Exploring Lifestyles and Sensory Processing Patterns of Toddlers in Relation to Sleep Patterns Using Body Movement Analysis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
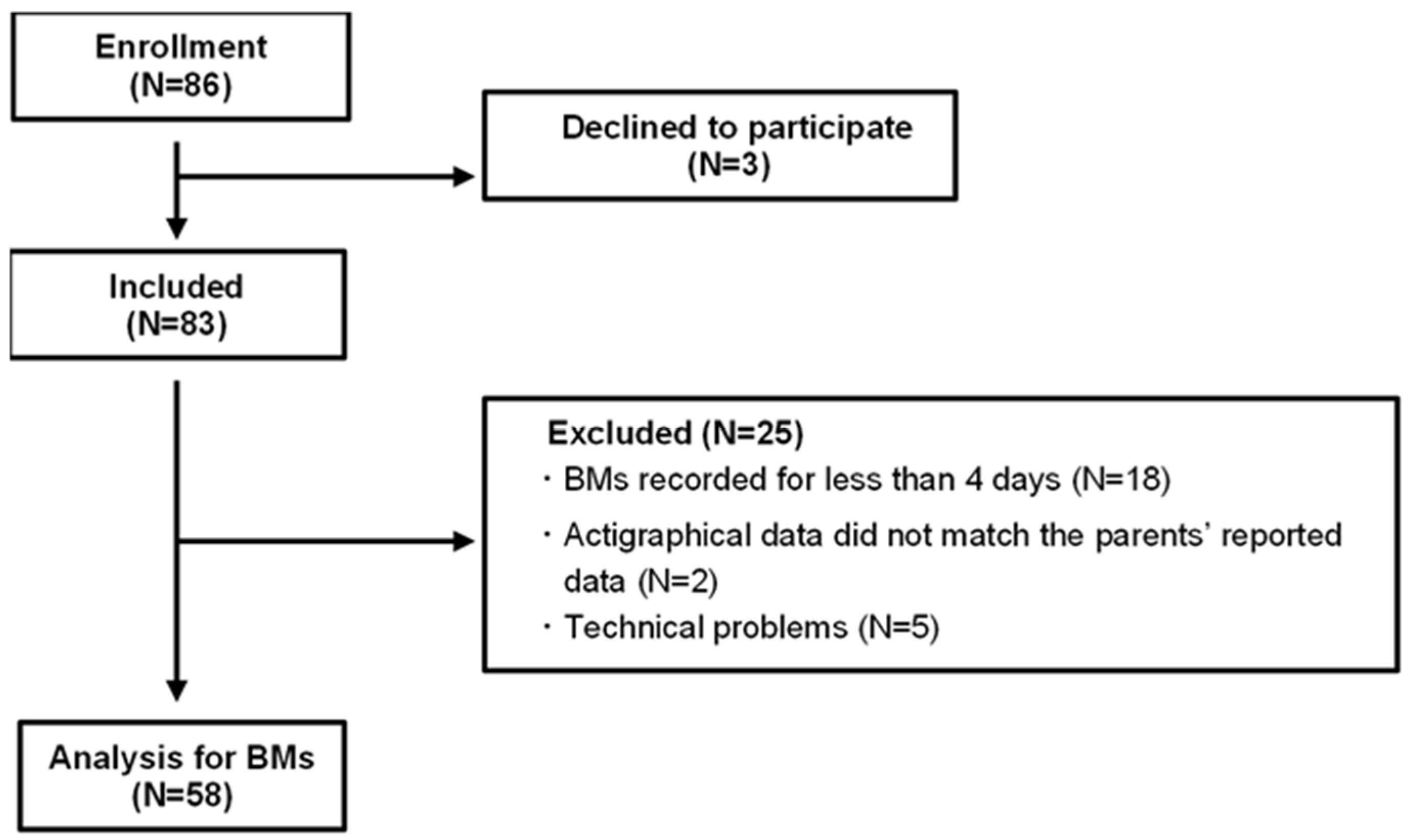
4.1. Participants
4.2. Actigraphy
4.3. Acquisition of Sleep-Related Habits and Sensory Processing Patterns
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SD | standard deviation |
| BMs | body movements |
| Total sleep BMs | BMs during overnight sleep |
| First 3 h BMs | BMs during the first 3 h of sleep from sleep onset |
| Last 3 h BMs | BMs during the last 3 h of sleep until the end of sleep |
References
- Walker, M.P.; van der Helm, E. Overnight therapy? The role of sleep in emotional brain processing. Psychol. Bull. 2009, 135, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; Stickgold, R. Sleep, memory, and plasticity. Annu. Rev. Psychol. 2006, 57, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Girardeau, G.; Lopes-Dos-Santos, V. Brain neural patterns and the memory function of sleep. Science 2021, 374, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflügers Arch.-Eur. J. Physiol. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Armstrong, J.M.; Ruttle, P.L.; Klein, M.H.; Essex, M.J.; Benca, R.M. Associations of child insomnia, sleep movement, and their persistence with mental health symptoms in childhood and adolescence. Sleep 2014, 37, 901–909. [Google Scholar] [CrossRef]
- Sadeh, A.; Mindell, J.A.; Luedtke, K.; Wiegand, B. Sleep and sleep ecology in the first 3 years: A web-based study. J. Sleep Res. 2009, 18, 60–73. [Google Scholar] [CrossRef]
- Berger, R.H.; Miller, A.L.; Seifer, R.; Cares, S.R.; Lebourgeois, M.K. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J. Sleep Res. 2012, 21, 235–246. [Google Scholar] [CrossRef]
- Huhdanpää, H.; Morales-Muñoz, I.; Aronen, E.T.; Pölkki, P.; Saarenpää-Heikkilä, O.; Paunio, T.; Kylliäinen, A.; Paavonen, E.J. Sleep difficulties in infancy are associated with symptoms of inattention and hyperactivity at the age of 5 years: A longitudinal study. J. Dev. Behav. Pediatr. 2019, 40, 432–440. [Google Scholar] [CrossRef]
- Morales-Muñoz, I.; Broome, M.R.; Marwaha, S. Association of parent-reported sleep problems in early childhood with psychotic and borderline personality disorder symptoms in adolescence. JAMA Psychiatry 2020, 77, 1256–1265. [Google Scholar] [CrossRef]
- Reynaud, E.; Forhan, A.; Heude, B.; Charles, M.-A.; Plancoulaine, S.; EDEN Mother−Child Cohort Study Group. Night-waking and behavior in preschoolers: A developmental trajectory approach. Sleep Med. 2018, 43, 90–95. [Google Scholar] [CrossRef]
- Sadeh, A.; Tikotzky, L.; Kahn, M. Sleep in infancy and childhood: Implications for emotional and behavioral difficulties in adolescence and beyond. Curr. Opin. Psychiatry 2014, 27, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Harvey, A.G.; Reichborn-Kjennerud, T.; Ystrom, E.; Hysing, M. Sleep problems and depressive symptoms in toddlers and 8-year-old children: A longitudinal study. J. Sleep Res. 2021, 30, e13150. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, S.; Spencer, R.M.C. Relations between sleep patterns early in life and brain development: A review. Dev. Cogn. Neurosci. 2022, 56, 101130. [Google Scholar] [CrossRef] [PubMed]
- Byars, K.C.; Yeomans-Maldonado, G.; Noll, J.G. Parental functioning and pediatric sleep disturbance: An examination of factors associated with parenting stress in children clinically referred for evaluation of insomnia. Sleep Med. 2011, 12, 898–905. [Google Scholar] [CrossRef]
- De Stasio, S.; Boldrini, F.; Ragni, B.; Gentile, S. Predictive factors of toddlers’ sleep and parental stress. Int. J. Environ. Res. Public Health 2020, 17, 2494. [Google Scholar] [CrossRef]
- Oyetunji, A.; Chandra, P. Postpartum stress and infant outcome: A review of current literature. Psychiatry Res. 2020, 284, 112769. [Google Scholar] [CrossRef]
- Moorman, J.D.; Harrison, K. Beyond access and exposure: Implications of sneaky media use for preschoolers’ sleep behavior. Health Commun. 2019, 34, 529–536. [Google Scholar] [CrossRef]
- Henderson, J.A.; Jordan, S.S. Development and preliminary evaluation of the bedtime routines questionnaire. J. Psychopathol. Behav. Assess. 2010, 32, 271–280. [Google Scholar] [CrossRef]
- Janssen, X.; Martin, A.; Hughes, A.R.; Hill, C.M.; Kotronoulas, G.; Hesketh, K.R. Associations of screen time, sedentary time and physical activity with sleep in under 5s: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 49, 101226. [Google Scholar] [CrossRef]
- Murata, E.; Yoshizaki, A.; Fujisawa, T.X.; Tachibana, M.; Taniike, M.; Mohri, I. What daily factors affect the sleep habits of Japanese toddlers? J. Clin. Sleep Med. 2023, 19, 1089–1101. [Google Scholar] [CrossRef]
- Ahn, R.R.; Miller, L.J.; Milberger, S.; McIntosh, D.N. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. Am. J. Occup. Ther. 2004, 58, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Galiana-Simal, A.; Vela-Romero, M.; Romero-Vela, V.M.; Oliver-Tercero, N.; García-Olmo, V.; Benito-Castellanos, P.J.; Muñoz-Martinez, V.; Beato-Fernandez, L. Sensory processing disorder: Key points of a frequent alteration in neurodevelopmental disorders. Cogent. Med. 2020, 7, 1736829. [Google Scholar] [CrossRef]
- Shochat, T.; Tzischinsky, O.; Engel-Yeger, B. Sensory hypersensitivity as a contributing factor in the relation between sleep and behavioral disorders in normal schoolchildren. Behav. Sleep. Med. 2009, 7, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, B.K.; Kayıhan, H.; Çifci, A. Sensory processing in typically developing toddlers with and without sleep problems. Infant Behav. Dev. 2024, 76, 101981. [Google Scholar] [CrossRef]
- Velluti, R.A. Interactions between sleep and sensory physiology. J. Sleep Res. 1997, 6, 61–77. [Google Scholar] [CrossRef]
- Fernández-Pires, P.; Valera-Gran, D.; Hurtado-Pomares, M.; Espinosa-Sempere, C.; Sánchez-Pérez, A.; Juárez-Leal, I.; Ruiz-Carbonell, M.P.; Peral-Gómez, P.; Campos-Sánchez, I.; Pérez-Vázquez, M.T.; et al. Sleep duration and quality and sensory reactivity in schoolaged children: The Spanish cross-sectional. Front. Pediatr. 2021, 9, 646011. [Google Scholar] [CrossRef]
- Allen, S.L.; Howlett, M.D.; Coulombe, J.A.; Corkum, P.V. ABCs of SLEEPING: A review of the evidence behind pediatric sleep practice recommendations. Sleep Med. Rev. 2016, 29, 1–14. [Google Scholar] [CrossRef]
- Okada, S.; Ohno, Y.; Shimizu, S.; Kato-Nishimura, K.; Mohri, I.; Taniike, M.; Makikawa, M. Development and preliminary evaluation of video analysis for detecting Gross movement during sleep in children. Biomed. Eng. Lett. 2011, 1, 220–225. [Google Scholar] [CrossRef]
- Wilde-Frenz, J.; Schulz, H. Rate and distribution of body movements during sleep in humans. Percept. Mot. Ski. 1983, 56, 275–283. [Google Scholar] [CrossRef]
- Krystal, A.D.; Edinger, J.D. Measuring sleep quality. Sleep Med. 2008, 9, S10–S17. [Google Scholar] [CrossRef]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2018, 14, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Mohri, I.; Yamamoto, T.; Shirota, A.; Okada, S.; Murata, E.; Hoshino, K.; Kato-Nishimura, K.; Matsuzawa, S.; Kato, T.; et al. An interactive smartphone app, Nenne Navi, for improving children’s sleep: Pilot usability study. JMIR Pediatr. Parent. 2020, 3, e22102. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, L.J.; Montgomery-Downs, H.E.; Insana, S.P.; Walsh, C.M. Use of actigraphy for assessment in pediatric sleep research. Sleep Med. Rev. 2012, 16, 463–475. [Google Scholar] [CrossRef]
- Ito, H.; Hirashima, T.; Hagiwara, T.; Iwanaga, R.; Tani, I.; Yikihiro, R. Standardization of the Japanese version of the sensory profile: Reliability and norms based on a community sample. Seishin Igaku 2013, 55, 537–548. [Google Scholar]
- Shimohira, M.; Shiiki, T.; Sugimoto, J.; Ohsawa, Y.; Fukumizu, M.; Hasegawa, T.; Iwakawa, Y.; Nomura, Y.; Segawa, M. Video analysis of gross body movements during sleep. Psychiatry Clin. Neurosci. 1998, 52, 176–177. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006, 10, 49–62. [Google Scholar] [CrossRef]
- Walker, M.P.; Stickgold, R. Sleep-dependent learning and memory consolidation. Neuron 2004, 44, 121–133. [Google Scholar] [CrossRef]
- Borbély, A.A. A two process model of sleep regulation. Hum Neurobiol 1982, 1, 195–204. [Google Scholar]
- Borbély, A. The two-process model of sleep regulation: Beginnings and outlook. J. Sleep Res. 2022, 31, e13598. [Google Scholar] [CrossRef]
- Liu, A.; Fan, J.; Ding, C.; Yuan, F.; Gong, W.; Zhang, Y.; Song, C.; Zhou, Y.; Ding, G. The association of sleep duration with breakfast patterns and snack behaviors among Chinese children aged 6 to 17 years: Chinese National Nutrition and Health Surveillance 2010–2012. Nutrients 2022, 14, 2247. [Google Scholar] [CrossRef] [PubMed]
- Tambalis, K.D.; Panagiotakos, D.B.; Psarra, G.; Sidossis, L.S. Insufficient sleep duration is associated with dietary habits, screen time, and obesity in children. J. Clin. Sleep Med. 2018, 14, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Hasegawa, T.; Kawahashi, I.; Imada, S. Preschool children’s eating and sleeping habits: Late rising and brunch on weekends is related to several physical and mental symptoms. Sleep Med. 2019, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; Levine, A.; Avni, H.; Nehama, H.; Greenfeld, M.; Sivan, Y. Coexistence of sleep and feeding disturbances in young children. Pediatrics 2011, 127, e615–e621. [Google Scholar] [CrossRef]
- BaHammam, A.S.; Pirzada, A. Timing matters: The interplay between early mealtime, circadian rhythms, gene expression, circadian hormones, and metabolism-A narrative review. Clocks Sleep 2023, 5, 507–535. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y.; et al. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: A randomized clinical trial. Diabetes Care 2017, 40, 1573–1579. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef]
- Ogata, H.; Horie, M.; Kayaba, M.; Tanaka, Y.; Ando, A.; Park, I.; Zhang, S.; Yajima, K.; Shoda, J.I.; Omi, N.; et al. Skipping breakfast for 6 days delayed the circadian rhythm of the body temperature without altering clock gene expression in human leukocytes. Nutrients 2020, 12, 2797. [Google Scholar] [CrossRef]
- Lane, S.J.; Reynolds, S.; Thacker, L. Sensory over-responsivity and ADHD: Differentiating using electrodermal responses, cortisol, and anxiety. Front. Integr. Neurosci. 2010, 4, 603. [Google Scholar] [CrossRef]
- Yang, C.M.; Lo, H.S. ERP evidence of enhanced excitatory and reduced inhibitory processes of auditory stimuli during sleep in patients with primary insomnia. Sleep 2007, 30, 585–592. [Google Scholar] [CrossRef]
- Movahed, E.; Moradi, S.; Mortezagholi, B.; Shafiee, A.; Moltazemi, H.; Hajishah, H.; Siahvoshi, S.; Monfared, A.B.; Amini, M.J.; Safari, F.; et al. Investigating oral health among US adults with sleep disorder: A cross-sectional study. BMC Oral. Health 2023, 23, 996. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A.; Meltzer, L.J.; Carskadon, M.A.; Chervin, R.D. Developmental aspects of sleep hygiene: Findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009, 10, 771–779. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.; Benedito-Silva, A.A.; Pires, M.L.N.; Poyares, D.; Tufik, S.; Calil, H.M. Further validation of actigraphy for sleep studies. Sleep 2003, 26, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L.; Study of Osteoporotic Fractures Research Group. Comparison of sleep parameters from actigraphy and polysomnography in older women: The SOF study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef]
- Martin, J.L.; Hakim, A.D. Wrist actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef]
- Rowe, M.; McCrae, C.; Campbell, J.; Horne, C.; Tiegs, T.; Lehman, B.; Cheng, J. Actigraphy in older adults: Comparison of means and variability of three different aggregates of measurement. Behav. Sleep Med. 2008, 6, 127–145. [Google Scholar] [CrossRef]
- Nakazaki, K.; Kitamura, S.; Motomura, Y.; Hida, A.; Kamei, Y.; Miura, N.; Mishima, K. Validity of an algorithm for determining sleep/wake states using a new actigraph. J. Physiol. Anthr. 2014, 33, 31. [Google Scholar] [CrossRef]
- Matsuo, M.; Masuda, F.; Sumi, Y.; Takahashi, M.; Yamada, N.; Ohira, M.H.; Fujiwara, K.; Kanemura, T.; Kadotani, H. Comparisons of portable sleep monitors of different modalities: Potential as naturalistic sleep recorders. Front. Neurol. 2016, 7, 110. [Google Scholar] [CrossRef]
- Dunn, W.; Daniels, D.B. Initial development of the infant/toddler sensory profile. J. Early Interv. 2002, 25, 27–41. [Google Scholar] [CrossRef]

| Toddler Demographics (n = 58) | |
|---|---|
| Age in months, mean ± SD | 22.0 ± 2.0 |
| Sex, male, n (%) | 30 (51.7%) |
| Sleep onset, mean ± SD | 9:24 PM ± 0:48 |
| Sleep end, mean ± SD | 6:49 AM ± 0:40 |
| Total sleep time (min), mean ± SD | 564.8 ± 43.9 |
| Sleep latency (min), mean ± SD | 33.3 ± 16.3 |
| Net sleep time (min), mean ± SD | 397.6 ± 65.1 |
| Sleep efficiency (%), mean ± SD | 64.5 ± 8.5 |
| Consuming a substantial breakfast, n (%) | 42 (72.4%) |
| Play outside in the morning, n (%) | 30 (51.7%) |
| Nap end time, mean ± SD | 3:17 PM ± 1:08 |
| Daily screen time (min), mean ± SD | 92.0 ± 69.0 |
| Screen time of 1 h before bedtime, n (%) | 10 (17.2%) |
| Amounts of BMs | mean ± SD |
| BMs 1 h before bedtime | 6.12 ± 2.17 |
| Total sleep BMs | 0.43 ± 0.15 |
| First 3 h BMs | 0.29 ± 0.13 |
| Last 3 h BMs | 0.48 ± 0.16 |
| Sensory processing scores | mean ± SD (normal range) |
| Auditory sensory | 17.0 ± 4.0 (13–21) |
| Visual sensory | 18.0 ± 3.0 (13–20) |
| Tactile sensory | 29.0 ± 8.0 (21–36) |
| Vestibular sensory | 15.0 ± 3.0 (12–17) |
| Oral sensory | 16.0 ± 5.0 (10–18) |
| Sleep Latency (Time) (Adjusted R² = 0.22) | |||||
|---|---|---|---|---|---|
| Coefficient | p | 95% CI | |||
| (n = 58) | Lower | Upper | |||
| Consuming a substantial breakfast | −7.43 | 0.089 | −16.0 | 1.18 | |
| Playing outside in the morning | −4.50 | 0.245 | −12.2 | 3.19 | |
| Nap end time | 2.43 | 0.537 | −5.42 | 10.3 | |
| Daily screen time | −0.66 | 0.875 | −8.94 | 7.63 | |
| before bedtime | Screen time | 12.7 | 0.016 * | −2.49 | 22.8 |
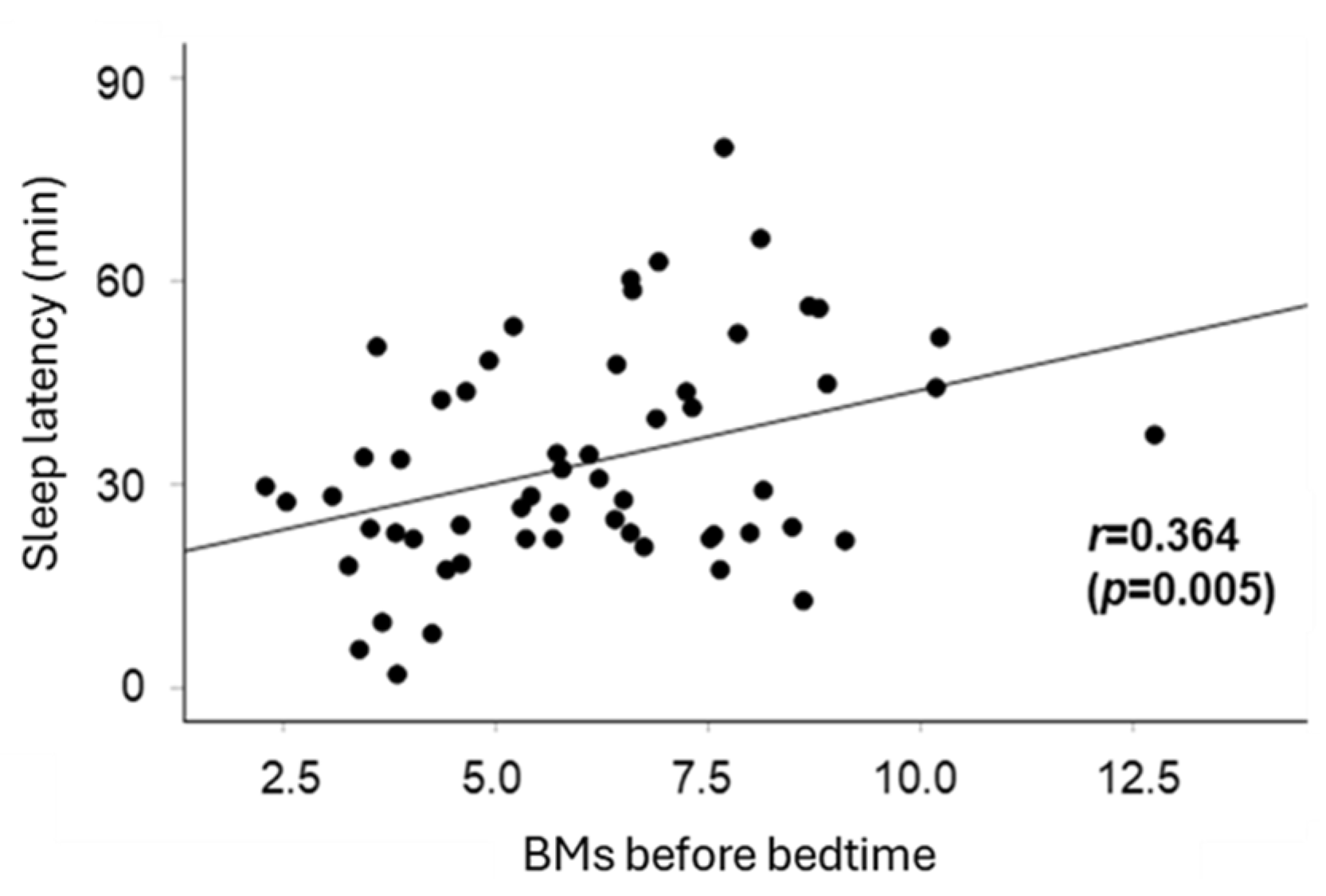
| BMs | 2.86 | 0.003 * | 1.05 | 4.67 | |
| First 3 h BMs (Adjusted R² = 0.16) | Last 3 h BMs (Adjusted R² = −0.005) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p | 95% CI | Coefficient | p | 95% CI | ||||
| (n = 58) | Lower | Upper | Lower | Upper | |||||
| Consuming a substantial breakfast | −0.096 | 0.009 ** | −0.17 | −0.03 | −0.097 | 0.317 | −1.50 | 0.05 | |
| Playing outside in the morning | 0.048 | 0.129 | −0.01 | 0.11 | 0.021 | 0.634 | −0.68 | 0.11 | |
| Nap end time | −0.066 | 0.046 * | −0.13 | −0.00 | −0.014 | −0.760 | −0.10 | 0.54 | |
| Daily screen time | −0.008 | 0.808 | −0.08 | −0.06 | 0.087 | 0.075 | −0.01 | 0.18 | |
| before bedtime | Screen time | 0.065 | 0.126 | −0.02 | 0.15 | 0.012 | 0.831 | −0.11 | 0.13 |
| BMs | 0.004 | 0.564 | −0.01 | −0.03 | 0.010 | 0.326 | −0.01 | 0.03 | |
| Sleep Latency | First 3 h BMs | Last 3 h BMs | |
|---|---|---|---|
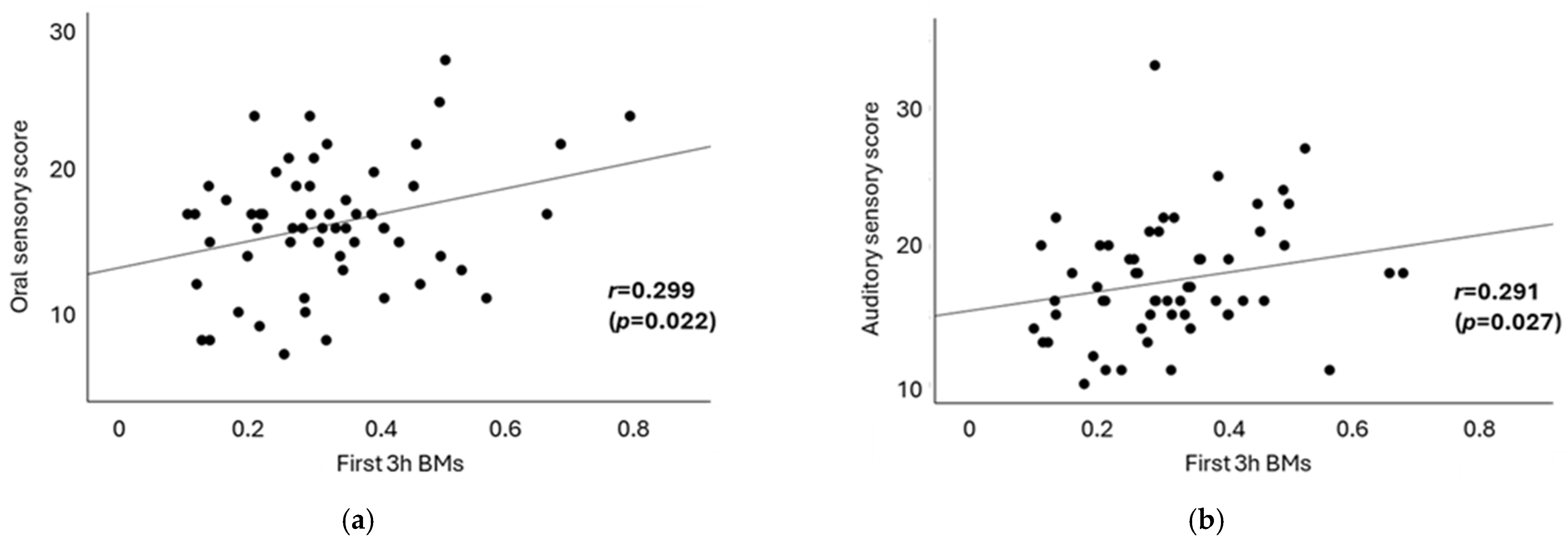
| Auditory sensory | 0.167 | 0.291 * | 0.039 |
| Visual sensory | −0.276 * | −0.062 | −0.008 |
| Tactile sensory | 0.040 | 0.031 | 0.074 |
| Vestibular sensory | 0.064 | 0.131 | 0.147 |
| Oral sensory | 0.204 | 0.299 * | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, A.; Iwatani, Y.; Yoshizaki, A.; Nishimura, T.; Mohri, I.; Kagitani-Shimono, K.; Taniike, M. Exploring Lifestyles and Sensory Processing Patterns of Toddlers in Relation to Sleep Patterns Using Body Movement Analysis. Clocks & Sleep 2025, 7, 25. https://doi.org/10.3390/clockssleep7020025
Ono A, Iwatani Y, Yoshizaki A, Nishimura T, Mohri I, Kagitani-Shimono K, Taniike M. Exploring Lifestyles and Sensory Processing Patterns of Toddlers in Relation to Sleep Patterns Using Body Movement Analysis. Clocks & Sleep. 2025; 7(2):25. https://doi.org/10.3390/clockssleep7020025
Chicago/Turabian StyleOno, Azusa, Yoshiko Iwatani, Arika Yoshizaki, Tomoko Nishimura, Ikuko Mohri, Kuriko Kagitani-Shimono, and Masako Taniike. 2025. "Exploring Lifestyles and Sensory Processing Patterns of Toddlers in Relation to Sleep Patterns Using Body Movement Analysis" Clocks & Sleep 7, no. 2: 25. https://doi.org/10.3390/clockssleep7020025
APA StyleOno, A., Iwatani, Y., Yoshizaki, A., Nishimura, T., Mohri, I., Kagitani-Shimono, K., & Taniike, M. (2025). Exploring Lifestyles and Sensory Processing Patterns of Toddlers in Relation to Sleep Patterns Using Body Movement Analysis. Clocks & Sleep, 7(2), 25. https://doi.org/10.3390/clockssleep7020025






