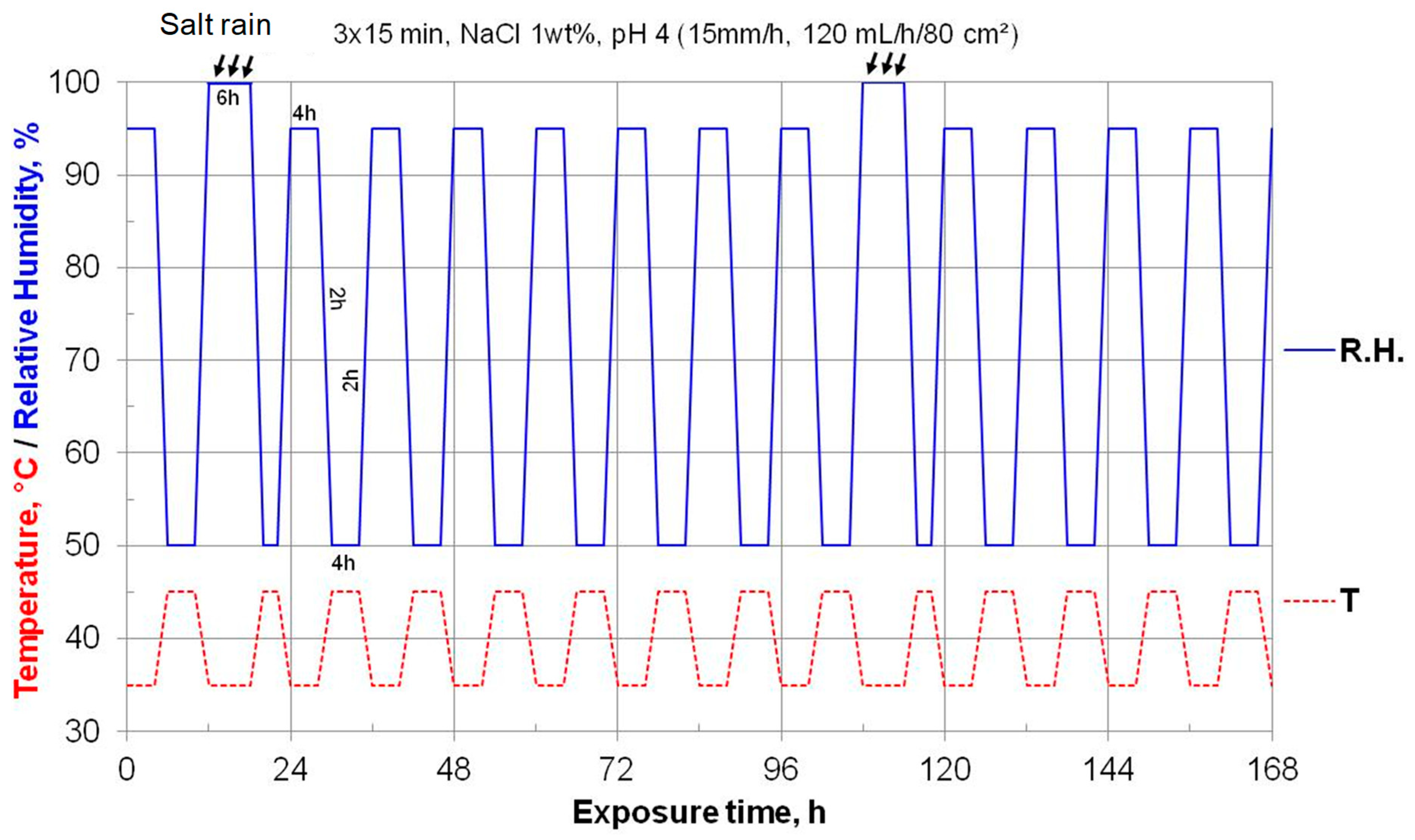
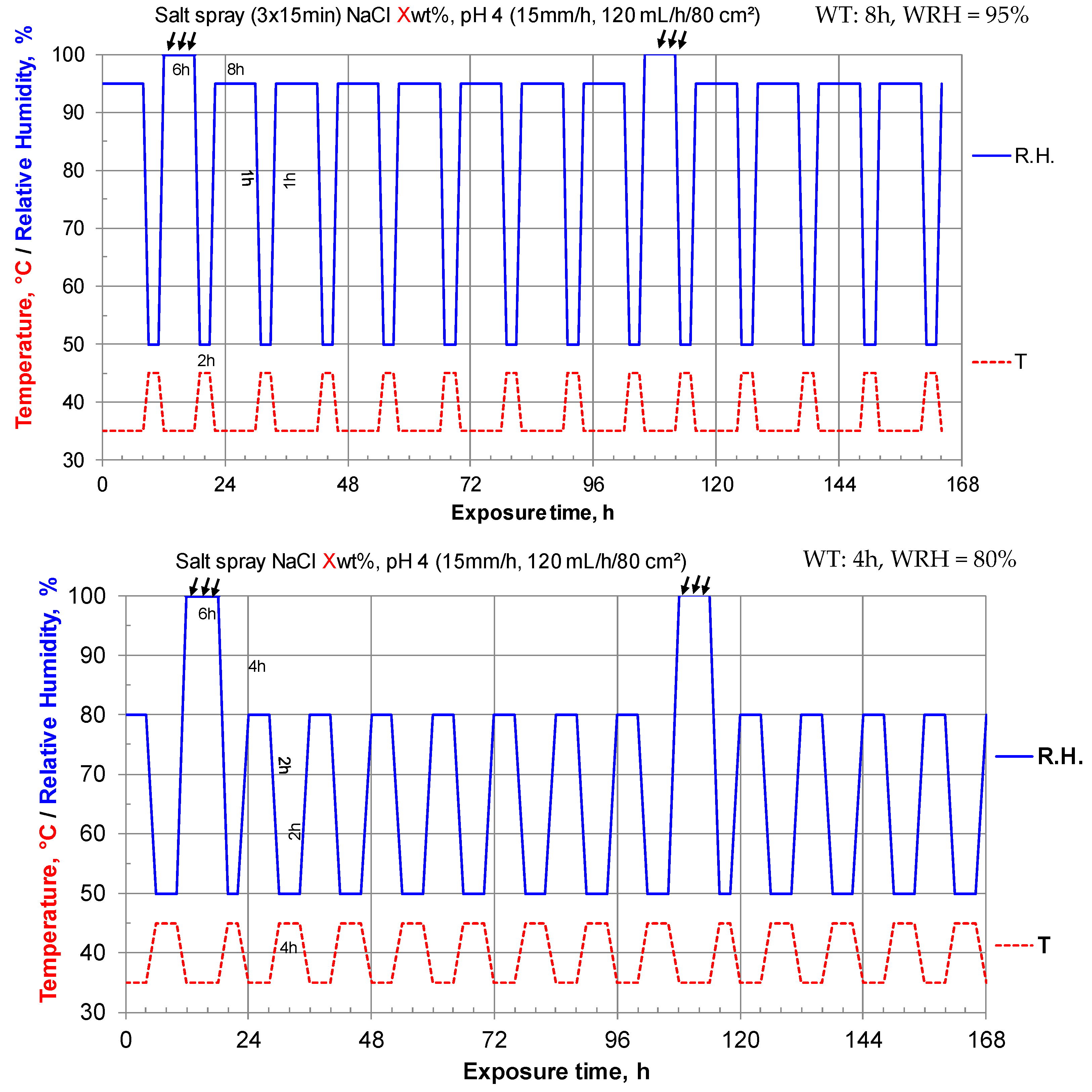
2.3.2. Accelerated Corrosion Test-Design of Experiment (DoE)
2.4. Evaluation Procedure
2.5. Statistical Analysis of the Results
3. Results
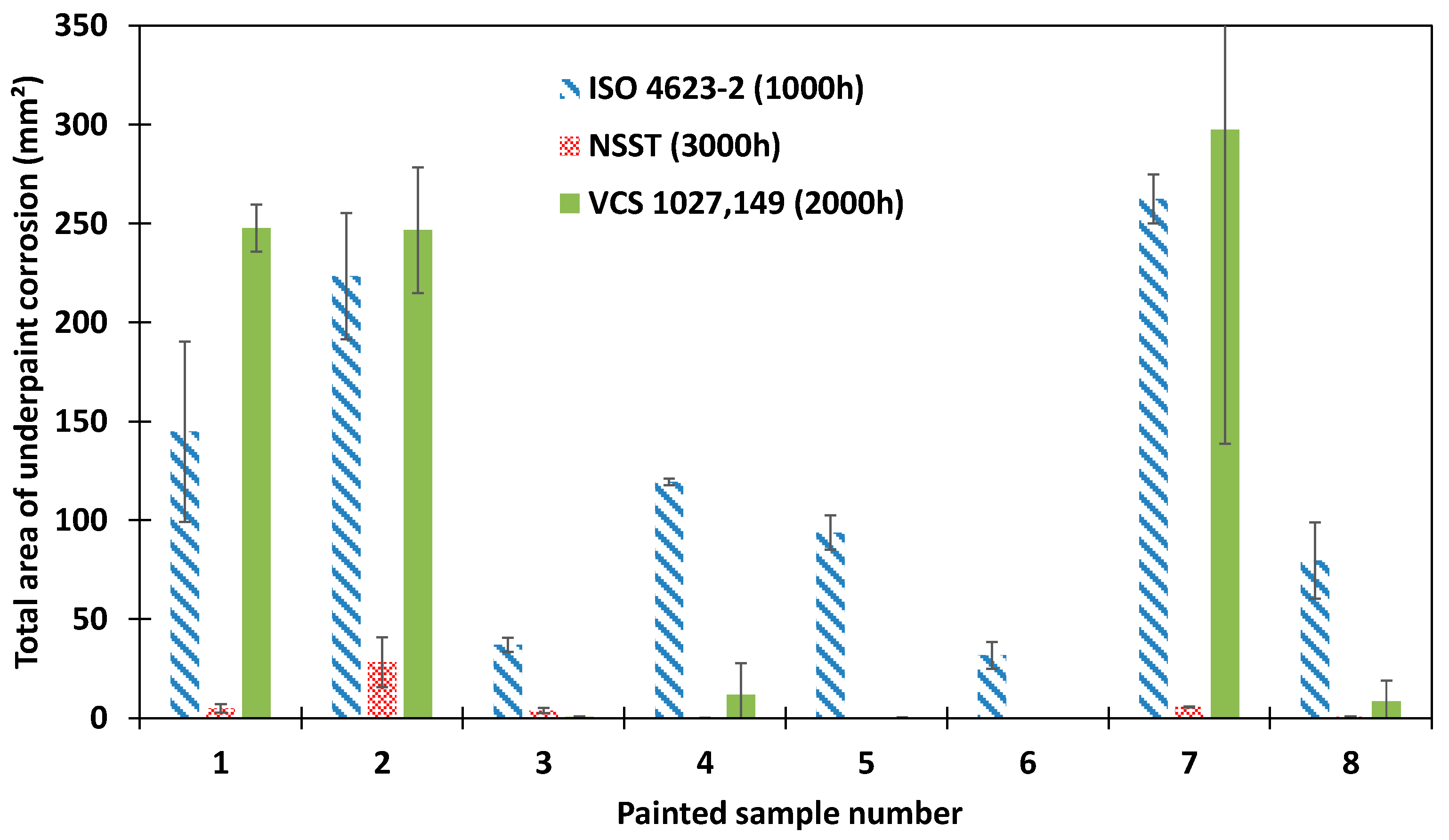
3.1. Standardized Accelerated Corrosion Tests
3.2. Accelerated Corrosion Tests—Design of Experiment (DoE)
3.3. Field Exposures
4. Discussion
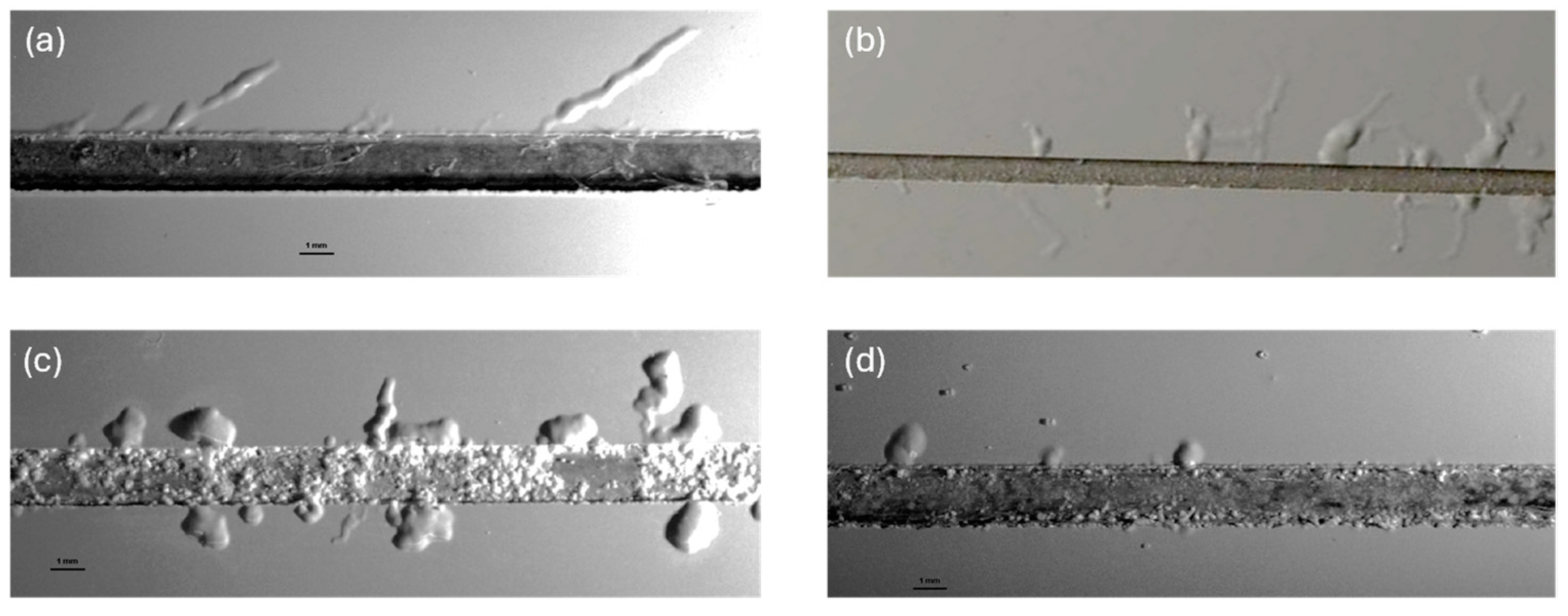
Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Long-term atmospheric corrosion of Cr-free painted aluminum alloys during outdoor worldwide exposures. Mater. Corros. 2023, 74, 1022–1029. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, Q.; Li, D.; Wen, J. Long-term atmospheric corrosion behaviour of aluminium alloys 2024 and 7075 in urban, coastal and industrial environments. Corros. Sci. 2009, 51, 719–727. [Google Scholar] [CrossRef]
- de la Fuente, D.; Otero-Huerta, E.; Morcillo, M. Studies of long-term weathering of aluminium in the atmosphere. Corros. Sci. 2007, 49, 3134–3148. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, C.; Niu, K.; Zhao, J.; Huang, Y.; Li, X. Long-term corrosion behavior of the 7A85 aluminum alloy in an industrial-marine atmospheric environment. J. Mater. Res. Technol. 2021, 12, 1350–1359. [Google Scholar] [CrossRef]
- Poltavtseva, M.; Heyn, A.; Boese, E. Long term corrosion behavior of clad aluminum materials under different atmospheric conditions. Mater. Corros. 2013, 64, 723–730. [Google Scholar] [CrossRef]
- Hu, S.; Sun, S.; Guo, A.; Jia, X.; Geng, Y. Atmospheric Corrosion Behavior of Extruded Aluminum Alloy 7075-T6 after Long-Term Field Testing in China. Corrosion 2011, 67, 106002–106002-10. [Google Scholar] [CrossRef]
- Kloet, J.V.; Schmidt, W.; Hassel, A.W.; Stratmann, M. The role of chromate in filiform corrosion inhibition. Electrochim. Acta 2004, 49, 1675–1682. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M.F. Smart composite coatings for corrosion protection of aluminium alloys in aerospace applications. In Smart Composite Coatings and Membranes: Transport, Structural, Environmental and Energy Applications, 1st ed.; Montemor, M.F., Ed.; Woodhead Publishing: Lisbon, Portugal, 2016; pp. 85–121. [Google Scholar] [CrossRef]
- U.R. [Online] European Chemicals Agency. 2016. Available online: https://echa.europa.eu/regulations/reach/understanding-reach (accessed on 1 September 2024).
- Visser, P.; Liu, Y.; Terryn, H.; Mol, J.M.C. Lithium salts as leachable corrosion inhibitors and potential replacement for hexavalent chromium in organic coatings for the protection of aluminum alloys. J. Coat. Technol. Res. 2016, 13, 557–566. [Google Scholar] [CrossRef]
- Visser, P.; Liu, Y.; Zhou, X.; Hashimoto, T.; Thompson, G.E.; Lyon, S.B.; Van Der Ven, L.G.J.; Mol, A.J.M.C.; Terryn, H.A. The corrosion protection of AA2024-T3 aluminium alloy by leaching of lithium-containing salts from organic coatings. Faraday Discuss. 2015, 180, 511–526. [Google Scholar] [CrossRef]
- Harvey, T.G.; Hardin, S.G.; Hughes, A.E.; Muster, T.H.; White, P.A.; Markley, T.A.; Corrigan, P.A.; Mardel, J.; Garcia, S.J.; Mol, J.M.C.; et al. The effect of inhibitor structure on the corrosion of AA2024 and AA7075. Corros. Sci. 2011, 53, 2184–2190. [Google Scholar] [CrossRef]
- Noiville, R.; Jaubert, O.; Gressier, M.; Bonino, J.-P. Ce(III) corrosion inhibitor release from silica and boehmite nanocontainers. Mater. Sci. Eng. B 2018, 229, 144–154. [Google Scholar] [CrossRef]
- Nanna, M.E.; Bierwagen-North, G.P.; Philadelphia, P.A. Mg-Rich Coatings: A New Paradigm for Cr-Free Corrosion Protection of Al Aerospace Alloys. JCT Res. 2004, 1, 69–80. [Google Scholar] [CrossRef]
- Kasten, L.S.; Grant, J.T.; Grebasch, N.; Voevodin, N.; Arnold, F.E.; Donley, M.S. An XPS study of cerium dopants in solgel coatings for aluminum 2024-T3. Surf Coat Technol 2001, 140, 1115. [Google Scholar] [CrossRef]
- Markley, T.A.; Mardel, J.I.; Hughes, A.E.; Hinton, B.R.W.; Glenn, A.M.; Forsyth, M. Chromate replacement in coatings for corrosion protection of aerospace aluminium alloys. Mater. Corros. 2011, 62, 836–840. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Lopez, V.; Gonzalez, J.A.; Otero, E.; Escudero, E.; Morcilló, M.; Morcilló, M. Atmospheric corrosion of bare and anodised aluminium in a wide range of environmental conditions. Part II Electrochem. Responses Surf. Coat. Technol. 2002, 153, 235–244. [Google Scholar] [CrossRef]
- ASTM B117:2017; Corrosion Tests in Artificial Atmospheres. American National Standards Institute: Washington, DC, USA, 2017.
- ISO 4623-2; Paints and Varnishes—Determination of Resistance to Filiform Corrosion. Part 2. Aluminum Substrates. AFNOR: Geneva, Switzerland, 2016.
- VDA 233-102:2013; Cyclic Corrosion Testing of Materials and Components in Automotive Construction. VDA: Berlin, Germany, 2013.
- Volvo STD 423-0014:2015; Accelerated Corrosion Test-Atmospheric Corrosion. Standard Volvo Group: Gothenburg, Sweden, 2015.
- Bautista, A. Filiform corrosion in polymer-coated metals. Prog. Org. Coat. 1996, 28, 49–58. [Google Scholar] [CrossRef]
- Kandell, A.Y.; Shawki, G.S.A. Material behaviour under corrosive environment -guide for material selection. Eng. J. Quatar Univ. 1988, 1, 59. [Google Scholar]
- Le Bozec, N.; Persson, D.; Nazarov, A.; Thierry, D. Investigation of Filiform Corrosion on Coated Aluminum Alloys by FTIR Microspectroscopy and Scanning Kelvin Probe. J. Electrochem. Soc. 2002, 149, B403. [Google Scholar] [CrossRef]
- Funke, W. Blistering of paint films and filiform corrosion. Prog. Org. Coat. 1981, 9, 29–46. [Google Scholar] [CrossRef]
- ISO 9225; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Measurement of Pollution. AFNOR: Geneva, Switzerland, 2012.
- ISO 9223; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. AFNOR: Geneva, Switzerland, 2012.
- Guérin, M.; Andrieu, E.; Odemer, G.; Alexis, J.; Blanc, C. Effect of varying conditions of exposure to an aggressive medium on the corrosion behavior of the 2050 Al-Cu-Li alloy. Corros. Sci. 2014, 85, 455–470. [Google Scholar] [CrossRef]
- Vera, R.; Delgado, D.; Rosales, B.M. Effect of atmospheric pollutants on the corrosion of high power electrical conductors: Part 1. Aluminium and AA6201 alloy. Corros. Sci. 2006, 48, 2882–2900. [Google Scholar] [CrossRef]
- Ambat, R.; Dwarakadasa, E.S. Studies on the influence of chloride ion and pH on the electrochemical behaviour of aluminium alloys 8090 and 2014. J. Appl. Electrochem. 1994, 24, 911–916. [Google Scholar] [CrossRef]


| Nr | Alloy | Surface Treatment | Primer | Top-Coat | Target Value of Thickness (µm) |
|---|---|---|---|---|---|
| 1 | AA 2024 T3 sheet | TSA | Cr-free primer 1 (epoxy primer) | Top-coat 1 (polyurethane) | 55 |
| 2 | CrIII conversion coating | ||||
| 3 | AA 2024 clad | TSA | CrVI primer 1 (2 or 3 components amine cured epoxy primer) | 55 | |
| 4 | AA 2024 T351 clad | Sol–gel | CrVI primer 2 (epoxy primer) | Top-coat 2 (polyurethane) | 69 |
| 5 | AA 2324 T39 | TSA | CrVI primer 3 (3 components amine cured epoxy primer) | 55 | |
| 6 | AA 7055 T7751 | TSA | CrVI primer 4 (epoxy primer) | 55 | |
| 7 | AA 2024 T351 clad | Sol–gel | CrVI primer 5 (epoxy primer) | 56 | |
| 8 | AA 2324 T39 | TSA | CrVI primer 4 (epoxy primer) | 55 |
| Average Temperature (°C) | Average Relative Humidity (%) | TOW * (%) Over the Period | Precipitation (mm) Over the Period (Sum) | Chloride Deposition (mg/m², d) (Mean) | Corrosivity Class (ISO 9223 [29] for Al AA1050) | Corrosivity Class (ISO 9223 for Carbon Steel) |
|---|---|---|---|---|---|---|
| 13 | 82 | 59 | 5485 | 960 | C2/C3 | C4/C5 |
| Level − | Level + | |
|---|---|---|
| Salt (NaCl) Concentration, %wt (SC) | 0.5 | 2 |
| Wet Time, h (WT) | 4 | 8 |
| Wet Relative humidity, % (WRH) | 80 | 95 |
| Test | Test Label | Salt Conc. Wt% | Type of Salt | Wet Time, h | Wet RH, % | Temperature, °C |
|---|---|---|---|---|---|---|
| SC | WT | WRH | T | |||
| 1 | SC0.5/WT4/WRH80 | 0.5 | NaCl | 4 | 80 | 35–45 °C |
| 2 | SC2/WT4/WRH80 | 2 | 4 | 80 | ||
| 3 | SC0.5/WT8/WRH80 | 0.5 | 8 | 80 | ||
| 4 | SC2/WT8/WRH80 | 2 | 8 | 80 | ||
| 5 | SC0.5/WT4/WRH95 | 0.5 | 4 | 95 | ||
| 6 | SC2/WT4/WRH95 | 2 | 4 | 95 | ||
| 7 | SC0.5/WT8/WRH95 | 0.5 | 8 | 95 | ||
| 8 | SC2/WT8/WRH95 | 2 | 8 | 95 | ||
| 9 | SC0.5/WT4/WRH80/T45 | 0.5 | 4 | 80 | 45 °C (Constant) | |
| 10 | SC0.5mix/WT4/WRH80 | 0.5 | NaCl + MgCl2 + CaCl2 | 4 | 80 | 35–45 °C |
| 11 | SC1/WT4/WRH80 | 1 | NaCl | 4 | 80 | |
| 12 | SC0.75/WT4/WRH95 | 0.75 | 4 | 95 |
| Mostly Filiform | Mostly Blistering | |||
|---|---|---|---|---|
| Very Thin Filaments | Wide Corrosion Filaments | Mostly Blisters + Scattered Filiform | Blistering | |
| ISO 4623-2 (1000 h) | 4, 7 | 1, 2, 6, 8 | 3, 5 | |
| NSST (3000 h) | 1, 2, 3, 4, 7, 8 | |||
| VCS 1027, 149 (2000 h) | 4, 7 | 2 | 1, 3, 5, 8 | |
| Mostly Filiform | Mostly Blistering | |||
|---|---|---|---|---|
| Very Thin Filaments | Wide Corrosion Filaments | Mostly Blisters + Scattered Filiform | Blistering | |
| SC0.5/WT4/WRH80 | 4, 7 | 1 | 2 | 3, 5, 6, 8 |
| SC0.5/WT4/WRH80/T45 | 4, 7 | 1 | 2, 5 | 3, 6, 8 |
| SC0.5mix/WT4/WRH80 | 4, 7 | 1, 2, 5 | 3, 8 | |
| SC1/WT4/WRH80 | 4, 7 | 1 | 2, 8 | 3, 5, 6 |
| SC2/WT4/WRH80 | 4, 7 | 1 | 2 | 3, 5, 6, 8 |
| SC0.5/WT8/WRH80 | 4, 7 | 1, 2 | 3, 6, 8 | |
| SC2/WT8/WRH80 | 4, 7 | 1, 2, 8 | 3, 5, 6 | |
| SC0.5/WT4/WRH95 | 4, 7 | 1, 2, 8 | 3, 5, 6 | |
| SC0.75/WT4/WRH95 | 4, 7 | 1, 2, 8 | 3, 5, 6 | |
| SC2/WT4/WRH95 | 4, 7 | 1 | 2, 8 | 3, 5, 6 |
| SC0.5/WT8/WRH95 | 4, 7 | 1, 2, 5, 6 | 3, 8 | |
| SC2/WT8/WRH95 | 4, 7 | 1, 2, 8 | 3, 6 | |
| Mostly Filiform | Mostly Blistering | |||
|---|---|---|---|---|
| Very Thin Filaments | Wide Corrosion Filaments | Mostly Blisters + Scattered Filiform | Blistering | |
| Field exposure (5 years) | 4, 7 | 5 | 1, 2, 3, 6, 8 | |
| Duration | Mostly Filiform | Mostly Blistering | |||
|---|---|---|---|---|---|
| Very Thin Filaments | Wide Corrosion Filaments | Mostly Blisters + Scattered Filiform | Blistering | ||
| ISO 4623-2 | 1000 h | 4, 7 | 1, 2, 6, 8 | 3, 5 | |
| NSST | 3000 h | 1, 2, 3, 4, 7, 8 | |||
| VCS 1027,149 | 2000 h | 4, 7 | 2 | 1, 3, 5, 8 | |
| SC0.5/WT4/WRH80 | 2000 h | 4, 7 | 1 | 2 | 3, 5, 6, 8 |
| SC0.5/WT4/WRH80/T45 | 2000 h | 4, 7 | 1 | 2, 5 | 3, 6, 8 |
| SC0.5mix/WT4/WRH80 | 2000 h | 4, 7 | 1, 2, 5 | 3, 8 | |
| SC1/WT4/WRH80 | 2000 h | 4, 7 | 1 | 2, 8 | 3, 5, 6 |
| SC2/WT4/WRH80 | 2000 h | 4, 7 | 1 | 2 | 3, 5, 6, 8 |
| SC0.5/WT8/WRH80 | 2000 h | 4, 7 | 1, 2 | 3, 6, 8 | |
| SC2/WT8/WRH80 | 2000 h | 4, 7 | 1, 2, 8 | 3, 5, 6 | |
| SC0.5/WT4/WRH95 | 2000 h | 4, 7 | 1, 2, 8 | 3, 5, 6 | |
| SC0.75/WT4/WRH95 | 2000 h | 4, 7 | 1, 2, 8 | 3, 5, 6 | |
| SC2/WT4/WRH95 | 2000 h | 4, 7 | 1 | 2, 8 | 3, 5, 6 |
| SC0.5/WT8/WRH95 | 2000 h | 4, 7 | 1, 2, 5, 6 | 3, 8 | |
| SC2/WT8/WRH95 | 2000 h | 4, 7 | 1, 2, 8 | 3, 6 | |
| Field exposure | 5 years | 4, 7 | 5 | 1, 2, 3, 6, 8 | |
| Duration | Coefficient of Variation | Acceleration Factor | |
|---|---|---|---|
| ISO 4623-2 | 1000 h | 0.9 | 1566 |
| NSST | 3000 h | 1.7 | 33 |
| VCS 1027,149 | 2000 h | 1.1 | 605 |
| SC0.5/WT4/WRH80 | 2000 h | 0.7 | 572 |
| SC0.5/WT4/WRH80/T45 | 2000 h | 1.0 | 473 |
| SC0.5mix/WT4/WRH80 | 2000 h | 1.1 | 169 |
| SC1/WT4/WRH80 | 2000 h | 0.8 | 1565 |
| SC2/WT4/WRH80 | 2000 h | 0.8 | 595 |
| SC0.5/WT8/WRH80 | 2000 h | 0.9 | 316 |
| SC2/WT8/WRH80 | 2000 h | 1.0 | 1220 |
| SC0.5/WT4/WRH95 | 2000 h | 1.3 | 661 |
| SC0.75/WT4/WRH95 | 2000 h | 0.4 | 496 |
| SC2/WT4/WRH95 | 2000 h | 0.6 | 703 |
| SC0.5/WT8/WRH95 | 2000 h | 0.4 | 487 |
| SC2/WT8/WRH95 | 2000 h | 1.2 | 695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peltier, F.; Thierry, D. Development of a Reliable Accelerated Corrosion Test for Painted Aluminum Alloys Used in the Aerospace Industry. Corros. Mater. Degrad. 2024, 5, 427-438. https://doi.org/10.3390/cmd5030019
Peltier F, Thierry D. Development of a Reliable Accelerated Corrosion Test for Painted Aluminum Alloys Used in the Aerospace Industry. Corrosion and Materials Degradation. 2024; 5(3):427-438. https://doi.org/10.3390/cmd5030019
Chicago/Turabian StylePeltier, Fabienne, and Dominique Thierry. 2024. "Development of a Reliable Accelerated Corrosion Test for Painted Aluminum Alloys Used in the Aerospace Industry" Corrosion and Materials Degradation 5, no. 3: 427-438. https://doi.org/10.3390/cmd5030019
APA StylePeltier, F., & Thierry, D. (2024). Development of a Reliable Accelerated Corrosion Test for Painted Aluminum Alloys Used in the Aerospace Industry. Corrosion and Materials Degradation, 5(3), 427-438. https://doi.org/10.3390/cmd5030019







