Abstract
This paper addresses the interplay between electrical fields in the human body and the corrosion behavior of Ti-6Al-4V alloy, a prevalent orthopedic material. The study investigates the impact of alternative electrical signals at different frequencies on the alloy’s electrochemical behavior in a simulated body environment. The human body always has natural sinusoidal potential due to, e.g., heart palpitations and brain/nervous system activities. Ignoring such natural activities may lead to underestimating the corrosion performance of the Ti-6Al-4V alloy in the body. By analyzing anodic and cathodic responses and the net faradaic current induced by alternating current potential, the research sheds light on the influence of electrical fields on corrosion rates. Understanding these dynamics could lead to improved implant materials, mitigating corrosion-related challenges and enhancing implant performance over the long term. Results of this work indicated that frequent oxidation and reduction at certain frequencies may induce corrosion and hinder biomimetic apatite formation, impacting osseointegration. Natural alternative currents in the body affect the corrosion performance of Ti-based implant alloys, highlighting the need for consideration in biomedical applications.
1. Introduction
Biocompatibility, mechanical performance, tribological features, and corrosion resistance are the main criteria in metallic material selection for biological applications [1]. Ti-based alloys are one of the best candidates for implant alloys. These alloys have been successfully implemented in orthopedic applications owing to excellent corrosion resistance, appropriate mechanical properties, and exceptional osseointegration rates [2,3,4]. Ti-6Al-4V alloy has high strength-to-weight ratios and is characterized by its high corrosion resistance. It is a two-phase α + β alloy and is characterized by the lamellar structure of the α phase separated by β-phase interlayers inside the β-grain [5,6]. α + β are stabilized by Al and V, respectively [7,8,9]. The nature of the passive film on the surface of these alloys is the key factor when considering the corrosion resistance and osseointegration performance of such an alloy [8,10,11,12,13,14]. This intrinsic passive film primarily consists of different crystallographic forms of TiO2 [15,16,17]. Reliable performance is the key factor in orthopedic alloy selection and evolution [18,19]. Despite the desirable chemical, physical, and biological properties of developed orthopedic alloys, numerous premature failures or incompatibility cases have been reported due to corrosion [20,21,22,23]. The corrosion behavior of metallic implants is influenced by numerous factors such as the chemical compound and microstructure of the material itself; the working conditions, including temperature; the physiological compound; construction, such as the formation of galvanic cells, crevices, and external factors, for instance; mechanical loading; wear; and thermal shocks [24].
The degradation of implant alloys may contribute to the late loosening of the prosthetic components and impact metabolism and immunity. In addition, corrosion and the release of ions such as Ti4+, Cr2+, V4+, and Al3+ from the dissolution of the multi-phasic alloys [25,26,27] promote inflammation, infection, and bone resorption [28,29]. For instance, trace elements incorporated in implantable alloys were detected in the blood, liver, and lung [30]. Decreases in DNA synthesis and the mineralization of alkaline phosphates [31] and cytotoxicity in osteoblasts [32] are other detrimental effects of alloying element release in the human body. Vanadium may also accumulate in some organs, such as the liver, and, in lower concentrations, in the kidneys, bones, and spleen [33].
Bioelectrochemical reactions in a physiological environment might affect the corrosion resistance properties of the Ti-6Al-4V evaluated in an under-controlled laboratory environment [20]. The corrosive environment of the human body, along with cyclic loads, wear, fretting, and temperature alternation, can significantly accelerate the degradation due to corrosion [2,8,34,35,36,37].
In addition, electrical currents in the body can influence the corrosion performance of an implant [20]. The electrical field induced in the human body can be classified into two main groups:
- External sources: Everyone is exposed inadvertently to alternative electrical currents and potential. Electric transmission lines with operating voltages higher than 765 kV raise serious questions about the impact of high-strength electric fields or shock on living organisms [38,39]. Many studies have investigated the harmful effects of power frequency (50/60 Hz) on the human body [38]. Depending on the size of the organ [40], the electric and magnetic fields (EMFs) induce biological effects, since electric and magnetic fields induce weak current flows in a body due to alternative current (AC), which may lead to brain malfunction and adverse effects on health [41,42,43]. Additionally, direct current (DC) and AC external sources (at a frequency range of 1 Hz to 1 MHz) such as electrical field inducers are used to stimulate injured tissue healing [44,45,46].
- Internal sources: Biopotentials are the internal and natural sources of electrical field in the human body. These potentials may originate from body motion [47], the growth and development of cells and tissues [48], and the heartbeat or brain [49,50]. In addition, injuries or any abnormal changes create a flux of various ions towards or outwards from the injured organ, which causes a stream of electrical current [51]. Considering the resistivity of tissues, these currents can produce voltage differences of between 10 and 100 mV/cm in the human body [20]. Strains in hard tissues like bones and teeth can also induce potential alternation due to the piezoelectric stimulation of dipolar collagens [20,52].
All the mentioned points highlight that tissues and organs in individuals are exposed to various alternative electrical signals. On the other hand, imposing an alternating electrical field can influence the corrosion activity of alloys [53,54,55]. For example, in a study on Ti-6Al-4V dental implants, Chen et al. showed that while the α phase portion of an alloy can promote the formation of insoluble Ti-Al passive film, under applied anodic potential conditions, the dissolution of β phase is favored, and the vanadium is released to the environment transpassively [56]. However, there is always a certain threshold for perturbation potential or frequency to observe tangible corrosion due to alternating electrical fields. Anodic or cathodic polarizing surfaces beyond the equilibrium potential range cause an extra faradaic reaction, providing a concentration of reactants and products on the surface. In the case of the alternative excitation of the surface, when the overvoltage reverses, the reverse reaction may not happen at the same rate due to the asymmetric kinetics of cathodic and anodic activation. As a result, repetitive forward and reversing overvoltage rectifies the net faradaic current, and corrosion ensues at an increasing rate [53,55,57,58]. However, the charge transfer at the interface is not significantly fast and may be slowed even more with mass transport barriers like passive film.
The current produced by the anodic half wave of alternating potential is not equal to the cathodic counterpart; as a result, the net current induced by an AC is greater than the free-corrosion current [54,59,60].
It is imperative to study the influence of the electrical field in the human body on the corrosion behavior of orthopedic alloys, which is the objective of this paper. This study aimed to analyze the electrochemical behavior of Ti-6Al-4V alloy, one of the most common orthopedic alloys, under different alternative electrical signals with different frequencies in a simulated body environment.
2. Experimental Section
As the working electrodes, 40 mm × 20 mm specimens were cut from a 2 mm thick Ti-6Al-4V sheet, which complied with the ASTM F1472-014 specified for surgical implant applications [61]. For each experiment, three specimens were prepared and tested. Specimens were ground using SiC paper up to #1200 grit and then polished with 0.5 and 0.05 μm alumina suspension. Then, they were rinsed with ethanol in an ultrasonic bath for 1 min and dried immediately by blowing cool air onto them. Specimens were epoxy-coated to provide insulation and a circular surface area of 1.0 cm2 was left in contact with the electrolyte. A 1X phosphate-buffered saline (PBS) solution of pH 7.4 [62], with the composition shown in Table 1, at a constant temperature of 37 °C with a stationary condition, was used in this study.

Table 1.
The composition of 1 L 1X-PBS solution used in this study.
It should be mentioned that no specific standard (e.g., ASTM) was followed for the electrochemical experiments since the application of an alternative potential is new. To evaluate the general polarization response of specimens exposed to PBS for 24 h at open circuit potential (OCP), potentiodynamic cyclic polarization tests were carried out by scanning potential from −200 mV vs. OCP to +700 mV vs. SCE and reversed to −100 mV vs. SCE with a scan rate of 1 mV/s.
Electrochemical measurements were carried out using a classical three-electrode arrangement consisting of a saturated calomel reference electrode (SCE), platinized platinum counter electrode, and the Ti-6Al-4V specimens as the working electrode. To ensure the reproducibility of the data, all measurements were repeated on three identical specimens. All electrochemical treatments and measurements were initiated after 24 h of exposure to a 37 °C PBS solution at OCP to reach a stable condition of the oxide film on working electrodes.
Electrochemical impedance spectroscopy (EIS) is a powerful technique to characterize surface conditions on metals and alloys. Conventional EIS can be employed only under small signal conditions, i.e., when the system response is linear. However, the kinetic information in the nonlinear part of the response would be missing under small signal conditions. In addition, small amplitude perturbation often leads to poor signal-to-noise ratio. The nonlinear electrochemical impedance spectroscopy (NLEIS) method can be considered an extended version of the EIS technique, wherein a large amplitude perturbation is applied to the system, and the response is recorded at the fundamental and higher harmonics [63].
In order to find the minimum adequate perturbation potential amplitude for the nonlinear distortion of current response, NLEIS measurements in the range of 100 kHz to 10 mHz with different sinusoidal perturbation amplitudes of 5, 10, 20, 50, 100, and 200 mV were carried out sequentially. For each exciting frequency with a particular perturbation potential, the nonlinearity response of the current was calculated using the total harmonic distortion (THD) of the current signal, as written in Equation (1), expressed as follows [64,65]:
where denotes the kth component from the Fourier transform of the current response. Thus, is the fundamental component of the current, while k ≥ 2 is associated with non-fundamental harmonics. The first to 10th harmonics are used to calculate THD in this study. The stimulus potential where THD exceeds the value of 5% was considered to be the potential that causes nonlinear behavior [66]. This value was 100 mV for this study. After specimens were immersed in the PBS solution for 24 h at their OCP, a 100 mV sinusoidal alternating voltage with various frequencies of 500, 50, 5, 0.5, and 0.05 Hz was superimposed on the specimens.
The chemical composition of the Ti oxide film formed on the specimens is expected to be transformed or indiscernible when using the ex situ examination methods [4,67]. Thus, in situ Raman spectroscopy was used to study and compare the formation and transformation of the oxide crystal modifications as a function of electrochemical parameters [10,68]. The films formed on the surface of the specimens after 24 h of immersion in the PBS solution at 37 °C at their OCP, and were investigated when they were perturbed with 100 mV sinusoidal potential vs. OCP with different frequencies. The Raman spectra obtained were corrected for the electrolyte background. The necessity of this correction was discussed elsewhere [68].
3. Results and Discussion
A cyclic potentiodynamic polarization curve of the specimens tested in PBS solution at 37 °C and immersed for 24 h is shown in Figure 1. The whole curve depicts characteristics of passive behavior in the test environment. Up to +0.7 V vs. SCE potential excursion, there is no evidence of an active–passive transition related to the initiation of localized corrosion processes. Negative hysteresis at reverse potential scan confirms the stability and resistance to passive film breakdown [69,70,71].
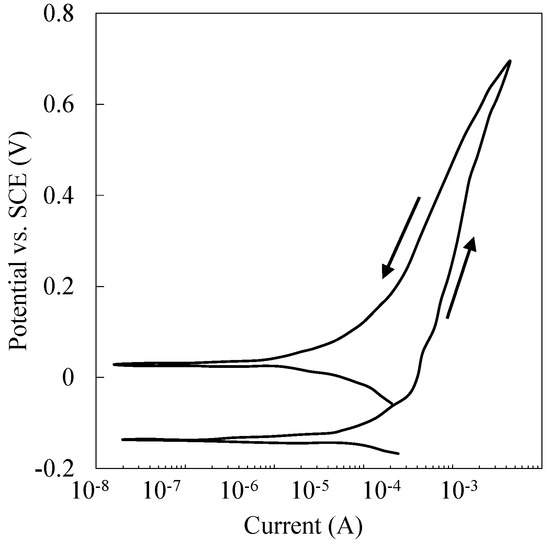
Figure 1.
Cyclic potentiodynamic polarization curve of a Ti-6A-4V specimen tested in 1X-PBS at 37 °C and immersed for 24 h.
It was found that the ratio of anodic to cathodic Tafel slope () determines a material’s susceptibility to AC-induced corrosion [68,69,70]. In this experiment, where r = 1.45 (βa = 0.725 V/decay, and βc = 0.500 V/decay), it is plausible that the asymmetry of cathodic and anodic activation polarization rectifies the net faradaic current [55,72]. The current produced by the anodic half-wave of alternating voltage is not equal to its cathodic counterpart; as a result, the net current induced by AC is greater than the free-corrosion current [54,60].
Figure 2a shows the Nyquist plots obtained from the different EIS measurements with different stimulus potentials on the specimens immersed at their OCP for 24 h in the PBS solution at 37 °C.
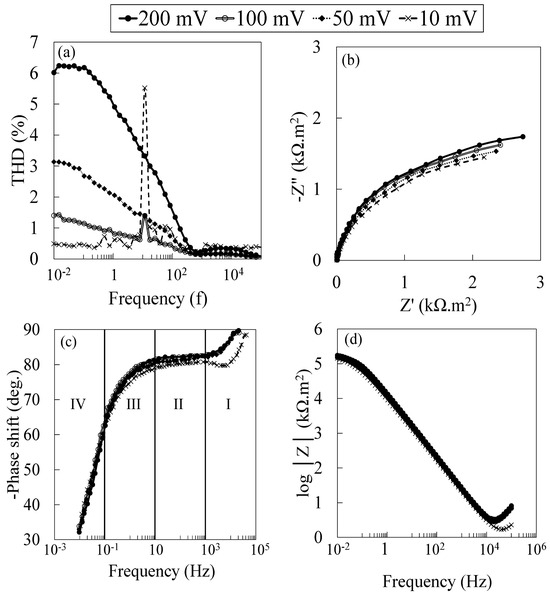
Figure 2.
(a) Total harmonic distortion, (b) Nyquist plots and (c) corrected Bode phase angle, and (d) Bode plot obtained from the NLEIS measured on the specimens immersed at their OCP for 24 h in 1X-PBS solution at 37 °C.
Looking at total harmonic distortions obtained with different perturbation potentials, it is evident that increasing stimulus potential results in a nonlinear polarization response from the surface, especially at lower frequencies. In Figure 2b, it appears that increasing the stimulus potential decreased all the impedances of the system. In the high order of the harmonic potential, the interface layer capacitance (Cdl) and charge transfer (Rct) response of the surface may yield nonlinear behavior to AC perturbation, and as a result, AC can potentially induce further dissolution [55,57,73]. As demonstrated in Figure 2b, a decrease in system impedances is observed by increasing potential perturbation in the whole spectrum. At high frequencies, the current is absorbed mainly by the interface capacitance, and the contribution of the nonlinear resistance of the charge transfer was negligible. However, by decreasing the frequency, the current flowed progressively through the nonlinear charge transfer resistor, increasing the nonlinearity at low frequencies [66]. In addition to the nonlinear behavior of the charge transfer resistance against high-order potential perturbations, as shown in Figure 2a, faradaic distortion can affect the capacitive behavior at the electrolyte/oxide film/substrate [74,75,76].
Figure 2c shows the corrected phase angle Bode plot obtained from the current response of the EIS measurements. Phase angle correction by subtracting the solution resistance ), yields valuable information concerning high and intermediate frequencies [77]. As shown in Figure 2c, the obtained phase angle spectra can be divided into four sections. At the highest frequencies of section I, the electrode geometry induced an inhomogeneous distribution of potential and current; as a result, phases were not steady [78,79]. This behavior was also attributed to the conductivity of the titanium oxide film formed on the surface of the specimens [76,80,81]. Increasing the excitation potential changed the behavior of the specimens, particularly at high frequencies. In section II, the phase angles showed constant values below 90° at intermediate frequencies, ascribing the constant phase element (CPE) behavior of the adsorptive layer at the electrolyte/oxide film [75,76,82]. In terms of CPE, the impedance associated with faradaic reaction without diffusion can be written as
where α is the CPE exponent, which can be obtained as follows:
Phase angle instability indicates inhomogeneous ohmic and kinetic distribution at the electrolyte/film/substrate. When α = 1, the dielectric characteristic of the surface can be assumed as ideal capacitance, while deviation from ideal values shows instability and/or inhomogeneous ohmic and kinetic distribution at the electrolyte/film/substrate [83]. As shown in Table 2, the graphically obtained values for α decrease when increasing the excitation potential’s amplitude. Increasing the excitation potential’s amplitude, even in the very low range of activation polarization, can change the specimens’ dielectric properties and charge transfer resistance. Decreasing values of α and instabilities in phase angles are indications of inhomogeneous ohmic and kinetic distribution at the electrolyte/film/substrate [83]. As a result, the surface can become vulnerable to film-dissolution-localized reactions after long-term exposure due to the detrimental effect of a high order of superimposed alternating potential [84,85,86]. Section III, known as the integration interval, mainly describes the relaxation time of the distributed capacitances [87,88]. In section IV, the Z approached the maximum value, and the frequency was sufficiently low to fully charge the capacitances [77].

Table 2.
The composition of 1 L 1X-PBS solution used in this study.
The effect of low potential excitation with different frequencies was investigated on the specimens after 24 h of immersion in the 1X-PBS electrolyte. The amplitude of 100 mV was chosen as the potential excitation, as it typically exists in the human body, tissue, and implant material [89]. For each condition, after preparation, the specimens were exposed to the 1X-PBS solution for 1 h at their OCP. Then, 100 mV sinusoidal potential vs. their OCP was applied to the specimens with constant frequency for 24 h. Here, 500, 50, 0.5, and 0.05 Hz were chosen as the frequencies. These frequencies were selected to represent one frequency in each section in Figure 2c. The application of the alternating potential was stopped for approximately 10 min, and standard/linear EIS (±10 mV perturbation at corresponding OCP, with a frequency range of 100 kHz to 1 mHz) was conducted on the specimens. Figure 3 shows the typical Nyquist and Bode phase (φadj) obtained from EIS measurements during 24 h exposure under different sinusoidal frequency perturbations.
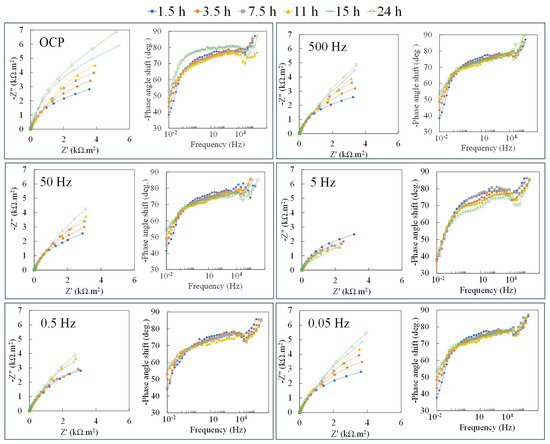
Figure 3.
Typical Nyquist and Bode phases obtained from EIS measurements during 24 h exposure of Ti-6Al-4V in the 1X-PBS solution at 37 °C superimposed by applying a constant 100 mV at OCP and different frequencies.
In all measurements, a single depressed arc can be observed in the Nyquist plot, indicating that electrochemical reactions in all examined conditions were governed by charge transfer control. This characteristic is also evident in Bode phase plots, where no distinct local minima in the medium to low frequency were observed. The non-steady phase angles at frequencies above 103 Hz mainly arise from surface geometry inducing an even potential. In the specimen at OCP, the impedance consistently rises upon immersion time, with the phase angles stabilizing at higher angles. However, this trend cannot be observed in other specimens exposed to frequent oxidation and reduction at different frequencies. To better understand the change and trend of the electrochemical behavior of the examined specimens, the specimens’ charge transfer resistances, i.e., Rt, were then obtained from the results of the EIS tests using the Brug method [90], presented in Figure 4.
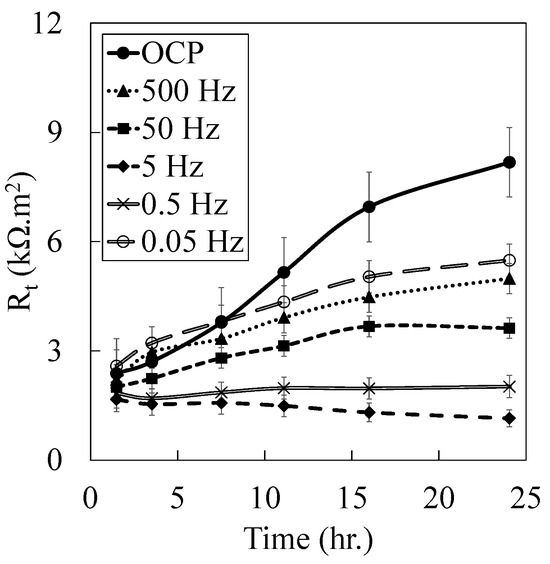
Figure 4.
The evolution of charge transfer during 24 h exposure in the 1X-PBS solution at 37 °C was superimposed by applying a constant 100 mV vs. OCP amplitude with different sinusoidal frequencies.
It is noteworthy that regressed physicochemical quantities should be taken carefully. The specimens indicated nonlinear behavior due to the activation polarization and mass transport phenomena in low frequencies [66,91,92]. Thus, any mechanism proposed with an equivalent circuit may become more complex or degenerate by giving it the same impedance [76,93]. As time passes, charge transfer resistance increases for the OCP condition due to the stabilizing of the interface capacitance and the aging of the oxide film [94]. However, applying the potential truncated this trend. Applying 100 mV with 500 and 50 Hz frequencies reduced the charge transfer resistance compared to the OCP condition; however, their trends were positive. As shown in Figure 4, in this range of frequencies (section II), the responses of the specimens were mainly due to the non-faradaic current of the double-layer capacitance. As a result, the double layer’s charge–discharge process consumed most of the current generated by the potential perturbation.
Additionally, further passivation and surface reduction could happen at slow rates. Decreasing the frequency to 5 Hz decreased the charge transfer with a negative slope vs. exposure time. Around this frequency, the dielectric capacitance of the double layer began to shift the phase and charge. Consequently, the involvement of the faradaic reactions increased. As a result, the oxide film formed during anodic activation reduced readily in the cathodic part, and the number of ions released from the surface increased [59]. Nevertheless, a further decrease in imposed frequency to 0.5 and 0.05 Hz again increased the charge transfer.
It is hypothesized that reducing the frequency provided sufficient time for the distribution of the dielectrics and relaxation. Consequently, the faradaic reaction consumed a larger portion of the current. As Huang and Blackwood proposed [67], a slow anodic scan rate (i.e., low frequencies) formed a thicker and more stable oxide film in each cycle.
Although, in this study, EIS measurements reflect the nonlinearities in relaxations due to high-amplitude perturbations, when the growth condition no longer applies, the electrochemical impedance of the electrode is mainly dominated by a space-charge layer at the solution–oxide film interface and thus not merely by the properties of oxide film [94,95]. As a result, the corrosion rate cannot readily be determined from impedance measurements. So, techniques such as Raman spectroscopy are required to qualitatively assess the oxide layer offered. Figure 5 shows the Raman spectra obtained from the in situ test on the specimens. Three strong emission bands at 145, 257, and 511 cm−1 and one weak band at 273 were detectable in all spectra. These Raman shifts correspond to typical Ti-O bending vibrations of very thin (around a few nanometers) Ti oxide film crystalline structures at the specimens’ surface, presumably TiO2 and Ti2O3 [10,68]. Due to the new structure formation, no apparent band was detected by applying potential perturbation on the surface of the specimens during 24 h of immersion in the PBS solution. However, increasing the immersion time from 1 h to 24 h at the OCP increased the intensity of the peaks, implying an increase in the crystallinity of the Ti oxide formed on the surface [68]. Specimens treated with 100 mV at 500 Hz for 24 h yielded relatively analogous results, although with shorter spikes of Ti2O3. Twenty-four hours of superposition of 100 mV at 5 Hz weakened and truncated the Raman bands. Peak broadening in the Raman bands ascribes the transformation of oxide film from crystalline structure to more disordered and defective crystals or an amorphous state [10,11].
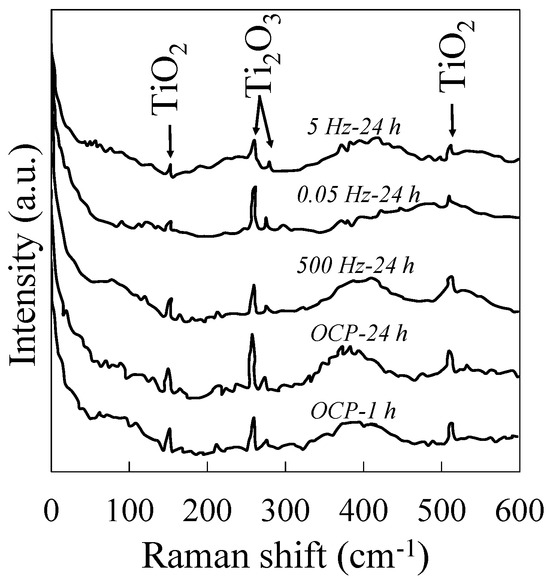
Figure 5.
Raman spectra obtained after 24 h of exposure in the PBS solution at 37 °C superimposed with a constant potential of ±100 mV amplitude vs. OCP and various frequencies.
Combined with the results from EIS tests, it was postulated that applying potential at certain intermediate frequencies did not allow the formation of a protective, stable, and integrated layer of oxide film on the surface of the specimens. As a result, the charge transfer decreased, and the interface dissolution increased. Potential perturbation applied with a frequency of 0.05 Hz was low enough to form a stable film that was not readily reducible in the cathodic part of each scan. Similar to the 24 h OCP condition, Ti2O3 peaks were more prominent than the TiO2 for this frequency condition.
The n-type semiconductive behavior of the Ti oxide film can explain this behavior. During the growth of the Ti oxide film, the Ti (II) and Ti (III) act as donors and migrate through the oxide toward the electrolyte [16]. However, the Ti species are gradually oxidized [17,67,94]. At low frequencies when all the dielectrics were charged, the applied potential did not allow the Ti(II) to migrate further from the substrate, and consequently, these species oxidized more in the form of Ti(III)/Ti2O3 rather than Ti(IV)/TiO2. With a higher growth rate, for example at 5Hz, when dielectric charge–discharge behavior was partly involved, high Ti(II) concentrations were trapped and partially reduced during the cathodic scan. The high donor densities were previously observed in a Ti film [96,97].
4. Conclusions
This study delved into the intricate electrochemical behavior of immersed specimens under various stimulus potentials and frequencies. The Nyquist plots demonstrated that higher potentials reduced impedance, implying a potential-dependent behavior. Different sections were identified through phase angle spectra analysis, each corresponding to distinct electrochemical processes. Applying 100 mV alternating potentials at different frequencies revealed complex charge transfer behaviors. While 500 and 50 Hz frequencies reduced charge transfer resistance due to dominant non-faradaic currents, lower frequencies (0.5 and 0.05 Hz) increased resistance through intensified faradaic reactions and relaxation effects.
Raman spectroscopy results aligned with EIS observations, providing insight into oxide film crystallinity and stability. The semiconductive behavior of the Ti oxide film and the migration of Ti species contributed to the observed effects. Moreover, it was revealed that frequent oxidation and reduction at a certain range of frequencies can induce corrosion and hinder the formation of biomimetic apatite, potentially affecting the osseointegration process.
There is always a natural alternative current or potential in the human body due to, e.g., heart palpitations and brain/nervous system activities. Ignoring such natural activities may lead to an underestimation of the corrosion performance of Ti-based implant alloys in the body. The study’s implications extend to the design and optimization of implant materials for biomedical applications. However, further investigation is warranted to uncover underlying mechanisms and validate findings through complementary techniques and in vivo studies. Overall, this research contributes to our understanding of electrochemical interactions, offering insights that can drive advancements in biomaterials and their successful integration within biological systems.
Author Contributions
Conceptualization, H.T.-S. and A.P.; methodology, H.T.-S.; formal analysis, H.T.-S., A.P., L.D. and G.D.; investigation, H.T.-S. and I.K.; writing, original draft preparation, H.T.-S.; writing, review and editing, H.T.-S., A.P., I.K., G.D. and L.D.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work was conducted in the Corrosion Research Laboratory (CorRLab) at Clemson University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials. Nanotechnol. Appl. Tissue Eng. 2015, 2, 21–44. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of Metal Orthopaedic Implants. J. Bone Jt. Surg. (Am. Vol.) 1998, 80, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarado, C.; Sundaram, P.A. Corrosion evaluation of Ti-48Al-2Cr-2Nb (at.%) in Ringer’s solution. Acta Biomater. 2006, 2, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Liu, R.L.; Majumdar, T.; Mantri, S.A.; Ravi, V.A.; Banerjee, R.; Birbilis, N. A closer look at the in vitro electrochemical characterisation of titanium alloys for biomedical applications using in-situ methods. Acta Biomater. 2017, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Klimova-Korsmik, O.G.; Turichin, G.A.; Shalnova, S.A.; Gushchina, M.O.; Cheverikin, V.V. Structure and properties of Ti-6Al-4V titanium alloy products obtained by direct laser deposition and subsequent heat treatment. J. Phys. Conf. Ser. 2018, 1109, 012061. [Google Scholar] [CrossRef]
- Vogel, S.C.; Bhattacharyy, D.; Viswanathan, G.B.; Williams, D.J.; Fraser, H.L. Phase transformation textures in Ti-6Al-4V alloy. Mater. Sci. Forum 2005, 495–497, 681–686. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–120. [Google Scholar] [CrossRef]
- Yu, F.; Addison, O.; Davenport, A.J. A synergistic effect of albumin and H2O2 accelerates corrosion of Ti6Al4V. Acta Biomater. 2015, 26, 355–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Addison, O.; Yu, F.; Troconis, B.C.R.; Scully, J.R.; Davenport, A.J. Time-dependent enhanced corrosion of Ti6Al4V in the presence of H2O2 and albumin. Sci. Rep. 2018, 8, 3185. [Google Scholar] [CrossRef]
- Pankuch, M.; Bell, R.; Melendres, C.A. Composition and structure of the anodic films on titanium in aqueous solutions. Electrochim. Acta 1993, 38, 2777–2779. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Guo, J.; Sato, N. Raman Spectra of the Anodic Oxide Film on Titanium in Acidic Sulfate and Neutral Phosphate Solutions. J. Electrochem. Soc. 1986, 133, 2473–2476. [Google Scholar] [CrossRef]
- Li, J.; Li, S.J.; Hao, Y.L.; Huang, H.H.; Bai, Y.; Hao, Y.Q.; Guo, Z.; Xue, J.Q.; Yang, R. Electrochemical and surface analyses of nanostructured Ti-24Nb-4Zr-8Sn alloys in simulated body solution. Acta Biomater. 2014, 10, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.; Guastaldi, A. Electrochemical stability and corrosion resistance of Ti–Mo alloys for biomedical applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Takadoum, J. Review on corrosion, tribocorrosion and osseointegration of titanium alloys as biomaterials. Corros. Mater. Degrad. 2023, 4, 644–658. [Google Scholar] [CrossRef]
- Gaintantzopoulou, M.; Zinelis, S.; Silikas, N.; Eliades, G. Micro-Raman spectroscopic analysis of TiO2 phases on the root surfaces of commercial dental implants. Dent. Mater. 2014, 30, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface modifications of titanium materials for developing corrosion behavior in human body environment: A review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Peter, L.M. The influence of growth rate on the properties of anodic oxide films on titanium. Electrochim. Acta 1989, 34, 1505–1511. [Google Scholar] [CrossRef]
- Properties of Materials. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 5–59. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef]
- Zainal Abidin, N.I.; Rolfe, B.; Owen, H.; Malisano, J.; Martin, D.; Hofstetter, J.; Uggowitzer, P.J.; Atrens, A. The in vivo and in vitro corrosion of high-purity magnesium and magnesium alloys WZ21 and AZ91. Corros. Sci. 2013, 75, 354–366. [Google Scholar] [CrossRef]
- Levine, D.L.; Staehle, R.W. Crevice corrosion in orthopedic implant metals. J. Biomed. Mater. Res. 1977, 11, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Gudić, S.; Vrsalović, L.; Kvrgić, D.; Nagode, A. Electrochemical behavior of Ti and Ti-6Al-4V alloy in phosphate buffered saline solution. Materials 2021, 14, 7495. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, A.; Milošev, I.; Pihlar, B. Cobalt-based alloys for orthopaedic applications studied by electrochemical and XPS analysis. J. Mater. Sci. Mater. Med. 2004, 15, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti–Ta–Nb–Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- McGuff, H.S.; Heim-Hall, J.; Holsinger, F.C.; Jones, A.A.; O’Dell, D.S.; Hafemeister, A.C. Maxillary Osteosarcoma Associated with a Dental Implant. J. Am. Dent. Assoc. 2008, 139, 1052–1059. [Google Scholar] [CrossRef]
- Poggio, C.E. Plasmacytoma of the mandible associated with a dental implant failure: A clinical report. Clin. Oral Implant. Res. 2007, 18, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Lugowski, S.J.; Smith, D.C.; McHugh, A.D.; Van Loon, J.C. Release of metal ions from dental implant materials in vivo: Determination of Al, Co, Cr, Mo, Ni, V, and Ti in organ tissue. J. Biomed. Mater. Res. 1991, 25, 1443–1458. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Schwartz, Z.; Köster, G.; Jahn, U.; Buchhorn, G.H.; MacDougall, M.J.; Casasola, D.; Liu, Y.; Sylvia, V.L.; Dean, D.D.; et al. Phagocytosis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle composition. Biomaterials 2000, 21, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Goc, A. Biological activity of vanadium compounds. Cent. Eur. J. Biol. 2006, 1, 314–332. [Google Scholar] [CrossRef]
- Alves, V.A.; Reis, R.Q.; Santos, I.C.B.; Souza, D.G.; de Gonçalves, T.F.; Pereira-da-Silva, M.A.; Rossi, A.; da Silva, L.A. In situ impedance spectroscopy study of the electrochemical corrosion of Ti and Ti–6Al–4V in simulated body fluid at 25 °C and 37 °C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- Swaminathan, V.; Gilbert, J.L. Fretting corrosion of CoCrMo and Ti6Al4V interfaces. Biomaterials 2012, 33, 5487–5503. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Orthopedic Implant Fretting Corrosion. Corros. Rev. 2003, 21, 183–214. [Google Scholar] [CrossRef]
- Lewis, A.C.; Kilburn, M.R.; Papageorgiou, I.; Allen, G.C.; Case, C.P. Effect of synovial fluid, phosphate-buffered saline solution, and water on the dissolution and corrosion properties of CoCrMo alloys as used in orthopedic implants. J. Biomed. Mater. Res. Part A 2005, 73A, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, R. Numerical Determination of Induced Currents in Humans and Baboons Exposed to 60-Hz Electric Fields. IEEE Trans. Electromagn. Compat. 1981, EMC-23, 382–390. [Google Scholar] [CrossRef]
- Wang, D.; Lu, T.; Li, X.; Chen, B.; Li, X.; Xie, L.; Ju, Y. Simulation and analysis of human body micro-shocks in the ion flow field near HVDC transmission lines. J. Electrost. 2018, 93, 10–16. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Peratta, A.; Poljak, D. Boundary Element Modeling of the Realistic Human Body Exposed to Extremely-Low-Frequency (ELF) Electric Fields: Computational and Geometrical Aspects. IEEE Trans. Electromagn. Compat. 2007, 49, 153–162. [Google Scholar] [CrossRef]
- Genuis, S.J. Fielding a current idea: Exploring the public health impact of electromagnetic radiation. Public Health 2008, 122, 113–124. [Google Scholar] [CrossRef]
- King, R.W.P. Electric current and electric field induced in the human body when exposed to an incident electric field near the resonant frequency. IEEE Trans. Microw. Theory Tech. 2000, 48, 1537–1543. [Google Scholar] [CrossRef]
- Poole, C.; Kavet, R.; Funch, D.P.; Donelan, K.; Charry, J.M.; Dreyer, N.A. Depressive Symptoms and Headaches in Relation to Proximity of Residence to an Alternating-Current Transmission Line Right-of-way. Am. J. Epidemiol. 1993, 137, 318–330. [Google Scholar] [CrossRef]
- Brighton, C.T.; Hunt, R.M. Ultrastructure of electrically induced osteogenesis in the rabbit medullary canal. J. Orthop. Res. 1986, 4, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lirani-Galvão, A.P.R.; Chavassieux, P.; Portero-Muzy, N.; Bergamaschi, C.T.; Silva, O.L.; Carvalho, A.B.; Lazaretti-Castro, M.; Delmas, P.D. Low-Intensity Electrical Stimulation Counteracts the Effects of Ovariectomy on Bone Tissue of Rats: Effects on Bone Microarchitecture, Viability of Osteocytes, and Nitric Oxide Expression. Calcif. Tissue Int. 2009, 84, 502–509. [Google Scholar] [CrossRef]
- Song, J.K.; Cho, T.H.; Pan, H.; Song, Y.M.; Kim, I.S.; Lee, T.H.; Hwang, S.J.; Kim, S.J. An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics 2009, 30, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. New estimation method for the electric potential of the human body under perfect noncontact conditions. IEEJ Trans. Electr. Electron. Eng. 2009, 4, 309–311. [Google Scholar] [CrossRef]
- Denaro, V.; Cittadini, A.; Barnaba, S.A.; Ruzzini, L.; Denaro, L.; Rettino, A.; Paola, B.D.; Papapietro, N.; Sgambato, A. Static Electromagnetic Fields Generated by Corrosion Currents Inhibit Human Osteoblast Differentiation. Spine 2008, 33, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Chirakanphaisarn, N.; Thongkanluang, T.; Chiwpreechar, Y. Heart rate measurement and electrical pulse signal analysis for subjects span of 20–80 years. J. Electr. Syst. Inf. Technol. 2018, 5, 112–120. [Google Scholar] [CrossRef]
- Helmreich, S. Potential energy and the body electric-Cardiac waves, brain waves, and the making of quantities into qualities. Curr. Anthropol. 2013, 54, S139–S148. [Google Scholar] [CrossRef]
- Levin, M. Large-scale biophysics: Ion flows and regeneration. Trends Cell Biol. 2007, 17, 261–270. [Google Scholar] [CrossRef]
- Norton, L.A.; Hanley, K.J.; Turkewicz, J. Bioelectric perturbations of bone: Research directions and clinical applications. Angle Orthod. 1984, 54, 73–87. [Google Scholar] [PubMed]
- Bosch, R.W.; Bogaerts, W.F. A theoretical study of AC-induced corrosion considering diffusion phenomena. Corros. Sci. 1998, 40, 323–336. [Google Scholar] [CrossRef]
- Bertocci, U. AC Induced Corrosion. The Effect of an Alternating Voltage on Electrodes Under Charge-Transfer Control. Corrosion 1979, 35, 211–215. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion—Part 1: Effects on overpotentials of anodic and cathodic processes. Corros. Sci. 2010, 52, 491–497. [Google Scholar] [CrossRef]
- Chena, X.; Shahb, K.; Donga, S.; Petersona, L.; Plantea, E.C.L.; Santa, G. Elucidating the corrosion-related degradationmechanisms of a Ti-6Al-4V dental implant. Dent. Mater. 2020, 36, 431–441. [Google Scholar] [CrossRef]
- Zhang, R.; Vairavanathan, P.R.; Lalvani, S.B. Perturbation method analysis of AC-induced corrosion. Corros. Sci. 2008, 50, 1664–1671. [Google Scholar] [CrossRef]
- Chin, D.T.; Venkatesh, S. A Study of Alternating Voltage Modulation on the Polarization of Mild Steel. J. Electrochem. Soc. 1979, 126, 1908–1913. [Google Scholar] [CrossRef]
- Wang, H.; Du, C.; Liu, Z.; Wang, L.; Ding, D. Effect of Alternating Current on the Cathodic Protection and Interface Structure of X80 Steel. Materials 2017, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Ouadah, M.h.; Touhami, O.; Ibtiouen, R.; Zergoug, M. Method for diagnosis of the effect of AC on the X70 pipeline due to an inductive coupling caused by HVPL. IET Sci. Meas. Technol. 2017, 11, 766–772. [Google Scholar] [CrossRef]
- ASTM F1472-02; Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400). ASTM: West Conshohocken, PA, USA, 2002.
- Asserghine, A.; Filotás, D.; Nagy, L.; Nagy, G. Scanning electrochemical microscopy investigation of the rate of formation of a passivating TiO2 layer on a Ti G4 dental implant. Electrochem. Commun. 2017, 83, 33–35. [Google Scholar] [CrossRef]
- Fasmin, F.; Srinivasan, R. Review—nonlinear electrochemical impedance spectroscopy. J. Electrochem. Soc. 2017, 164, H443–H455. [Google Scholar] [CrossRef]
- Dong, Z.; Torbati-Sarraf, H.; Poursaee, A. Determining the optimal frequency and perturbation amplitude for AC electrical resistance measurements of cement-based materials using harmonic analysis. Adv. Civ. Eng. Mater. 2022, 11, 339–353. [Google Scholar] [CrossRef]
- Dong, Z.; Torbati-Sarraf, H.; Hussein, H.; Poursaee, A. Harmonic analysis on the effect of potential perturbations and electrode arrangements on the electrochemical impedance (EIS) measurement of cementitious material. Constr. Build. Mater. 2021, 273, 121701. [Google Scholar] [CrossRef]
- Kiel, M.; Bohlen, O.; Sauer, D.U. Harmonic analysis for identification of nonlinearities in impedance spectroscopy. Electrochim. Acta 2008, 53, 7367–7374. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Blackwood, D.J. Characterisation of titanium oxide film grown in 0.9% NaCl at different sweep rates. Electrochim. Acta 2005, 51, 1099–1107. [Google Scholar] [CrossRef]
- Arsov, L.D.; Kormann, C.; Plieth, W. In Situ Raman Spectra of Anodically Formed Titanium Dioxide Layers in Solutions of H2SO4, KOH, and HNO3. J. Electrochem. Soc. 1991, 138, 2964–2970. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of cyclic potentiodynamic polarization test results for study of corrosion behavior of metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 1, 976–989. [Google Scholar] [CrossRef]
- Souza, M.E.P.; Lima, L.; Lima, C.R.P.; Zavaglia, C.A.C.; Fieire, C.M.A. Effects of pH on the electrochemical behaviour of titanium alloys for implant applications. J. Mater. Sci. Mater. Med. 2009, 20, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Chelariu, R.; Bolat, G.; Izquierdo, J.; Mareci, D.; Gordin, D.M.; Gloriant, T.; Souto, R.M. Metastable beta Ti-Nb-Mo alloys with improved corrosion resistance in saline solution. Electrochim. Acta 2014, 137, 280–289. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion. Part 2: Parameters influencing corrosion rate. Corros. Sci. 2010, 52, 916–922. [Google Scholar] [CrossRef]
- Bosch, R.W.; Bogaerts, W.F. Harmonic analysis of corroding systems considering diffusion phenomena. J. Electrochem. Soc. 1996, 143, 4033. [Google Scholar] [CrossRef]
- Darowicki, K.; Majewska, J. Harmonic Analysis of Electrochemical and Corrosion Systems—A Review. Corros. Rev. 1999, 17, 383–400. [Google Scholar] [CrossRef]
- Tjelta, M.; Sunde, S. Comment on “Current-distribution effects on the impedance of porous electrodes and electrodes covered with films” by M. Tjelta and S. Sunde [J. Electroanal. Chem. 737 (2015) 65–77]. J. Electroanal. Chem. 2016, 763, 79–80. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Oliveira, N.T.C.; Bolfarini, C.; Roche, V.; Nogueira, R.P. Electrochemical impedance analysis of TiO2 nanotube porous layers based on an alternative representation of impedance data. J. Electroanal. Chem. 2015, 737, 54–64. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Córdoba-Torres, P.; Mesquita, T.J.; Nogueira, R.P. Toward a better characterization of constant-phase element behavior on disk electrodes from direct impedance analysis: Methodological considerations and mass transport effects. Electrochim. Acta 2013, 92, 323–334. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Mesquita, T.J.; Nogueira, R.P. Influence of geometry-induced current and potential distributions on the characterization of constant-phase element behavior. Electrochim. Acta 2013, 87, 676–685. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Frateur, I.; Orazem, M.E.; Tribollet, B. The Global and Local Impedance Response of a Blocking Disk Electrode with Local Constant-Phase-Element Behavior. J. Electrochem. Soc. 2007, 154, C89. [Google Scholar] [CrossRef]
- Bojinov, M.; Stancheva, M. Coupling between dissolution and passivation revisited—Kinetic parameters of anodic oxidation of titanium alloys in a fluoride-containing electrolyte. J. Electroanal. Chem. 2015, 737, 150–161. [Google Scholar] [CrossRef]
- Baux, J.; Caussé, N.; Esvan, J.; Delaunay, S.; Tireau, J.; Roy, M.; You, D.; Pébère, N. Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochim. Acta 2018, 283, 699–707. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Mesquita, T.J.; Devos, O.; Tribollet, B.; Roche, V.; Nogueira, R.P. On the intrinsic coupling between constant-phase element parameters α and Q in electrochemical impedance spectroscopy. Electrochim. Acta 2012, 72, 172–178. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.W. A new understanding on AC corrosion of pipeline steel in alkaline environment. J. Mater. Eng. Perform. 2017, 26, 221–228. [Google Scholar] [CrossRef]
- Qiu, W.W.; Pagano, M.; Zhang, G.; Lalvani, S.B. A periodic voltage modulation effect on the corrosion oF Cu-Ni Alloy. Corros. Sci. 1995, 37, 97–110. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.; Li, X.; Liu, Z.; Wang, S.; Li, J.; Zhang, D. Effect of AC current density on stress corrosion cracking behavior of X80 pipeline steel in high pH carbonate / bicarbonate solution. Electrochim. Acta 2014, 117, 351–359. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Characterization of frequency dispersion in the impedance response of a distributed model from the mathematical properties of the distribution function of relaxation times. Electrochim. Acta 2015, 180, 591–603. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. A general theory for the impedance response of dielectric films with a distribution of relaxation times. Electrochim. Acta 2018, 282, 892–904. [Google Scholar] [CrossRef]
- Peterka, D.S.; Takahashi, H.; Yuste, R. Imaging voltage in neurons. Neuron 2011, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Srinivasan, R.; Pachimatla, R. Nonlinear Electrochemical Impedance Spectroscopic Analysis of Ti Dissolution in HF. ECS Trans. 2017, 80, 1039–1048. [Google Scholar] [CrossRef]
- Ghez, R. Simple Time-Dependent Examples. In Diffusion Phenomena: Case and Studies; Springer Science & Business Media: Berlin, Germany, 2001; pp. 103–143. [Google Scholar] [CrossRef]
- Fletcher, S. Tables of Degenerate Electrical Networks for Use in the Equivalent-Circuit Analysis of Electrochemical Systems. J. Electrochem. Soc. 1994, 141, 1823–1826. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Peter, L.M.; Williams, D.E. Stability and open circuit breakdown of the passive oxide film on titanium. Electrochim. Acta 1988, 33, 1143–1149. [Google Scholar] [CrossRef]
- Blackwood, D.J. Influence of the space-charge region on electrochemical impedance measurements on passive oxide films on titanium. Electrochim. Acta 2000, 46, 563–569. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Otsuki, T. The influence of the growth rate on the semiconductive properties of titanium anodic oxide films. Corros. Sci. 1998, 40, 951–958. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Greef, R.; Peter, L.M. An ellipsometric study of the growth and open-circuit dissolution of the anodic oxide film on titanium. Electrochim. Acta 1989, 34, 875–880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).