Carbonation-Induced Corrosion of Reinforced Concrete Elements according to Their Positions in the Buildings
Abstract
1. Introduction
1.1. Concrete and Corrosion
1.2. Carbonation
1.3. Norm Codes
1.4. Objectives
2. Methodology
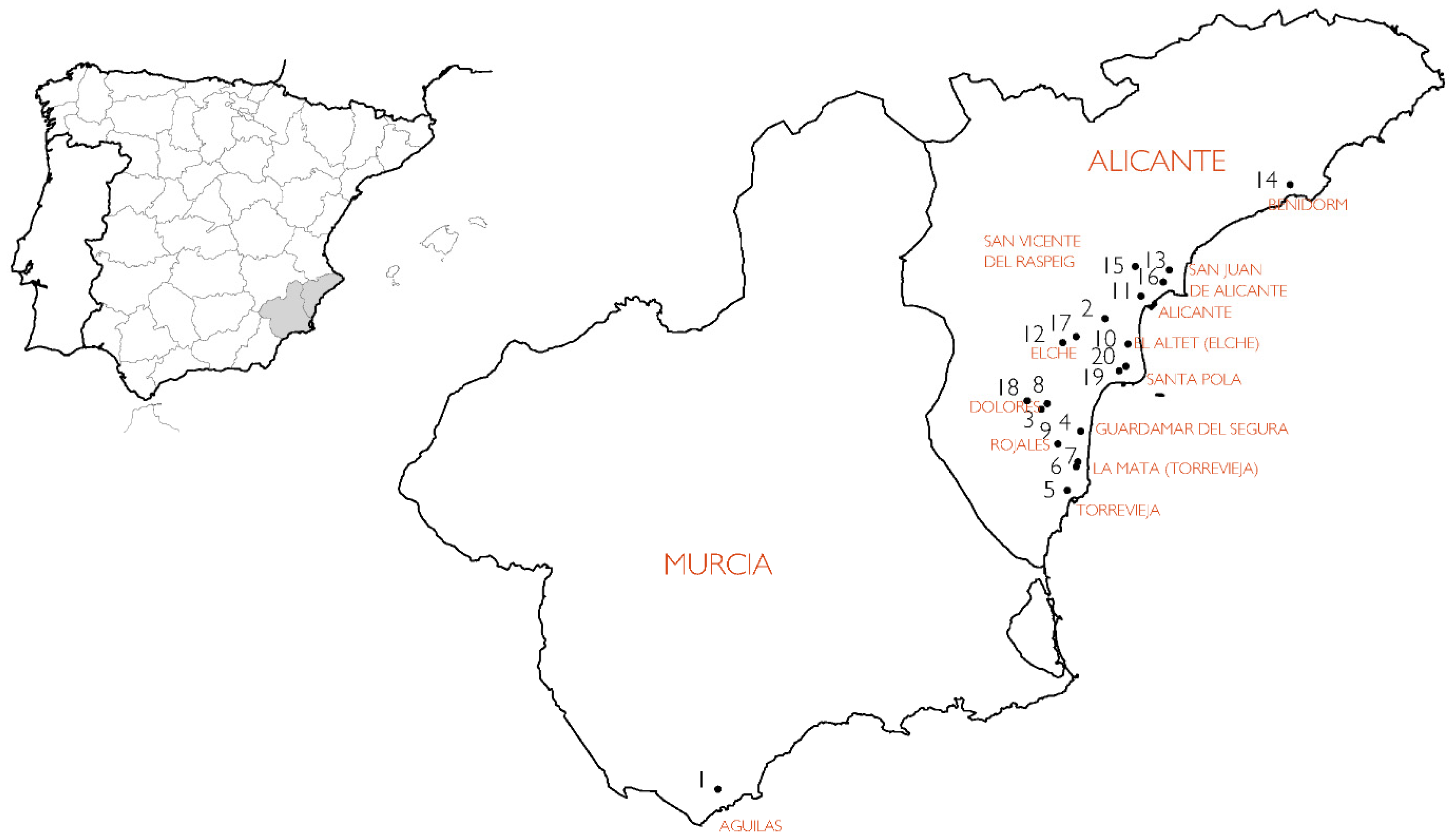
2.1. Buildings and Construction Elements Studied
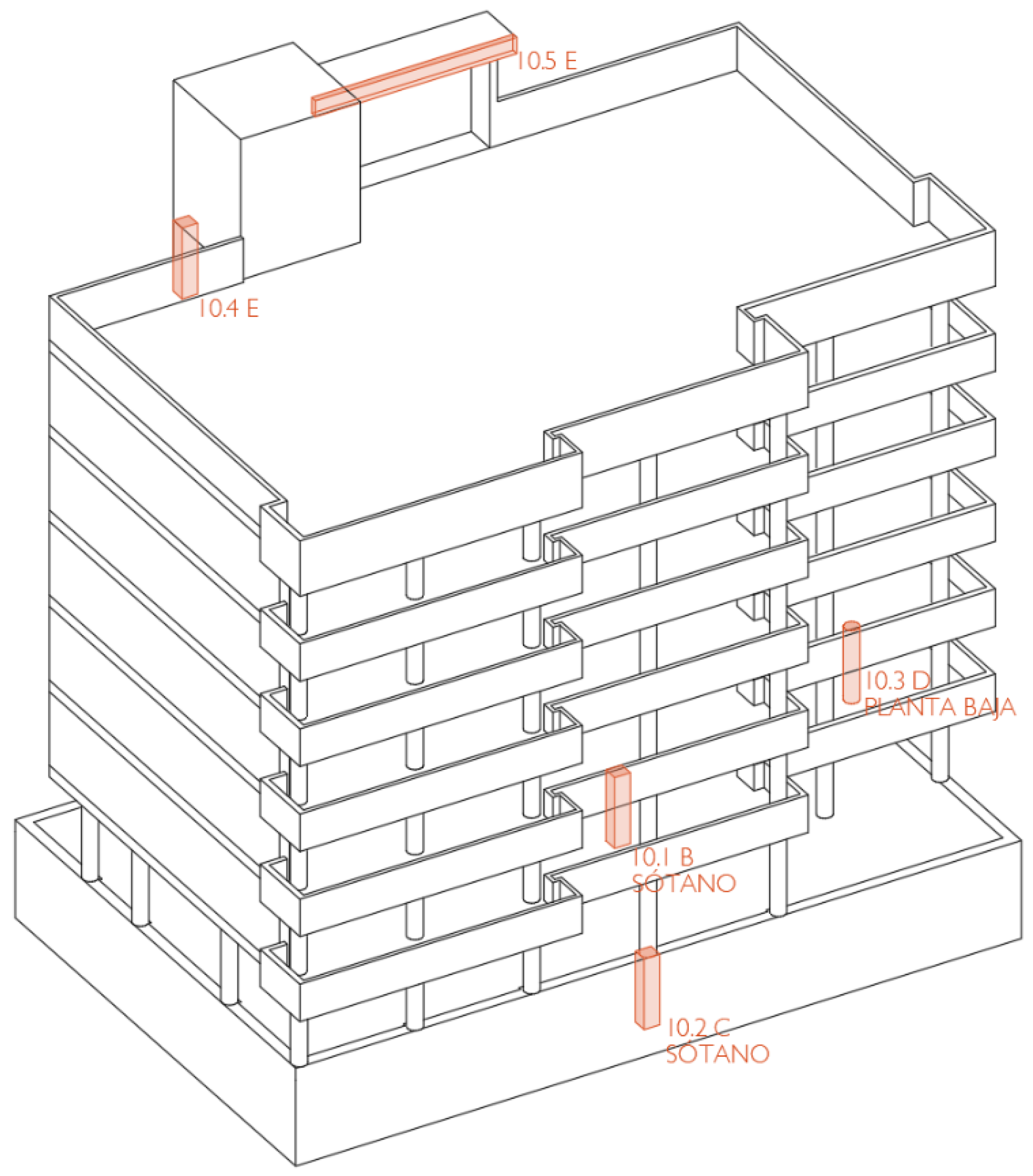
2.2. Variable According to the Location of the Element in the Building
- Façade columns in contact with the ground
- Interior columns in contact with the ground
- Wall columns in contact with the ground
- External columns and beams protected from rain
- External columns and beams exposed to rain
- Columns and beams in air chambers beneath sanitary slabs
- Interior columns and beams
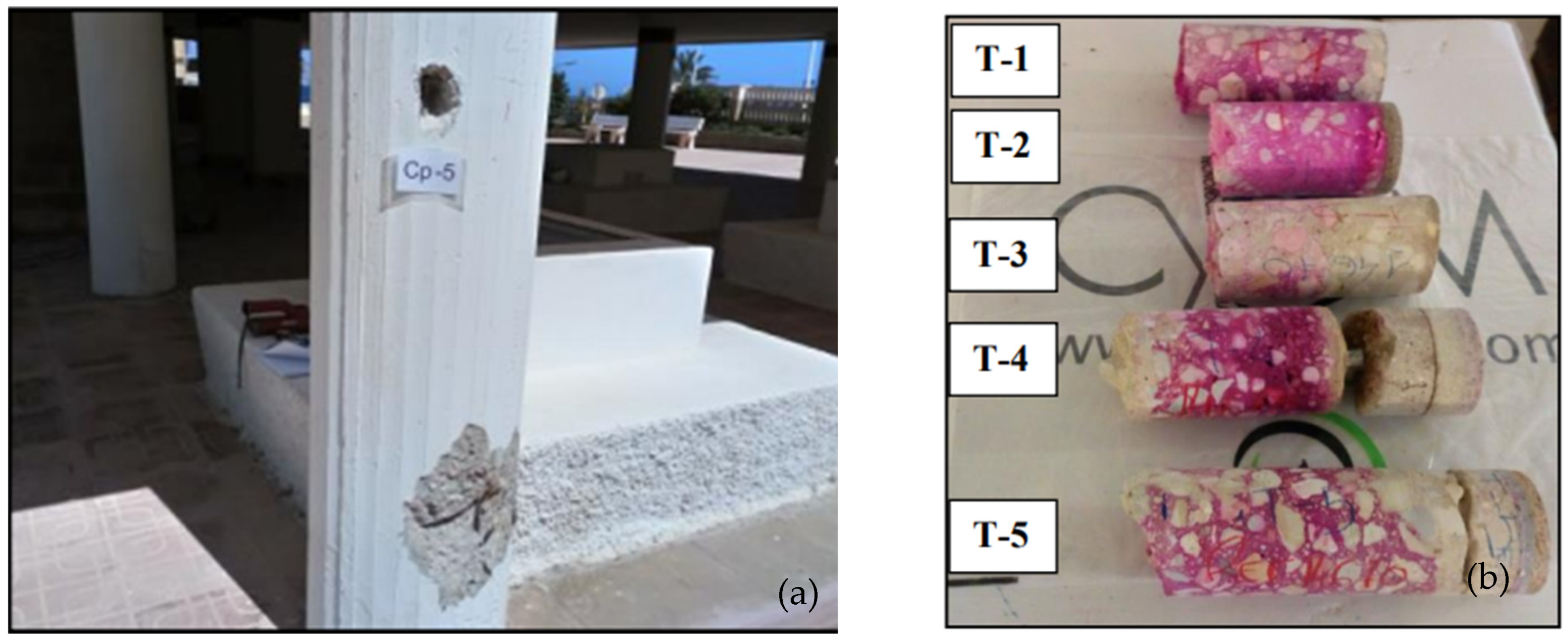
2.3. Testing
2.4. Calculation of Kap,carb, and KCO2—Models and Statistical Processing
2.4.1. Calculation of Kap,carb
2.4.2. Calculation of KCO2
2.4.3. Models and Statistical Processing
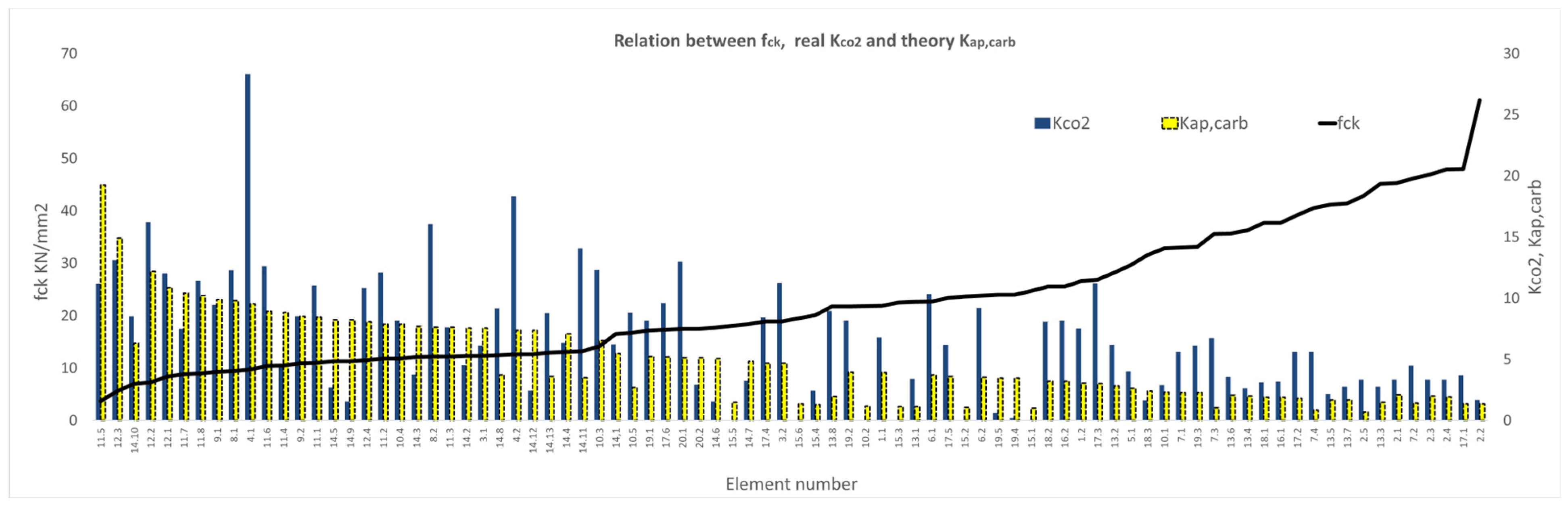
3. Results and Discussion
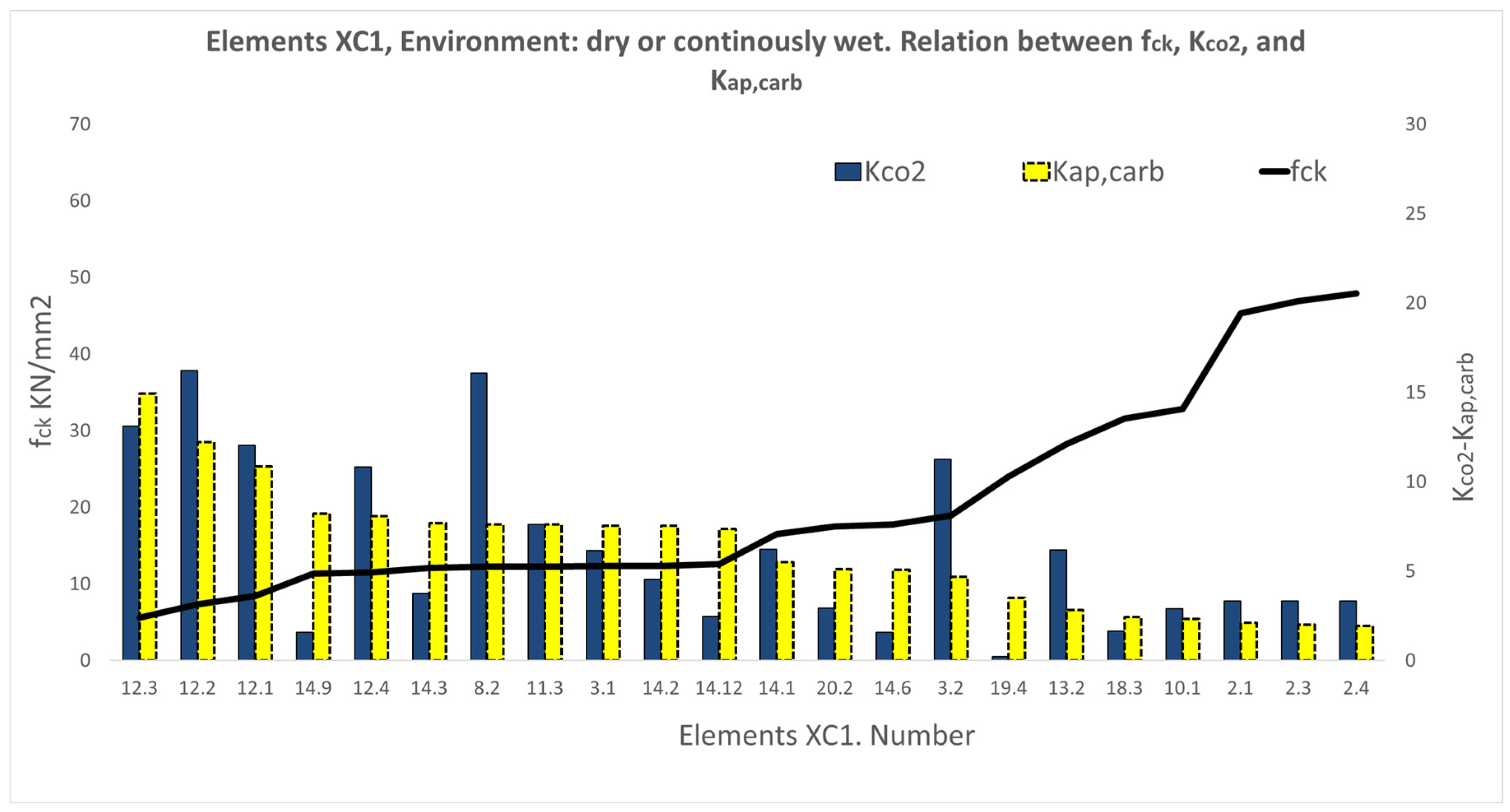
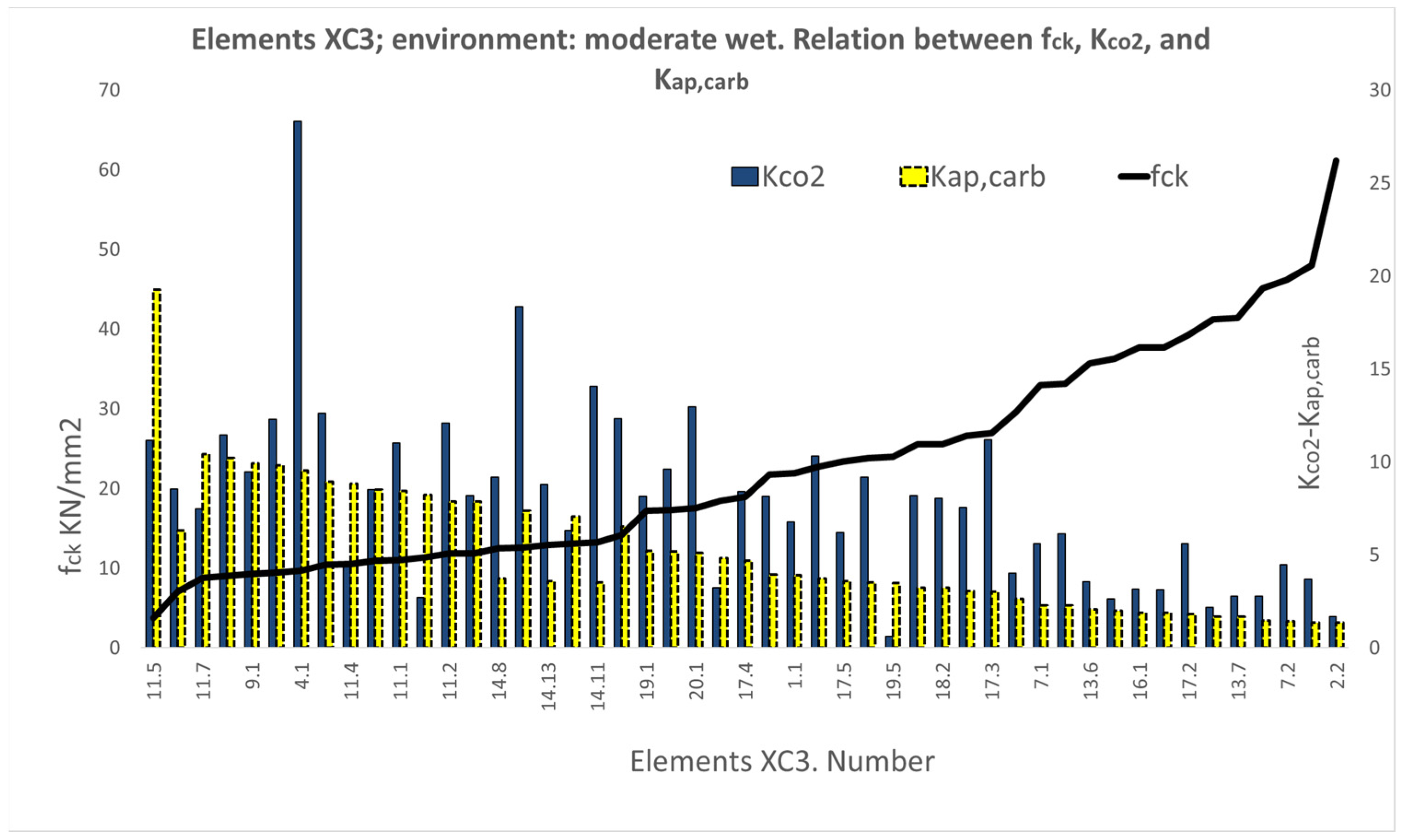
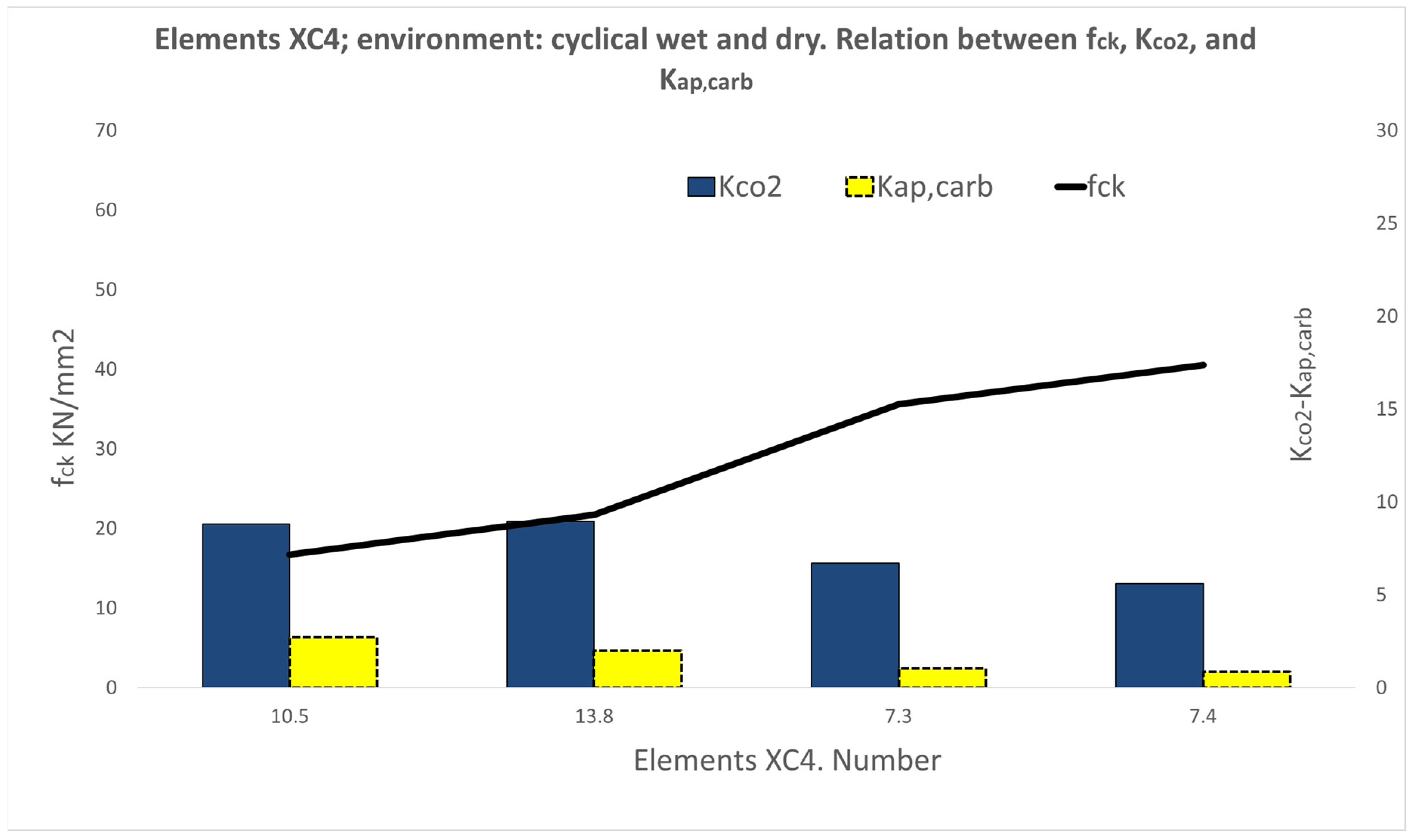
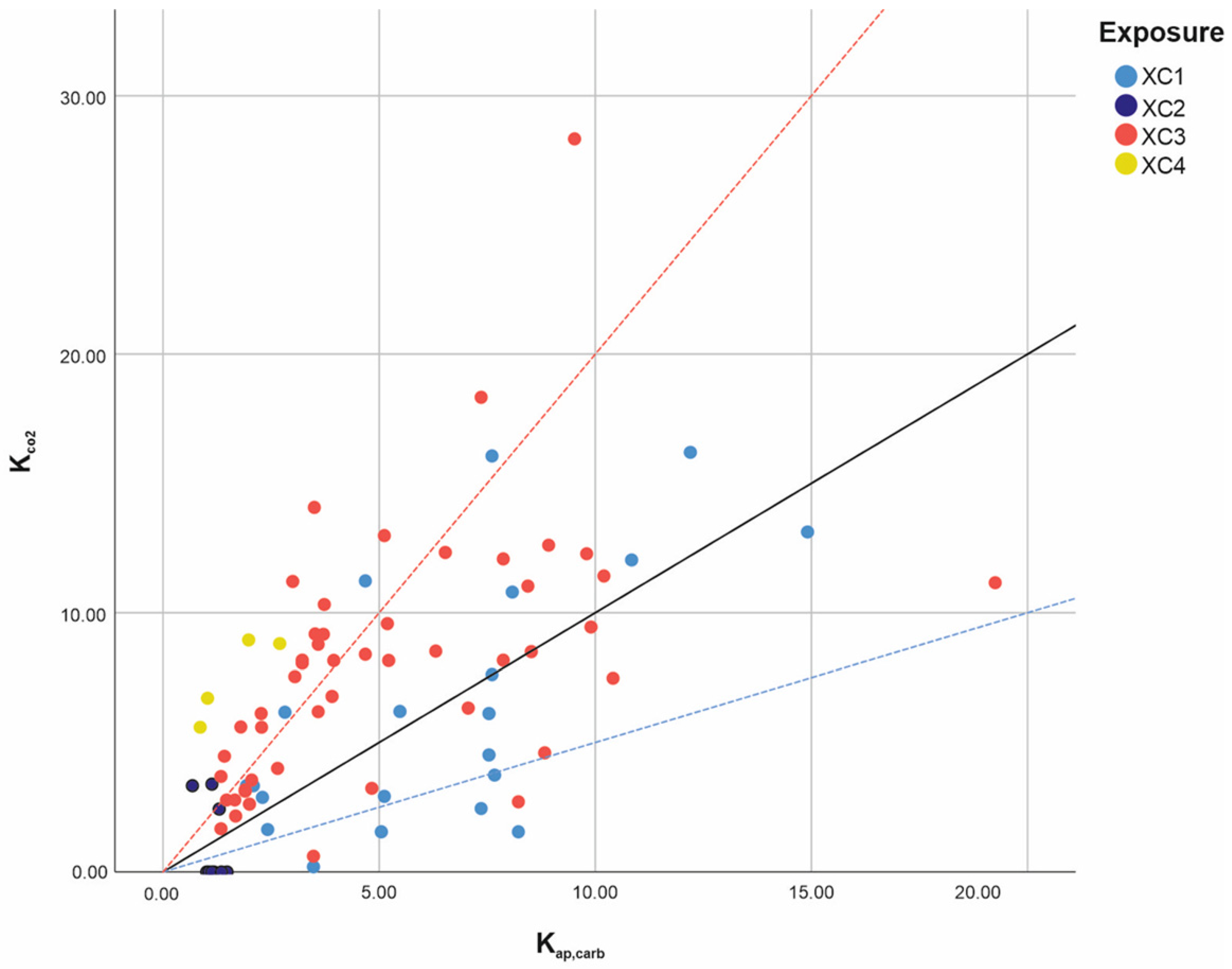
3.1. Results of the XC Variable
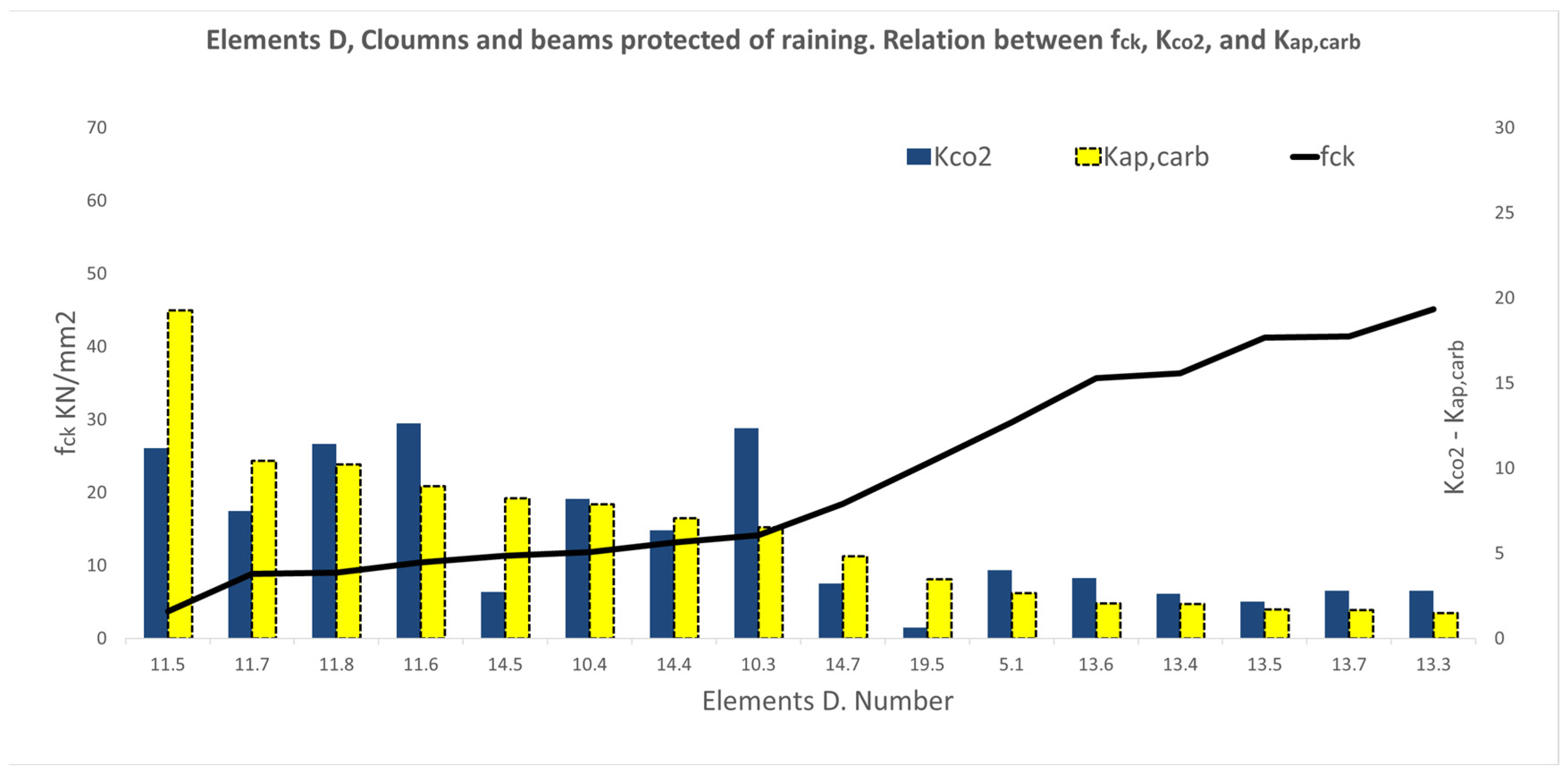
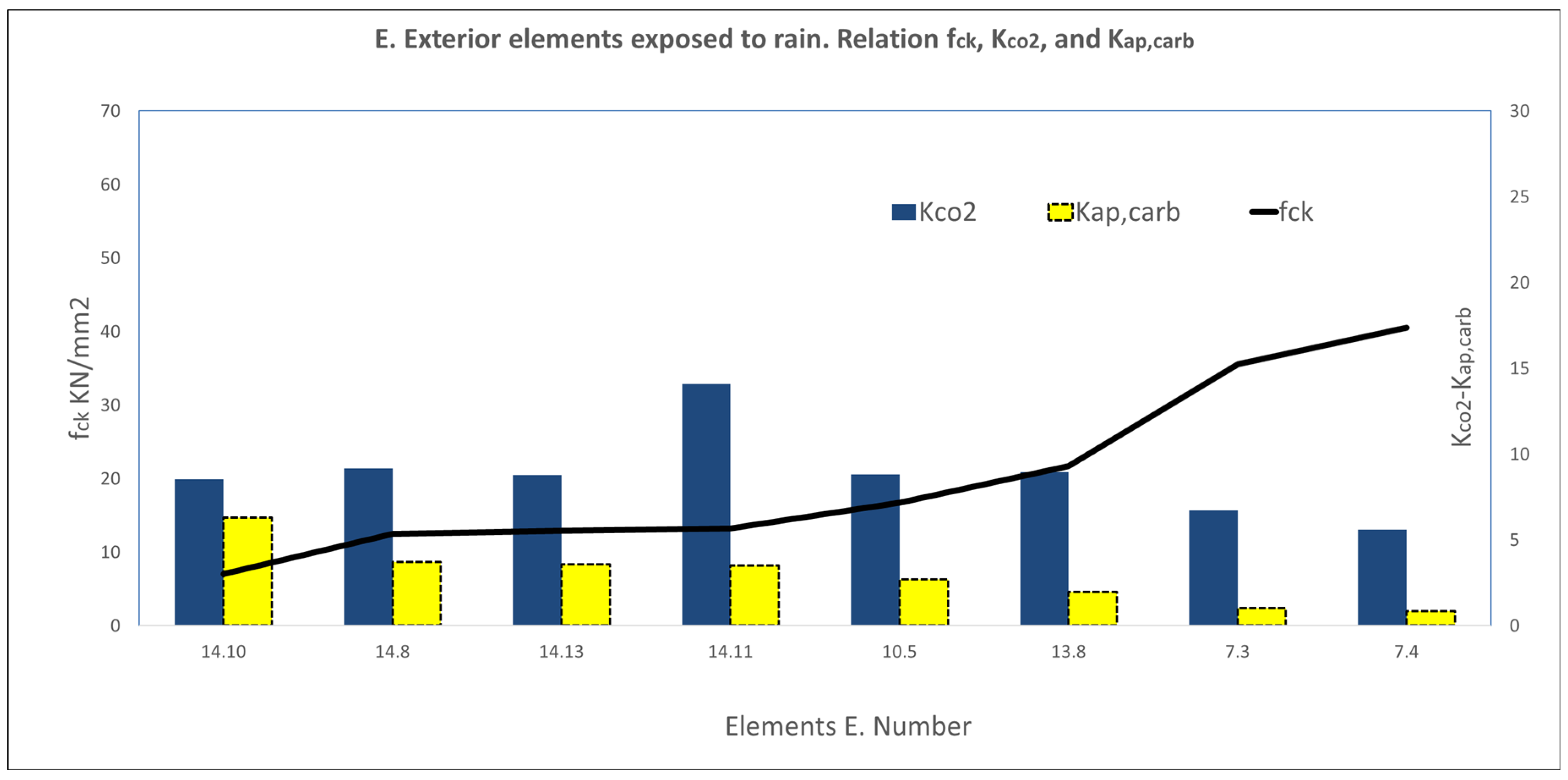
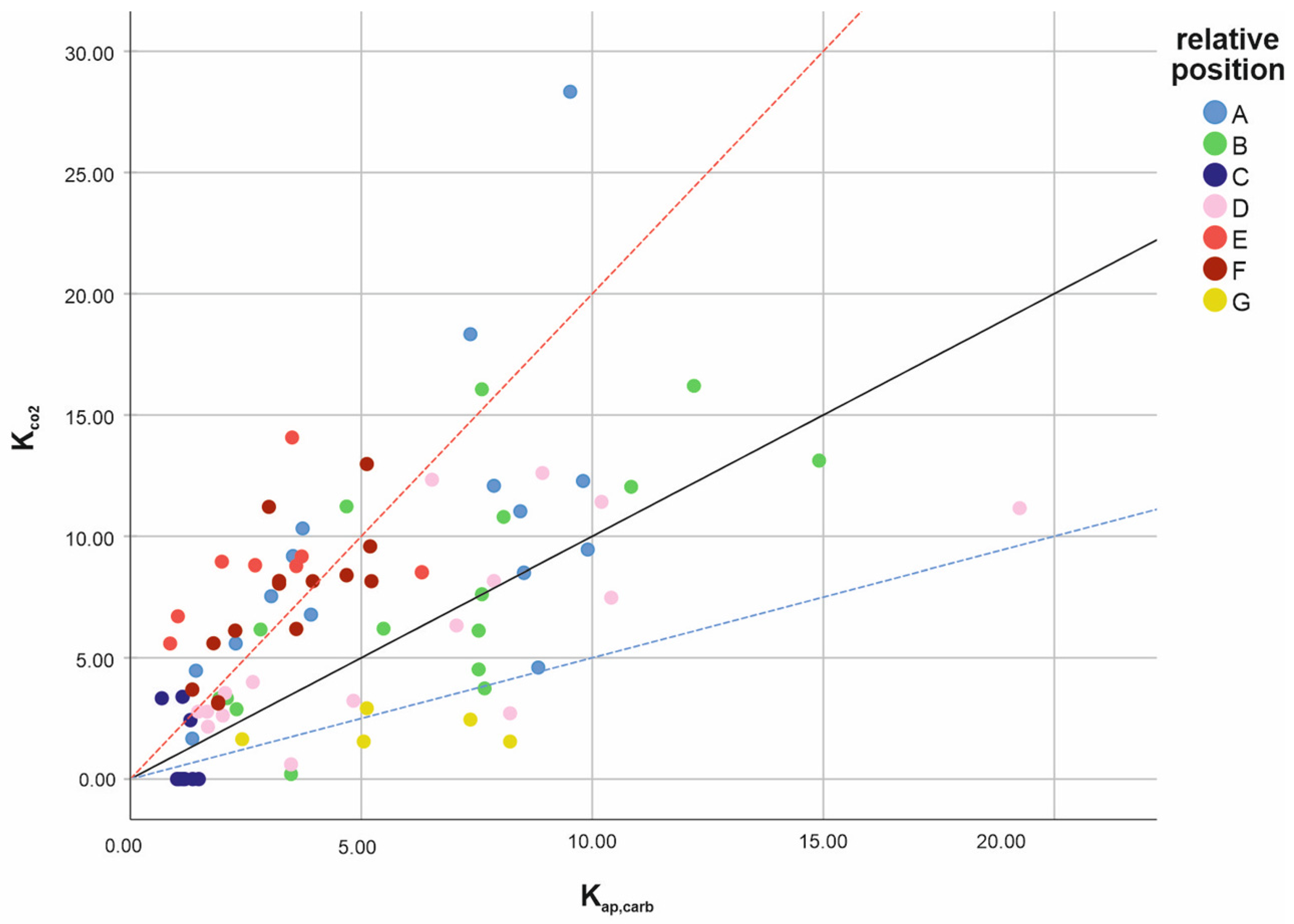
3.2. Results of the Variable Series A, B, C, D, E, F, and G
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourbaix, M. Thermodynamics and Corrosion. Corros. Sci. 1990, 30, 963–988. [Google Scholar] [CrossRef]
- Al-Kadhimi, T.K.H.; Banfill, P.F.G.; Millard, S.G.; Bungey, J.H. An Accelerated Carbonation Procedure for Studies on Concrete. Adv. Cem. Res. 1996, 8, 47–59. [Google Scholar] [CrossRef]
- Chávez-Ulloa, E.; Pérez López, T.; Reyes Trujeque, J.; Corvo Pérez, F.; Osorno Carrillo, J.B. Carbonatación de concreto en atmósfera natural y cámara de carbonatación acelerada. Rev. CENIC Cienc. Químicas 2010, 41. ISSN: 1015-8553. Available online: https://www.redalyc.org/articulo.oa?id=181620500029 (accessed on 2 April 2023).
- Ho, D.W.S.; Lewis, R.K. Carbonation of Concrete and Its Prediction. Cem. Concr. Res. 1987, 17, 489–504. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C. Life Time of Rebars in Carbonated Concrete. In Proceedings of the 10th European Corrosion Congress—Progress in the Understanding and Prevention of Corrosion, Barcelona, Spain, 31 May–4 June 1993; Volume 1, pp. 634–641. [Google Scholar]
- Chen, C.; Liu, R.; Zhu, P.; Liu, H.; Wang, X. Carbonization Durability of Two Generations of Recycled Coarse Aggregate Concrete with Effect of Chloride Ion Corrosion. Sustainability 2020, 12, 10544. [Google Scholar] [CrossRef]
- Geng, J.; Liu, J.; Yan, J.; Ba, M.; He, Z.; Li, Y. Chemical Composition of Corrosion Products of Rebar Caused by Carbonation and Chloride. Int. J. Corros. 2018, 2018, 7479383. [Google Scholar] [CrossRef]
- Sanchez, J.; Fullea, J.; Andrade, C. Corrosion-Induced Brittle Failure in Reinforcing Steel. Theor. Appl. Fract. Mech. 2017, 92, 229–232. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosión of Steel in Concrete. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, October 1982. [Google Scholar]
- Kim, C.; Choe, D.-E.; Castro-Borges, P.; Castaneda, H. Probabilistic Corrosion Initiation Model for Coastal Concrete Structures. Corros. Mater. Degrad. 2020, 1, 328–344. [Google Scholar] [CrossRef]
- Otieno, M.B.; Beushausen, H.D.; Alexander, M.G. Modelling Corrosion Propagation in Reinforced Concrete Structures—A Critical Review. Cem. Concr. Compos. 2011, 33, 240–245. [Google Scholar] [CrossRef]
- Andrade, C.; Menéndez, E.; Lima, L.; Luchtenberg, C. Vida Útil de la Estructuras de Hormigón. Proyecto y Modelización; Fundación Rogelio Segovia para el Desarrollo de las Telecomunicaciones: Madrid, Spain, 2013. [Google Scholar]
- Andrade, C.; Menéndez, E.; Lima, L.; Villagrán, Y.; Luchtenberg, C. Diseño Prestacional para la Durabilidad de Estructuras de Hormigón Armado. Vida Útil de Estructuras Existentes. Monitoreo, Intervención y Rehabilitación; Fundación Rogelio Segovia para el Desarrollo de las Telecomunicaciones: Madrid, Spain, 2013. [Google Scholar]
- Torres Martín, J.E.; Rebolledo Ramos, N.; Chinchón-Payá, S.; Helices Arcila, I.; Silva Toledo, A.; Sánchez Montero, J.; Otero García, F.; Llorente Sanjuán, M.; Agulló Soto, S.; de Haan, L. Durability of a Reinforced Concrete Structure Exposed to Marine Environment at the Málaga Dock. Case Stud. Constr. Mater. 2022, 17, e01582. [Google Scholar] [CrossRef]
- Galan, I.; Sanchez, J.; Andrade, C.; Evans, A. Carbonation Profiles in Cement Paste Analyzed by Neutron Diffraction. J. Phys. Conf. Ser. 2012, 340, 12108. [Google Scholar] [CrossRef]
- Galán, I.; Perdrix, C.A.; Castellote, M.; Rebolledo, N.; Sánchez-Montero, J.; Toro, L.; Puente-Orench, I.; Campo, J.; Fabelo, O. Neutron Diffraction for Studying the Influence of the Relative Humidity on the Carbonation Process of Cement Pastes. J. Phys. Conf. Ser. 2011, 325, 012015. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Andrade, C.; Chinchón, S. Indicator of Carbonation Front in Concrete as Substitute to Phenolphthalein. Cem. Concr. Res. 2016, 82, 87–91. [Google Scholar] [CrossRef]
- Chinchón-Payá, S.; Andrade, C.; Chinchón, S. Use of Anthocyanin Solutions in Portland Cement Concrete to Identify Carbonation Depth. Mater. Struct./Mater. Constr. 2020, 53, 4–9. [Google Scholar] [CrossRef]
- Código Estructural, Real Decreto 470/2021. 2021. (In Spanish). Available online: https://www.boe.es/eli/es/rd/2021/06/29/470 (accessed on 2 April 2023).
- Yoon, I.-S.; Chang, C.-H. Time Evolution of CO2 Diffusivity of Carbonated Concrete. Appl. Sci. 2020, 10, 8910. [Google Scholar] [CrossRef]
- Garces, P.E. Procesos de Degradación Físico-Químicos en Estructuras de Hormigón Armado; Publicacions Universitat Alacant: Alicante, Spain, 2021; ISBN 9788497177450. [Google Scholar]
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion Rate of Carbon Steel in Carbonated Concrete—A Critical Review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Parrott, L.J. Carbonation, Moisture and Empty Pores. Adv. Cem. Res. 1992, 4, 111–118. [Google Scholar] [CrossRef]
- Page, C.L.; Treadaway, K.W.J. Aspects of the Electrochemistry of Steel in Concrete. Nature 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Balayssac, J.P.; Détriché, C.H.; Grandet, J. Effects of Curing upon Carbonation of Concrete. Constr. Build. Mater. 1995, 9, 91–95. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the Carbonation of Concrete with Supplementary Cementitious Materials: A Critical Review by RILEM TC 281-CCC. Mater. Struct./Mater. Constr. 2020, 53, 136. [Google Scholar] [CrossRef]
- Melchers, R.E.; Richardson, P.J. Carbonation, Neutralization and reinforcement corrosion for concrete in long-term atmospheric exposures. Corrosion 2023, 79, 395–404. [Google Scholar] [CrossRef]
- Ministerio de Obras Públicas. Orden de 3 de Febrero de 1939 Instrucción para el Proyecto y Ejecución de Obras de Hormigón; Ministerio de Obras Públicas: Madrid, Spain, 1939. [Google Scholar]
- Ministerio de Obras Públicas. Orden de 23 de Marzo de 1944 Instrucción Definitiva para el Proyecto y Ejecución de Obras de Hormigón; Ministerio de Obras Públicas: Madrid, Spain, 1944. [Google Scholar]
- Presidencia del Gobierno. Decreto 2987/1968 de 20 de Febrero Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa y Armado; Presidencia del Gobierno: Madrid, Spain, 1968. [Google Scholar]
- Presidencia del Gobierno. Decreto 3062/1973 de 20 de Febrero Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado. Instrucción EH-73; Presidencia del Gobierno: Madrid, Spain, 1973. [Google Scholar]
- Ministerio de Obras Públicas y Urbanismo. Real Decreto 2868/1980 de 17 de Octubre Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado (EH-80); Ministerio de Obras Públicas y Urbanismo: Madrid, Spain, 1980. [Google Scholar]
- Ministerio de Obras Públicas y Urbanismo. Real Decreto 2262/1982 de 24 de julio Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado (EH-82); Ministerio de Obras Públicas y Urbanismo: Madrid, Spain, 1982. [Google Scholar]
- Ministerio de Obras Públicas y Urbanismo. Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado (EH-88); Ministerio de Obras Públicas y Urbanismo: Madrid, Spain, 1988. [Google Scholar]
- EHE-91 REAL DECRETO 1039/1991, de 28 de Junio, Por El Que Se Aprueba La «Instrucción Para El Proyecto y La Ejecución de Obras de Hormigón En Masa o Armado (EH-91)». 1991. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-1991-17068 (accessed on 2 June 2023).
- Ministerio de Fomento. Real Decreto 2661/1998 de 11 de Diciembre Instrucción de Hormigón Estructural (ECH-99); Ministerio de Fomento: Madrid, Spain, 1998. [Google Scholar]
- Ministerio de la Presidencia. Real Decreto 1247/2008 de 18 de Julio. Instrucción de Hormigón Estructural (EHE-08); Ministerio de la Presidencia: Madrid, Spain, 2008. [Google Scholar]
- EN 1992-1-1; European Standard Eurocode 2: Design of Concrete Structures-Part 1-1: General Rules and Rules for Buildings. European Committee for Standardization: Brussels, Belgium, 2004.
- Liu, X.; Niu, D.; Li, X.; Lv, Y.; Fu, Q. Pore Solution PH for the Corrosion Initiation of Rebars Embedded in Concrete under a Long-Term Natural Carbonation Reaction. Appl. Sci. 2018, 8, 128. [Google Scholar] [CrossRef]
- Sohail, M.G.; Laurens, S.; Deby, F.; Balayssac, J.P. Significance of Macrocell Corrosion of Reinforcing Steel in Partially Carbonated Concrete: Numerical and Experimental Investigation. Mater. Struct. 2013, 48, 217–233. [Google Scholar] [CrossRef]
- Sohail, M.G.; Laurens, S.; Deby, F.; Balayssac, J.P.; Al Nuaimi, N. Electrochemical Corrosion Parameters for Active and Passive Reinforcing Steel in Carbonated and Sound Concrete. Mater. Corros. 2021, 72, 1854–1871. [Google Scholar] [CrossRef]
- Bossio, A.; Faella, G.; Frunzio, G.; Guadagnuolo, M.; Serpieri, R. Diagnostic Reliability in the Assessment of Degradation in Precast Concrete Elements. Infrastructures 2021, 6, 164. [Google Scholar] [CrossRef]
- Wang, C. Explicitly Assessing the Durability of RC Structures Considering Spatial Variability and Correlation. Infrastructures 2021, 6, 156. [Google Scholar] [CrossRef]







| Statistical Indicators. KCO2/Kap,carb | ||||
|---|---|---|---|---|
| Variable | N | Median = M | Standard Deviation = σ | |
| XC1 | 22 | 1.06 | 1.10 | 0.66 |
| XC2 | 9 | 0.00 | 1.09 | 1.80 |
| XC3 | 49 | 1.72 | 1.82 | 0.87 |
| XC4 | 4 | 5.51 | 5.20 | 1.60 |
| Statistical Indicators. KCO2/Kap,carb | ||||
|---|---|---|---|---|
| Variable | N | Median = M | Standard Deviation = σ | |
| A | 15 | 1.73 | 1.90 | 0.84 |
| B | 17 | 1.25 | 1.30 | 0.61 |
| C | 9 | 0.00 | 1.09 | 1.80 |
| D | 16 | 1.20 | 1.14 | 0.54 |
| E | 8 | 3.64 | 3.89 | 1.89 |
| F | 14 | 2.28 | 2.30 | 0.65 |
| G | 5 | 0.33 | 0.42 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saura Gómez, P.; Sánchez Montero, J.; Torres Martín, J.E.; Chinchón-Payá, S.; Rebolledo Ramos, N.; Galao Malo, Ó. Carbonation-Induced Corrosion of Reinforced Concrete Elements according to Their Positions in the Buildings. Corros. Mater. Degrad. 2023, 4, 345-363. https://doi.org/10.3390/cmd4030018
Saura Gómez P, Sánchez Montero J, Torres Martín JE, Chinchón-Payá S, Rebolledo Ramos N, Galao Malo Ó. Carbonation-Induced Corrosion of Reinforced Concrete Elements according to Their Positions in the Buildings. Corrosion and Materials Degradation. 2023; 4(3):345-363. https://doi.org/10.3390/cmd4030018
Chicago/Turabian StyleSaura Gómez, Pascual, Javier Sánchez Montero, Julio Emilio Torres Martín, Servando Chinchón-Payá, Nuria Rebolledo Ramos, and Óscar Galao Malo. 2023. "Carbonation-Induced Corrosion of Reinforced Concrete Elements according to Their Positions in the Buildings" Corrosion and Materials Degradation 4, no. 3: 345-363. https://doi.org/10.3390/cmd4030018
APA StyleSaura Gómez, P., Sánchez Montero, J., Torres Martín, J. E., Chinchón-Payá, S., Rebolledo Ramos, N., & Galao Malo, Ó. (2023). Carbonation-Induced Corrosion of Reinforced Concrete Elements according to Their Positions in the Buildings. Corrosion and Materials Degradation, 4(3), 345-363. https://doi.org/10.3390/cmd4030018









