Corrosion Modeling of Magnesium and Its Alloys for Biomedical Applications: Review
Abstract
1. Introduction
2. Corrosion of Magnesium and Its Alloys
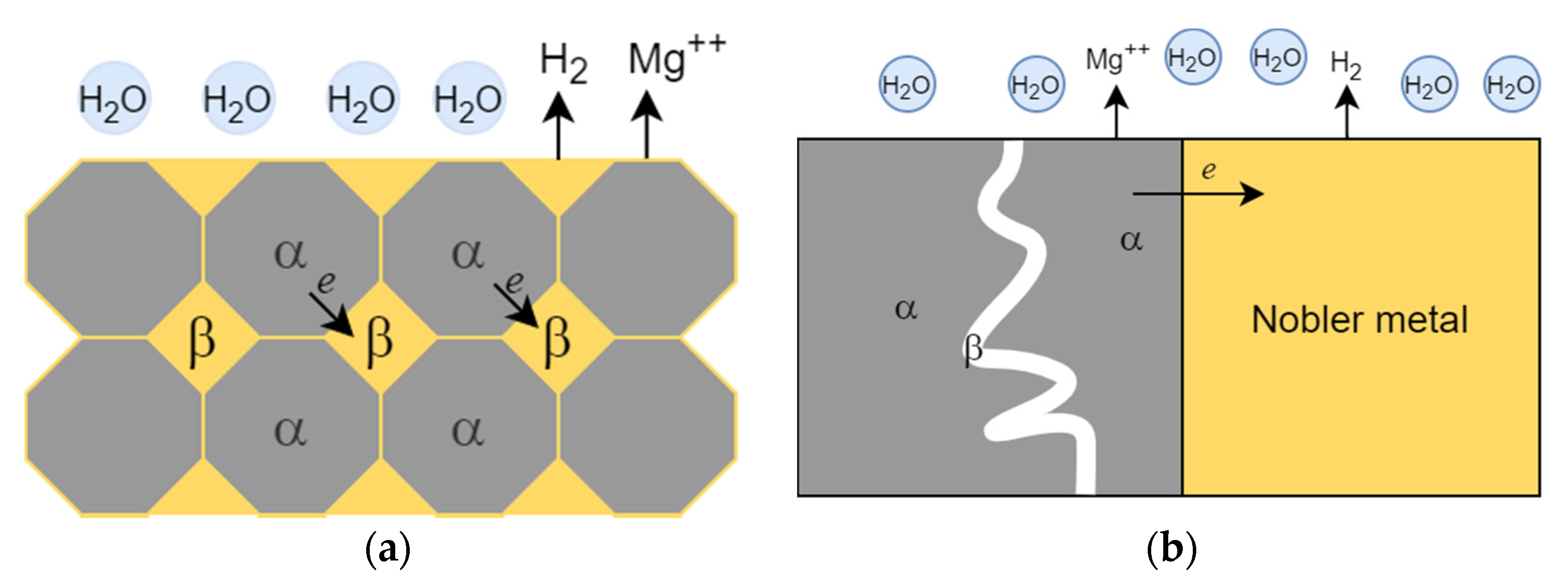
2.1. Galvanic Corrosion
2.2. Pitting Corrosion
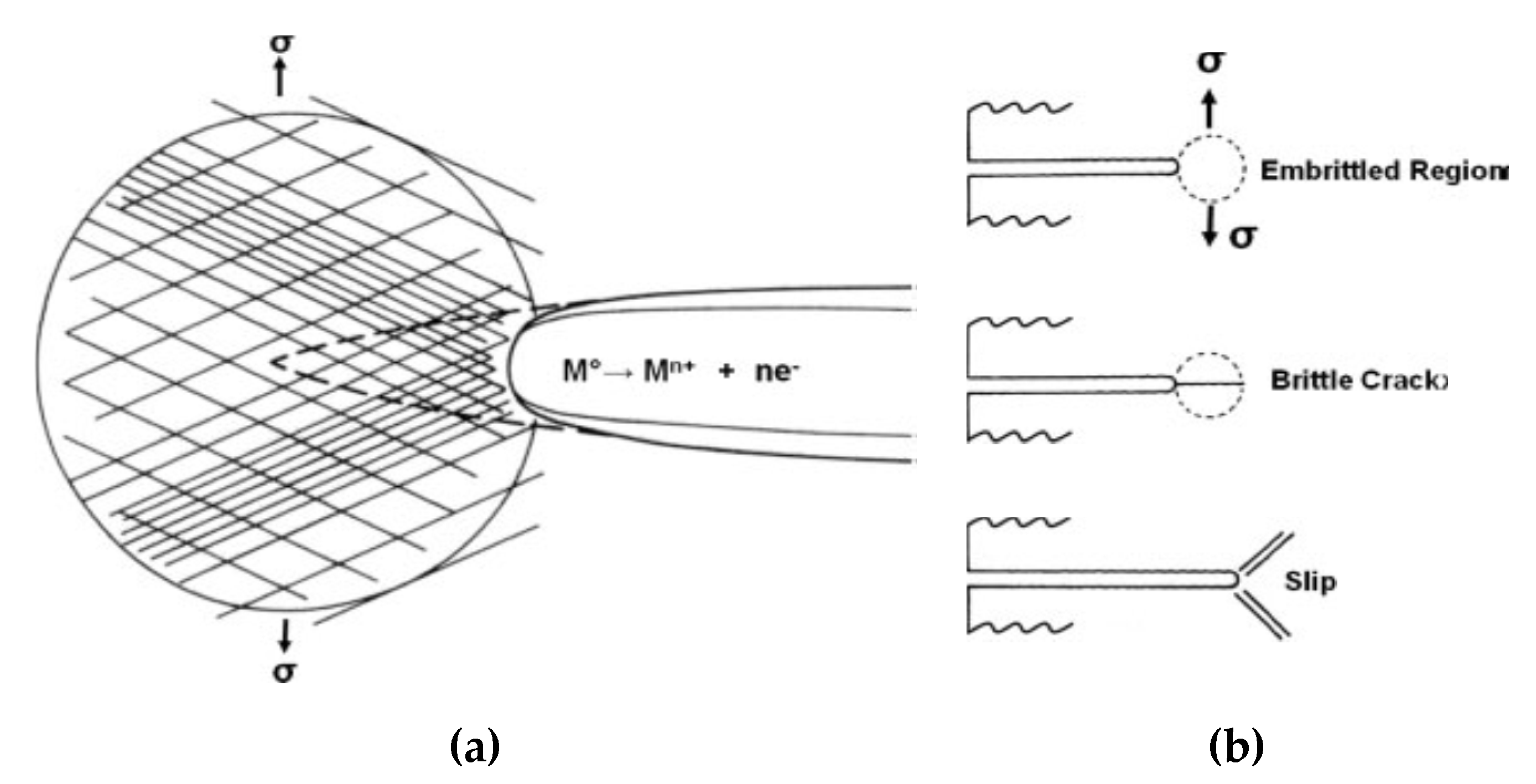
2.3. Stress Corrosion Cracking
- (i)
- Preferential attack (i.e., near the surface), in which the matrix is preferentially attacked and the adjacent grain boundaries to cathodic phases corrode creating small cracks near the surface. Applied stress opens the cracks and allows species in the solution to flow towards the crack tip which accelerates the crack growth due to the galvanic corrosion [25].
- (ii)
- Galvanic corrosion due to passive film rupture, in which strains cause rupture of the protective oxide film and expose parts of the anode matrix. This creates a galvanic cell with covered cathodic regions which in return dissolves the matrix grains and initiates a crack through the grains.
- (iii)
- IGSCC is initiated due to tunneling, which is a tubular pitting corrosion. These tunnels can be near each other leading to a ductile tear of the metal in between due to stress, which initiates cracks on the surface. Opened cracks will continue growing under cyclic loading and are also subjected to the formation of new pits.
- (i)
- The first is cleavage fracture due to stages of electrochemical and mechanical effects. Electrochemical effects cause the initiation of pits that is followed by a mechanical effect in high-stress concentration that starts a cleavage crack. The crack propagates through the grain until it reaches an obstruction such as the grain boundary. Pitting corrosion then continues to initiate another crack in a different direction.
- (ii)
- The second main mode is hydrogen embrittlement. The evolution of hydrogen due to the electrochemical reaction (12) during the galvanic corrosion leads to embrittlement of a crack tip and propagation of cracks. Another hypothesis is that hydrogen reacts with magnesium resulting in a brittle magnesium hydride.
3. Modeling Methods
3.1. Phenomenological Modeling
3.1.1. Uniform Corrosion Modeling
3.1.2. Modeling of Pitting Corrosion
3.1.3. Modeling of Stress Corrosion Cracking
3.2. Physical Modeling
3.2.1. Activation Controlled Modeling
3.2.2. Transport-Controlled Modeling
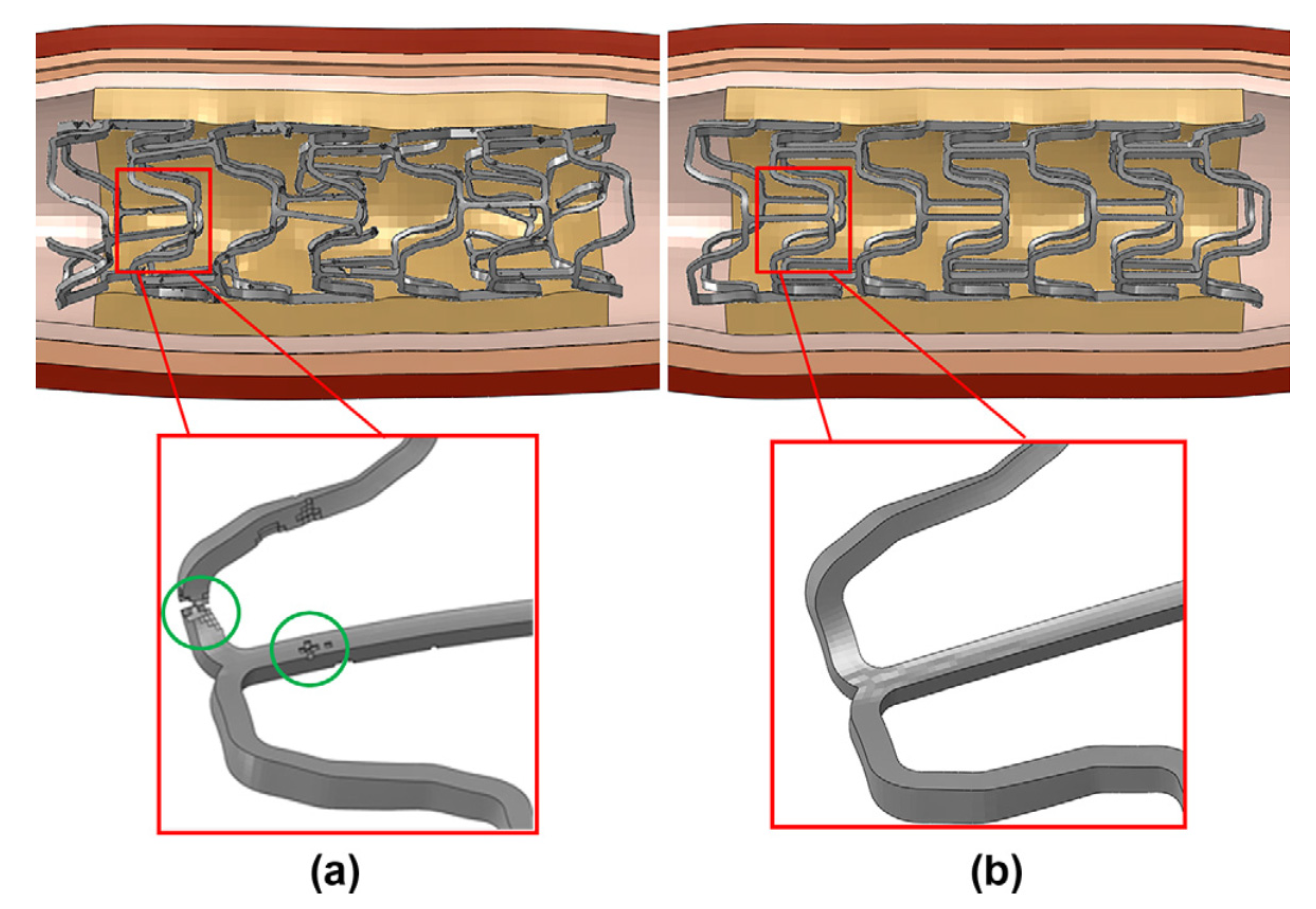
3.2.3. Modeling of Coating Effect
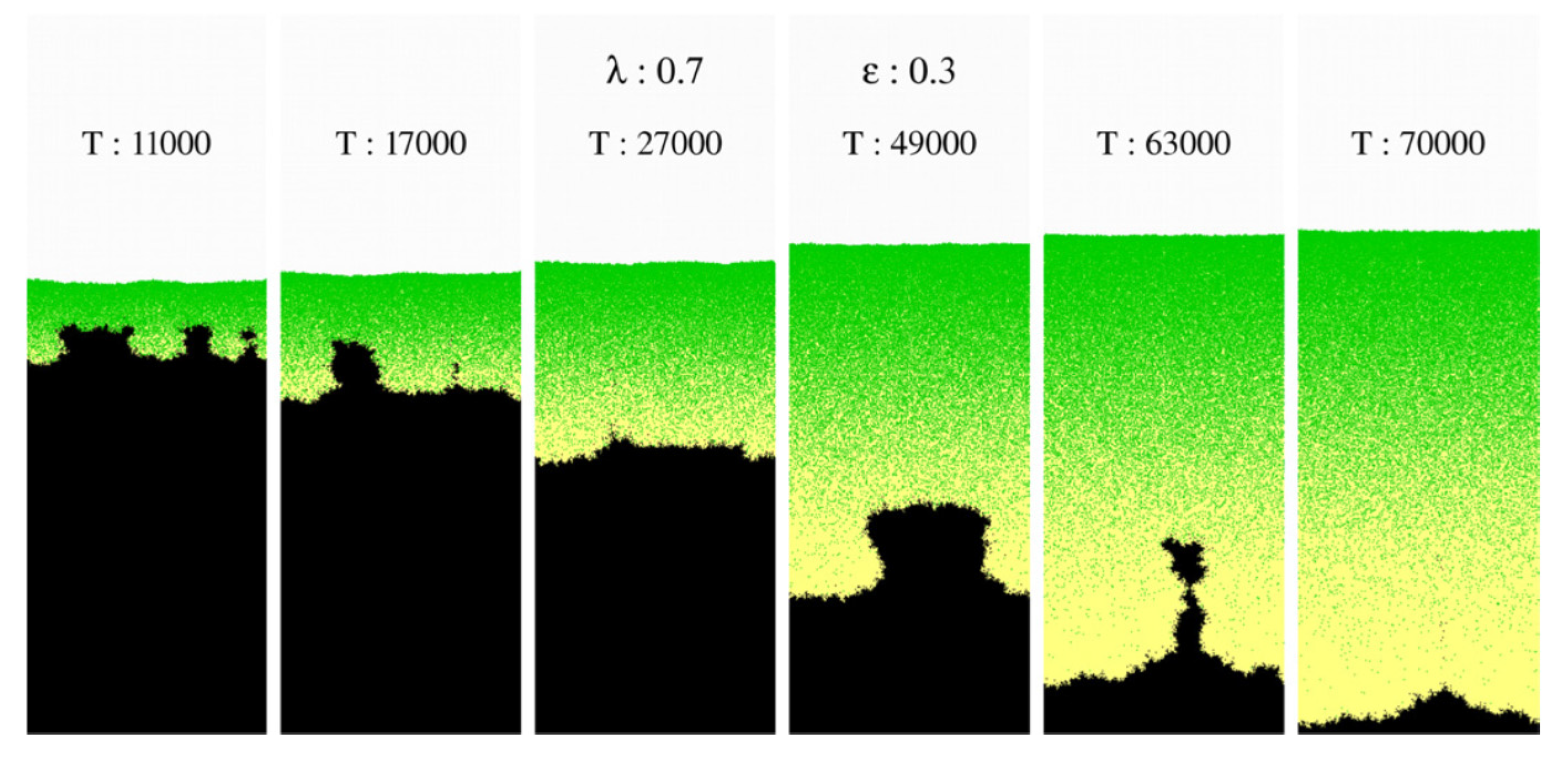
3.3. Cellular Automata Corrosion Modeling Approach
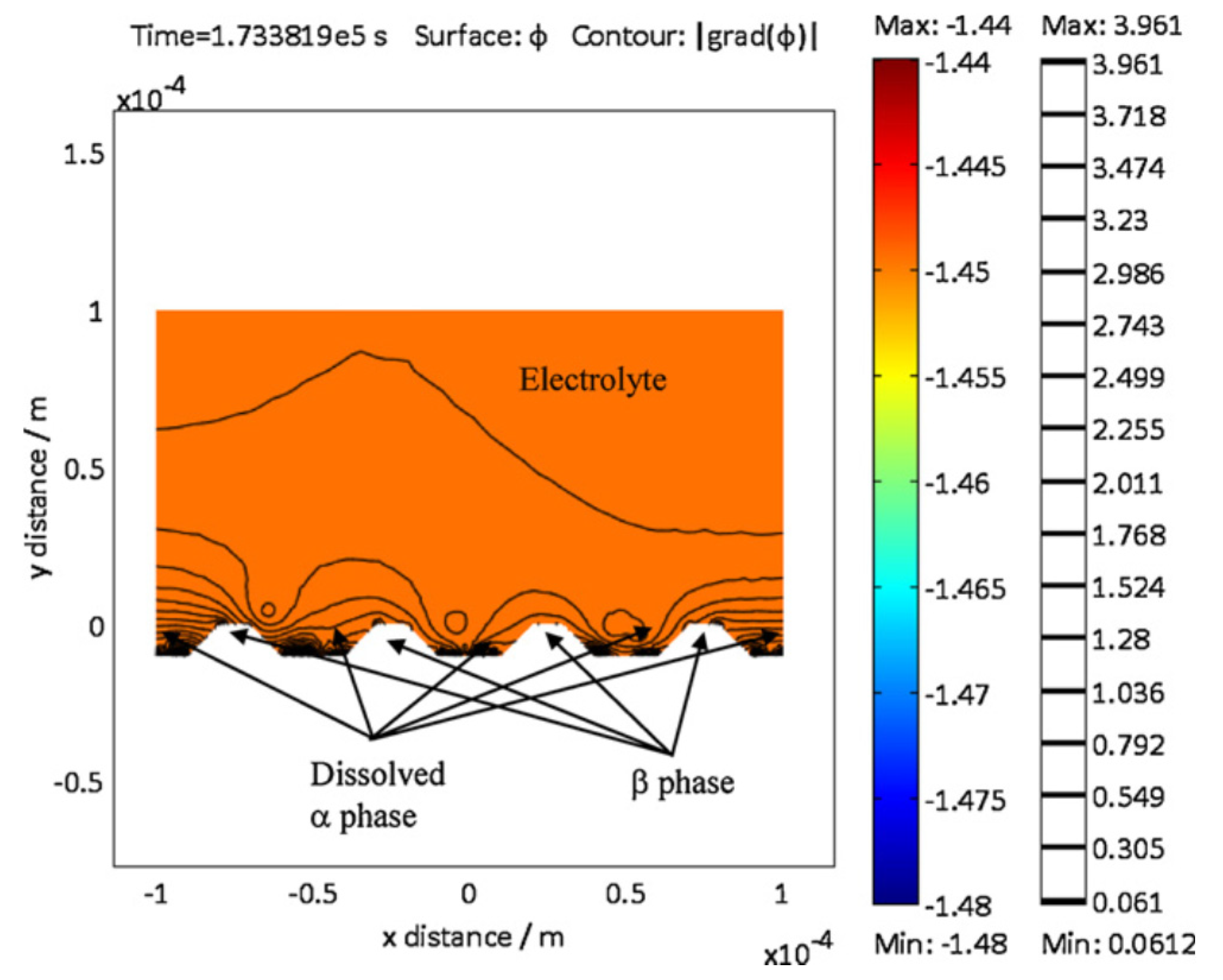
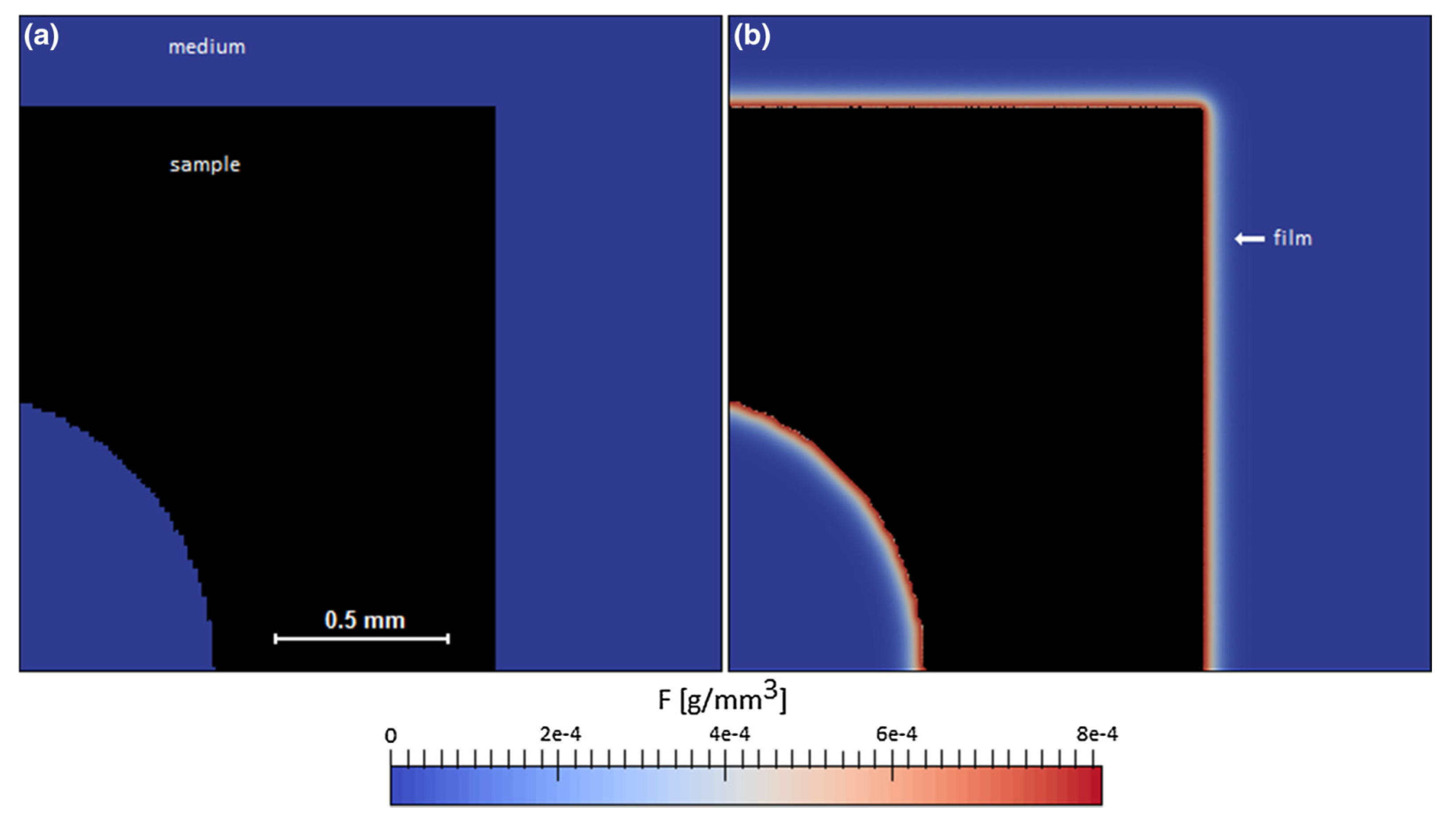
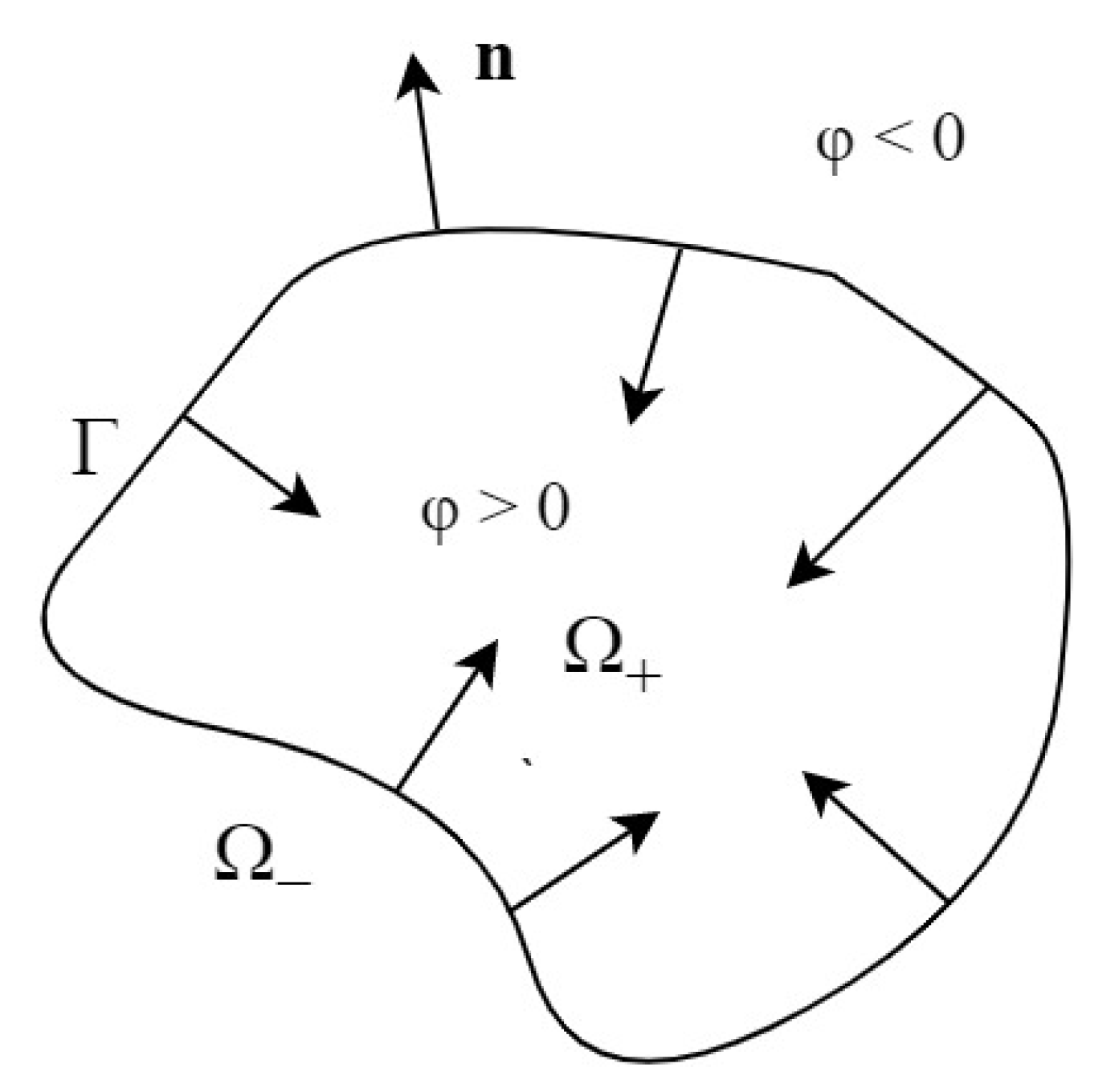
4. Corrosion Surface Tracking: Level Set Method
5. Calibration Test Methods
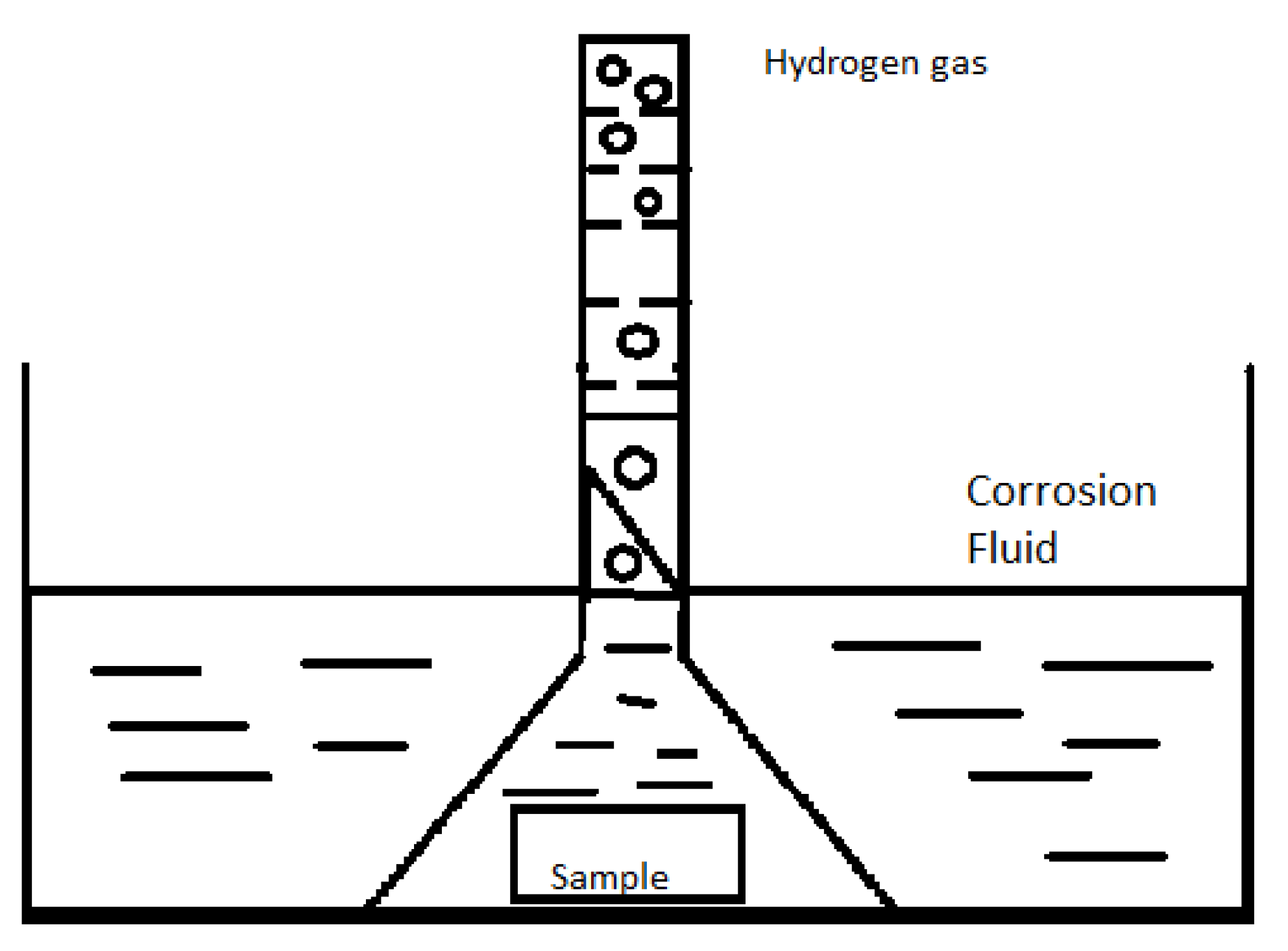
5.1. In Vitro Testing
5.1.1. Unpolarized tests
Mass Loss
Hydrogen Evolution Measurement
pH Monitoring
5.1.2. Polarized (Electrochemical) Method
Potentiodynamic Polarization
Electrochemical Impedance Spectroscopy (EIS)
5.2. In Vivo Testing
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ibrahim, H.; Mehanny, S.; Darwish, L.; Farag, M. A comparative study on the mechanical and biodegradation characteristics of starch-based composites reinforced with different lignocellulosic fibers. J. Polym. Environ. 2018, 26, 2434–2447. [Google Scholar] [CrossRef]
- Ibrahim, H.; Farag, M.; Megahed, H.; Mehanny, S. Characteristics of starch-based biodegradable composites reinforced with date palm and flax fibers. Carbohydr. Polym. 2014, 101, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Guo, H.; Xia, D.; Zheng, Y.; Jauer, L.; Poprawe, R.; Voshage, M.; Schleifenbaum, J.H. Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta Biomater. 2019, 98, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Esfahani, S.N.; Poorganji, B.; Dean, D.; Elahinia, M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater. Sci. Eng. C 2017, 70, 870–888. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- The American Academy of Orthopaedic Surgeons. Internal Fixation for Fractures. Available online: http://orthoinfo.aaos.org/topic.cfm?topic=A00196 (accessed on 19 January 2020).
- Transparency Market Research. Orthopedic Trauma Fixation Devices Market. Analysis, Market. Size, Application Analysis, Regional Outlook, Competitive Strategies and Forecasts, 2014 to 2020; Transparency Market Research: Albany, NY, USA, July 2014; p. 94. [Google Scholar]
- Huang, Z.-M.; Fujihara, K. Stiffness and strength design of composite bone plates. Compos. Sci. Technol. 2005, 65, 73–85. [Google Scholar] [CrossRef]
- Rahmanian, R.; Shayesteh Moghaddam, N.; Haberland, C.; Dean, D.; Miller, M.; Elahinia, M. Load bearing and stiffness tailored NiTi implants produced by additive manufacturing: A simulation study. In Proceedings of the International Society for Optical Engineering, San Diego, CA, USA, 11–15 August 2019. [Google Scholar]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C 2017, 71, 1253–1266. [Google Scholar] [CrossRef]
- Yelin, E.H. The Burden of Musculoskeletal Diseases in the United States. Available online: https://www.boneandjointburden.org/fourth-edition/vh0/economic-burden-injuries (accessed on 26 July 2020).
- Grand View Research, Inc. Trauma Devices Market Size, Share & Trends Analysis. Available online: https://www.grandviewresearch.com/industry-analysis/trauma-devices-market (accessed on 23 June 2020).
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Durlach, J. Recommended dietary amounts of magnesium: Mg RDA. Magnes. Res. 1989, 2, 195–203. [Google Scholar] [PubMed]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Acad. Nutr. Diet. 2001, 101, 294. [Google Scholar]
- Zeng, R.-C.; Zhang, J.; Huang, W.-J.; Dietzel, W.; Kainer, K.; Blawert, C.; Wei, K. Review of studies on corrosion of magnesium alloys. Trans. Nonferrous Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Ibrahim, H.; Klarner, A.D.; Poorganji, B.; Dean, D.; Luo, A.A.; Elahinia, M. Microstructural, mechanical and corrosion characteristics of heat-treated Mg-1.2 Zn-0.5 Ca (wt%) alloy for use as resorbable bone fixation material. J. Mech. Behav. Biomed. Mater. 2017, 69, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Luo, A.; Dean, D.; Elahinia, M. Effect of Zn content and aging temperature on the in-vitro properties of heat-treated and Ca/P ceramic-coated Mg-0.5% Ca-x% Zn alloys. Mater. Sci. Eng. C 2019, 103, 109700. [Google Scholar] [CrossRef] [PubMed]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Elahinia, M. Biodegradable magnesium alloys. In Metals for Biomedical Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–289. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Liu, L.; Schlesinger, M. Corrosion of magnesium and its alloys. Corros. Sci. 2009, 51, 1733–1737. [Google Scholar] [CrossRef]
- Lynch, S.; Trevena, P. Stress corrosion cracking and liquid metal embrittlement in pure magnesium. Corrosion 1988, 44, 113–124. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A critical review of the stress corrosion cracking (SCC) of magnesium alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Duddu, R. Numerical modeling of corrosion pit propagation using the combined extended finite element and level set method. Comput. Mech. 2014, 54, 613–627. [Google Scholar] [CrossRef]
- Lemaitre, J. A Course on Damage Mechanics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Amerinatanzi, A.; Mehrabi, R.; Ibrahim, H.; Dehghan, A.; Shayesteh Moghaddam, N.; Elahinia, M. Predicting the biodegradation of magnesium alloy implants: Modeling, parameter identification, and validation. Bioengineering 2018, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, D.; Sassi, V.; Petrini, L.; Vedani, M.; Trasatti, S.; Migliavacca, F. Continuum damage model for bioresorbable magnesium alloy devices—Application to coronary stents. J. Mech. Behav. Biomed. Mater. 2011, 4, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.; O’Brien, B.; Leen, S.; McHugh, P. A corrosion model for bioabsorbable metallic stents. Acta Biomater. 2011, 7, 3523–3533. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, J.; Hermawan, H.; Dubé, D.; Mantovani, D. Design of a pseudo-physiological test bench specific to the development of biodegradable metallic biomaterials. Acta Biomater. 2008, 4, 284–295. [Google Scholar] [CrossRef]
- Wu, W.; Gastaldi, D.; Yang, K.; Tan, L.; Petrini, L.; Migliavacca, F. Finite element analyses for design evaluation of biodegradable magnesium alloy stents in arterial vessels. Mater. Sci. Eng. B 2011, 176, 1733–1740. [Google Scholar] [CrossRef]
- Wenman, M.; Trethewey, K.; Jarman, S.; Chard-Tuckey, P. A finite-element computational model of chloride-induced transgranular stress-corrosion cracking of austenitic stainless steel. Acta Mater. 2008, 56, 4125–4136. [Google Scholar] [CrossRef]
- Debusschere, N.; Segers, P.; Dubruel, P.; Verhegghe, B.; De Beule, M. A computational framework to model degradation of biocorrodible metal stents using an implicit finite element solver. Ann. Biomed. Eng. 2016, 44, 382–390. [Google Scholar] [CrossRef]
- da Costa-Mattos, H.; Bastos, I.; Gomes, J. A simple model for slow strain rate and constant load corrosion tests of austenitic stainless steel in acid aqueous solution containing sodium chloride. Corros. Sci. 2008, 50, 2858–2866. [Google Scholar] [CrossRef]
- Bajger, P.; Ashbourn, J.; Manhas, V.; Guyot, Y.; Lietaert, K.; Geris, L. Mathematical modelling of the degradation behaviour of biodegradable metals. Biomech. Model. Mechanobiol. 2017, 16, 227–238. [Google Scholar] [CrossRef]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. A physical corrosion model for bioabsorbable metal stents. Acta Biomater. 2014, 10, 2313–2322. [Google Scholar] [CrossRef]
- Deshpande, K.B. Numerical modeling of micro-galvanic corrosion. Electrochim. Acta 2011, 56, 1737–1745. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]
- Wilder, J.W.; Clemons, C.; Golovaty, D.; Kreider, K.L.; Young, G.W.; Lillard, R.S. An adaptive level set approach for modeling damage due to galvanic corrosion. J. Eng. Math. 2015, 91, 121–142. [Google Scholar] [CrossRef]
- Montoya, R.; Iglesias, C.; Escudero, M.; García-Alonso, M. Modeling in vivo corrosion of AZ31 as temporary biodegradable implants. Experimental validation in rats. Mater. Sci. Eng. C 2014, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Hellmich, C. Stable pitting corrosion of stainless steel as diffusion-controlled dissolution process with a sharp moving electrode boundary. Corros. Sci. 2007, 49, 319–346. [Google Scholar] [CrossRef]
- Gartzke, A.-K.; Julmi, S.; Klose, C.; Waselau, A.-C.; Meyer-Lindenberg, A.; Maier, H.J.; Besdo, S.; Wriggers, P. A simulation model for the degradation of magnesium-based bone implants. J. Mech. Behav. Biomed. Mater. 2019, 101, 103411. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Hunt, A.G.; Ewing, R.P.; Sahimi, M. Tortuosity in porous media: A critical review. Soil Sci. Soc. Am. J. 2013, 77, 1461–1477. [Google Scholar] [CrossRef]
- Dietzel, W.; Pfuff, M.; Winzer, N. Testing and mesoscale modelling of hydrogen assisted cracking of magnesium. Eng. Fract. Mech. 2010, 77, 257–263. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Ibrahim, H.; Moghaddam, N.; Elahinia, M. Mechanical and in vitro corrosion properties of a heat-treated Mg-Zn-Ca-Mn alloy as a potential bioresorbable material. Adv. Metall. Mater. Eng. 2017, 1. [Google Scholar] [CrossRef]
- Ibrahim, H.; Dehghanghadikolaei, A.; Advincula, R.; Dean, D.; Luo, A.; Elahinia, M. Ceramic coating for delayed degradation of Mg-1.2 Zn-0.5 Ca-0.5 Mn bone fixation and instrumentation. Thin Solid Film. 2019, 687, 137456. [Google Scholar] [CrossRef]
- Li, L.-H.; Narayanan, T.S.; Kim, Y.K.; Kong, Y.-M.; Park, I.S.; Bae, T.S.; Lee, M.H. Deposition of microarc oxidation–polycaprolactone duplex coating to improve the corrosion resistance of magnesium for biodegradable implants. Thin Solid Film. 2014, 562, 561–567. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.-f.; Bandopadhyay, S.; Ning, C.; Zhang, Y.; Guo, Y. Corrosion mechanism and model of pulsed DC microarc oxidation treated AZ31 alloy in simulated body fluid. Appl. Surf. Sci. 2012, 258, 6116–6126. [Google Scholar] [CrossRef]
- Pidaparti, R.M.; Fang, L.; Palakal, M.J. Computational simulation of multi-pit corrosion process in materials. Comput. Mater. Sci. 2008, 41, 255–265. [Google Scholar] [CrossRef]
- Codd, E. Cellular Automata; Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Di Caprio, D.; Vautrin-Ul, C.; Stafiej, J.; Saunier, J.; Chaussé, A.; Féron, D.; Badiali, J. Morphology of corroded surfaces: Contribution of cellular automaton modelling. Corros. Sci. 2011, 53, 418–425. [Google Scholar] [CrossRef]
- Stafiej, J.; Di Caprio, D.; Bartosik, Ł. Corrosion-passivation processes in a cellular automata based simulation study. J. Supercomput. 2013, 65, 697–709. [Google Scholar] [CrossRef]
- Lishchuk, S.; Akid, R.; Worden, K.; Michalski, J. A cellular automaton model for predicting intergranular corrosion. Corros. Sci. 2011, 53, 2518–2526. [Google Scholar] [CrossRef]
- Di Caprio, D.; Stafiej, J.; Luciano, G.; Arurault, L. 3D cellular automata simulations of intra and intergranular corrosion. Corros. Sci. 2016, 112, 438–450. [Google Scholar] [CrossRef]
- Osher, S.; Sethian, J.A. Fronts propagating with curvature-dependent speed: Algorithms based on Hamilton-Jacobi formulations. J. Comput. Phys. 1988, 79, 12–49. [Google Scholar] [CrossRef]
- Sethian, J.A. Level Set Methods and Fast Marching Methods: Evolving Interfaces in Computational Geometry, Fluid Mechanics, Computer Vision, and Materials Science; Cambridge University Press: Cambridge, UK, 1999; Volume 3. [Google Scholar]
- Osher, S.; Fedkiw, R.; Piechor, K. Level set methods and dynamic implicit surfaces. Appl. Mech. Rev. 2004, 57, B15. [Google Scholar] [CrossRef]
- Guyot, Y.; Papantoniou, I.; Chai, Y.C.; Van Bael, S.; Schrooten, J.; Geris, L. A computational model for cell/ECM growth on 3D surfaces using the level set method: A bone tissue engineering case study. Biomech. Model. Mechanobiol. 2014, 13, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, C.; Gui, C.; Fox, M.D. Level set evolution without re-initialization: A new variational formulation. In Proceedings of the 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05), San Diego, CA, USA, 20–25 June 2005; pp. 430–436. [Google Scholar]
- Kirkland, N.T.; Birbilis, N. Magnesium Biomaterials: Design, Testing, and Best Practice; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Mohamed, A.; EI-Aziz, A.M.; Breitinger, H.-G. Study of the degradation behavior and the biocompatability of Mg-0.8Ca alloy for othropedic implant applications. J. Magnes. Alloy. 2019, 7, 249–257. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2009, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Myrissa, A.; Agha, N.A.; Lu, Y.; Martinelli, E.; Eichler, J.; Szakacs, G.; Klienhans, C.; Willumeit-Romer, R.; Schafer, U.; Weinberg, A.-M. In vitro and in vivo comparison of binary Mg alloys and pur Mg. Mater. Sci. Eng. 2016, 61, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocapatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Hou, L.; Zhen, L.; Pan, Y.; Du, L.; Li, X.; Zheng, Y.; Li, L. In vitro and in vivo studies on biodegradable magnesium alloy. Prog. Mater. Sci. Mater. Int. 2014, 24, 466–471. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- ASTM International. Standard practice for laboratory immersion corrosion testing of metals. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2004. [Google Scholar]
- ASTM International. Standard Practice for Preparing, Cleaning, and Evaluating. Corrosion Test Specimens. G1-03. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Gonzales, J.H.; Rui, Q.; Nidadavolu, E.P.S.; Willumeit-Romer, R.; Feyerabend, F. Magnesium degradation under physiological conditions-Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Xu, H.; Hu, T.; Wang, M.; Zheng, Y.; Qin, H.; Cao, H.; An, Z. Degradability and Biocompatibility of magnesium-MAO: The consistency and contradiction between in-vitro and in-vivo outcomes. Arab. J. Chem. 2018, 13, 2795–2805. [Google Scholar]
- Ren, Y.B.; Wang, H.; Huang, J.J.; Yang, K.; Zhang, B.C. Study of Biodegradation of Pure Magnesium. Key Eng. Mater. 2007, 342, 601–604. [Google Scholar] [CrossRef]
- Wang, J.; Cui, L.; Ren, Y.; Zou, Y.; Ma, J.; Wang, C.; Zheng, Z.; Chen, X.; Zeng, R.; Zheng, Y. In Vitro and in vivo biodegradation and biocapatibility of an MMT/BSA composite coating upon magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 47, 52–67. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Tan, Z.; Dong, Z.; Schutz, M.J. In Vivo and In Vitro Degradation Behavior of Magnesium Alloys as Biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Yfantis, C.D.; Yfantis, D.K.; Anastassopoulou, J.; Theophanides, T.; Staiger, M. In Vitro Corrosion Behavior of New Magnesium Alloys for Bone Regeneration. In Proceedings of the 4th WSEAS International Conference on Environment, Ecosystems and Development, Venice, Italy, 20–22 November 2006; pp. 186–189. [Google Scholar]
- Xin, Y.; Hu, T.; Chu, P.K. In Vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2010, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, J.; Wang, Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coat. Technol. 2004, 185, 92–98. [Google Scholar] [CrossRef]
- Liu, C.; Xin, Y.; Tang, G.; Chu, P.K. Influence of heat treatment on degradation behavior of bio-degradable die-cast AZ63 magnesium alloy in simulated body fluid. Mater. Sci. Eng. A 2007, 456, 350–357. [Google Scholar] [CrossRef]
- López, H.Y.; Cortés-Hernández, D.A.; Escobedo, S.; Mantovani, D. In vitro bioactivity assessment of metallic magnesium. Key Eng. Mater. 2006, 309–311, 453–456. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion; Macmillan: New York, NY, USA, 1992. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A theoretical analysis of the shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar]
- Jianlu Zhang, H.Z.; Jinfeng, W.; Juijun, Z. Chapter 10—High Temperature PEM Fuel Cells. In PEM Fuel Cell Testing and Diagnosis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 243–282. [Google Scholar]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current Status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2016, 112, 287–302. [Google Scholar] [CrossRef]
- Windhagen, H.; Weizbaur, K.R.A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Strukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Outl. 2013, 12, 62. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2015, 81, 84–92. [Google Scholar] [CrossRef]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechansm of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, C.; Lou, T.; Chen, B.; Yi, R.; Wang, W.; Zheng, R.; Zou, M.; Xu, H.; Han, P.; et al. Crevice corrosion—A newly observed mechanism of degradation in biomedical magnesium. Acta Biomater. 2019, 98, 152–159. [Google Scholar] [CrossRef] [PubMed]












| Research Group | Material | |||
|---|---|---|---|---|
| Gastaldi et al. [29] | AZ31, AZ61, AZ80, ZK60 and ZM21 | 100 | 40 | 0.01–0.1 |
| Grogan et al. [30] | AZ31 | 170 | 70 | 0.026 |
| Wu et al. [32] | AZ31 | 100 | 40 | 0.005 |
| Research Group | Material | γ | ψ | β | |||
|---|---|---|---|---|---|---|---|
| Grogan et al. [30] | AZ31 | 170 | 70 | 0.00042 | 0.2 | – | 0.8 |
| Amerinatanzi et al. [28] | AZ31, Mg–Zn–Ca | – | – | 0.1005 | 2.748 | 2.60477 | 5.1 |
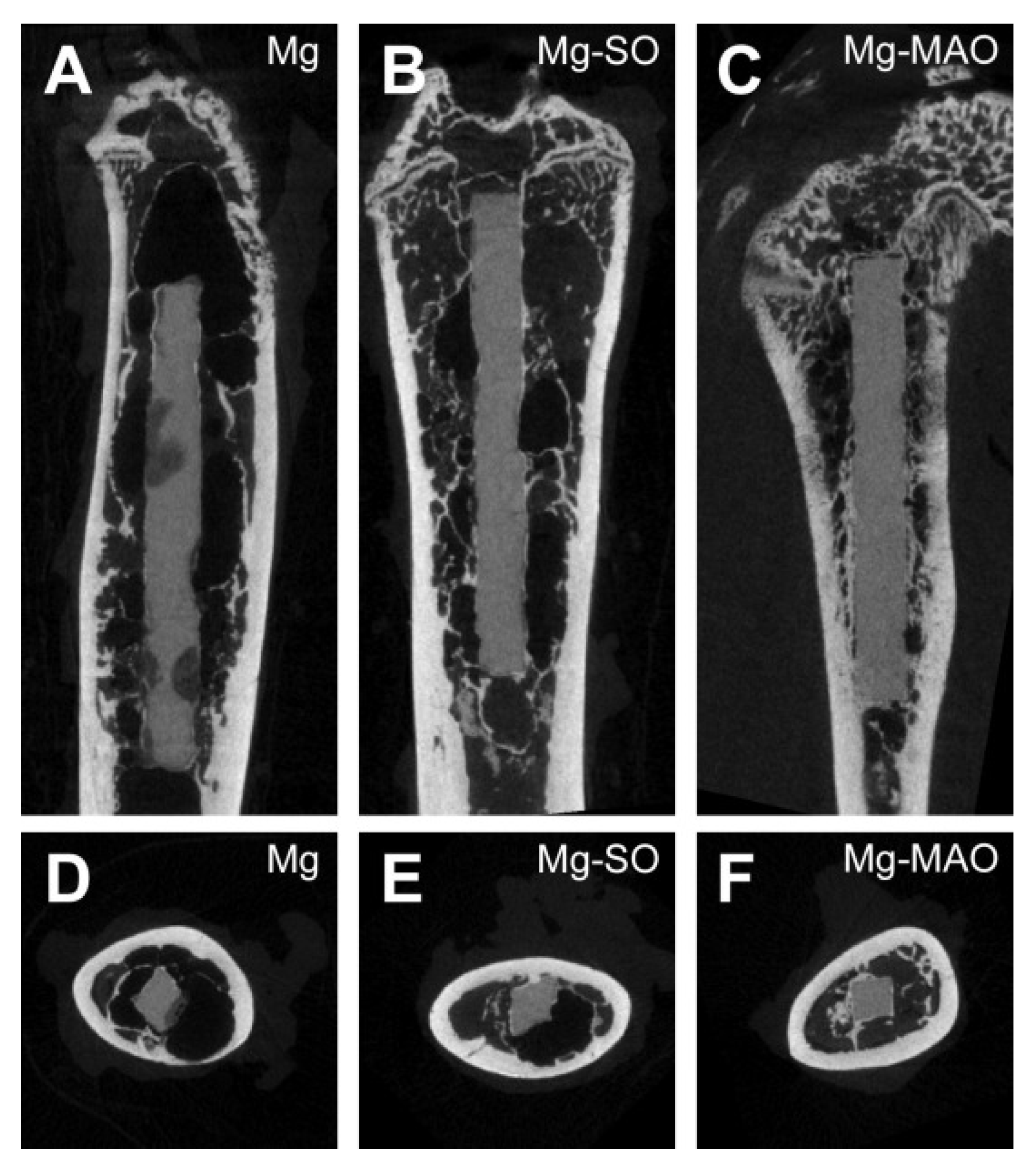
| Coating Type | New Bone Volume (mm3) | Initial Implant Volume (mm3) | Final Implant Volume (mm3) | Implant Volume Change (%) |
|---|---|---|---|---|
| Mg | 0.56 | 28.35 | 24.28 | −14.36% |
| Mg–SO | 1.52 | 28.35 | 26.32 | −7.17% |
| Mg–MAO | 4.72 | 28.35 | 27.68 | −2.38% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.; Joplin, A.; Elahinia, M.; Ibrahim, H. Corrosion Modeling of Magnesium and Its Alloys for Biomedical Applications: Review. Corros. Mater. Degrad. 2020, 1, 219-248. https://doi.org/10.3390/cmd1020011
Abdalla M, Joplin A, Elahinia M, Ibrahim H. Corrosion Modeling of Magnesium and Its Alloys for Biomedical Applications: Review. Corrosion and Materials Degradation. 2020; 1(2):219-248. https://doi.org/10.3390/cmd1020011
Chicago/Turabian StyleAbdalla, Moataz, Alexander Joplin, Mohammad Elahinia, and Hamdy Ibrahim. 2020. "Corrosion Modeling of Magnesium and Its Alloys for Biomedical Applications: Review" Corrosion and Materials Degradation 1, no. 2: 219-248. https://doi.org/10.3390/cmd1020011
APA StyleAbdalla, M., Joplin, A., Elahinia, M., & Ibrahim, H. (2020). Corrosion Modeling of Magnesium and Its Alloys for Biomedical Applications: Review. Corrosion and Materials Degradation, 1(2), 219-248. https://doi.org/10.3390/cmd1020011







