Mapping the Neural Basis of Wake Onset Regularity and Its Effects on Sleep Quality and Positive Affect
Abstract
1. Introduction
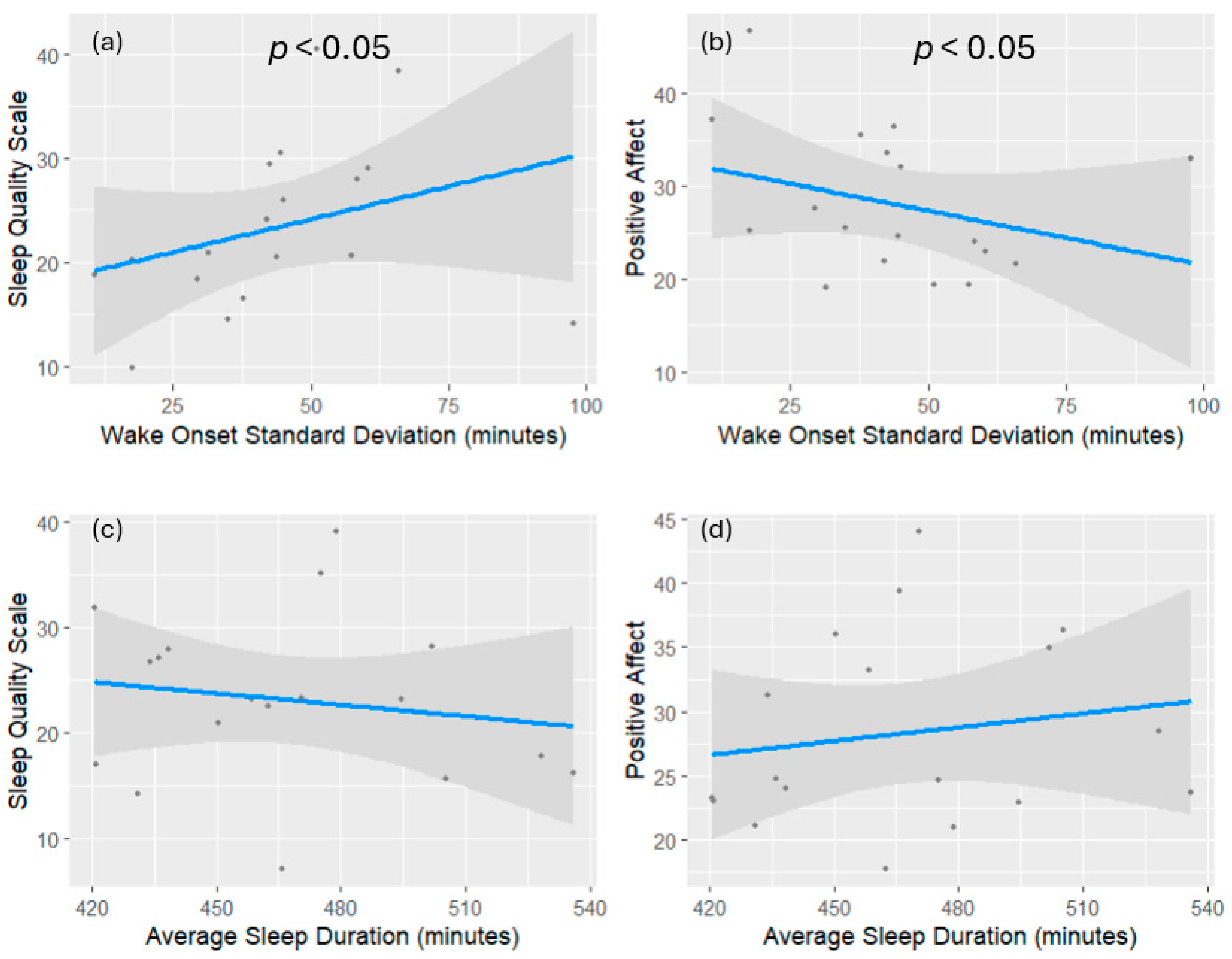
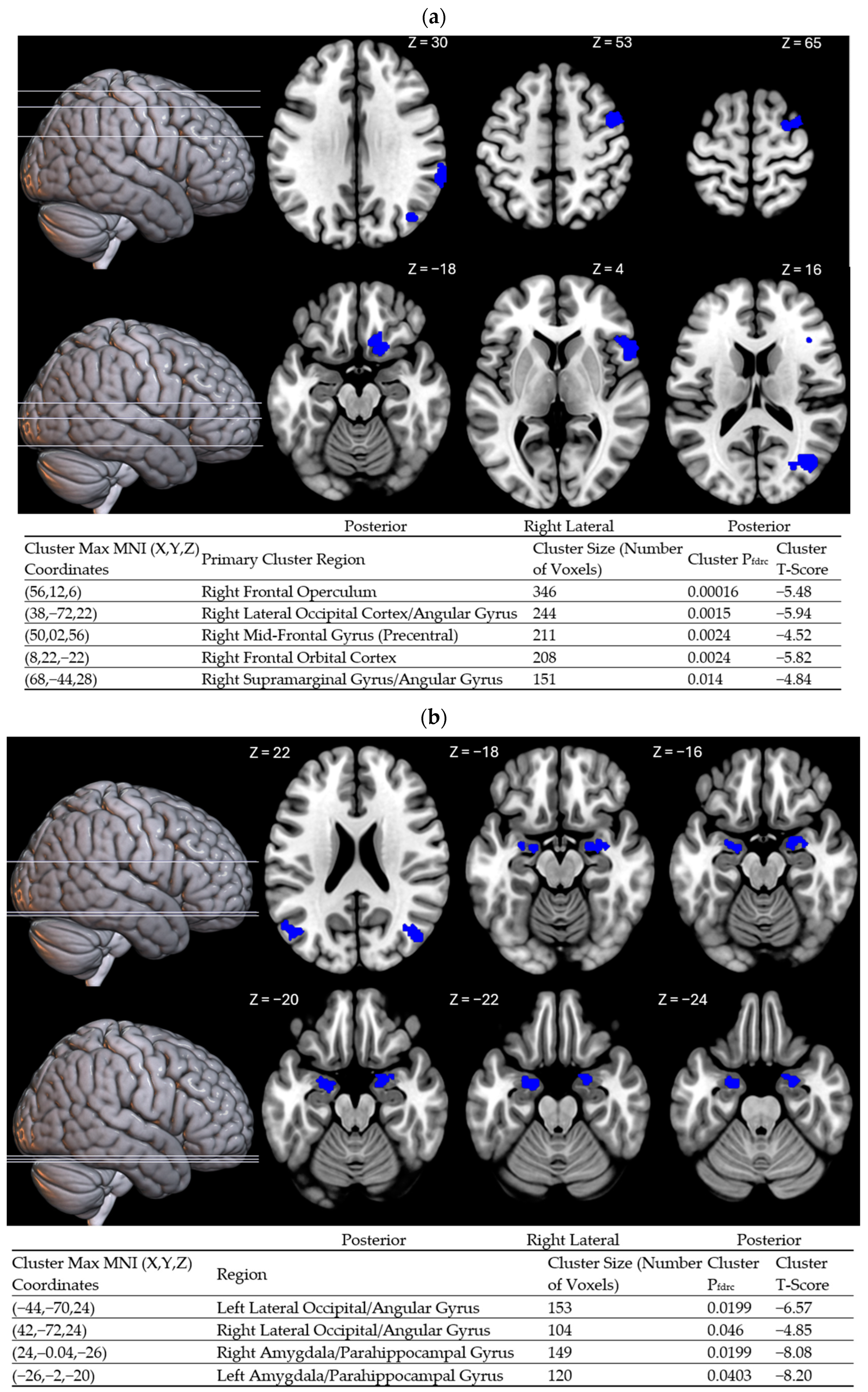
2. Results
3. Discussion
4. Strengths and Limitations
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and synaptic homeostasis: A hypothesis. Brain Res. Bull. 2003, 62, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef]
- Frank, M.G. Erasing Synapses in Sleep: Is It Time to Be SHY? Neural Plast. 2012, 2012, 264378. [Google Scholar] [CrossRef] [PubMed]
- BuzsÁk, G. Memory consolidation during sleep: A neurophysiological perspective. J. Sleep Res. 1998, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Rasch, B.; Gais, S. Sleep to Remember. Neuroscientist 2006, 12, 410–424. [Google Scholar] [CrossRef]
- Rudoy, J.D.; Voss, J.L.; Westerberg, C.E.; Paller, K.A. Strengthening Individual Memories by Reactivating Them During Sleep. Science 2009, 326, 1079. [Google Scholar] [CrossRef]
- Wilson, M.A.; McNaughton, B.L. Reactivation of Hippocampal Ensemble Memories During Sleep. Science 1994, 265, 676–679. [Google Scholar] [CrossRef]
- Hudson, A.N.; Van Dongen, H.P.A.; Honn, K.A. Sleep deprivation, vigilant attention, and brain function: A review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef]
- Frenda, S.J.; Fenn, K.M. Sleep less, think worse: The effect of sleep deprivation on working memory. J. Appl. Res. Mem. Cogn. 2016, 5, 463–469. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, C.U.; Lim, H.K. Role of Sleep Disturbance in the Trajectory of Alzheimer’s Disease. Clin. Psychopharmacol. Neurosci. 2017, 15, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sadeghmousavi, S.; Eskian, M.; Rahmani, F.; Rezaei, N. The effect of insomnia on development of Alzheimer’s disease. J. Neuroinflammation 2020, 17, 289. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep Med. 2015, 11, 591–592. [Google Scholar] [CrossRef]
- Ayas, N.T.; White, D.P.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Malhotra, A.; Hu, F.B. A Prospective Study of Sleep Duration and Coronary Heart Disease in Women. Arch. Intern. Med. 2003, 163, 205. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Kario, K. Sleep Duration as a Risk Factor for Cardiovascular Disease—A Review of the Recent Literature. Curr. Cardiol. Rev. 2010, 6, 54–61. [Google Scholar] [CrossRef]
- Covassin, N.; Singh, P. Sleep Duration and Cardiovascular Disease Risk. Sleep Med. Clin. 2016, 11, 81–89. [Google Scholar] [CrossRef]
- Li, Q. The association between sleep duration and excess body weight of the American adult population: A cross-sectional study of the national health and nutrition examination survey 2015–2016. BMC Public Health 2021, 21, 335. [Google Scholar] [CrossRef]
- Nôga, D.A.; Meth, E.D.M.E.S.; Pacheco, A.P.; Tan, X.; Cedernaes, J.; Van Egmond, L.T.; Xue, P.; Benedict, C. Habitual Short Sleep Duration, Diet, and Development of Type 2 Diabetes in Adults. JAMA Netw. Open 2024, 7, e241147. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Araujo, A.B.; McKinlay, J.B. Sleep Duration as a Risk Factor for the Development of Type 2 Diabetes. Diabetes Care 2006, 29, 657–661. [Google Scholar] [CrossRef]
- Grandner, M.A.; Seixas, A.; Shetty, S.; Shenoy, S. Sleep Duration and Diabetes Risk: Population Trends and Potential Mechanisms. Curr. Diabetes Rep. 2016, 16, 106. [Google Scholar] [CrossRef]
- Gallicchio, L.; Kalesan, B. Sleep duration and mortality: A systematic review and meta-analysis. J. Sleep Res. 2009, 18, 148–158. [Google Scholar] [CrossRef]
- Yin, J.; Jin, X.; Shan, Z.; Li, S.; Huang, H.; Li, P.; Peng, X.; Peng, Z.; Yu, K.; Bao, W.; et al. Relationship of Sleep Duration with All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005947. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Mariani, S.; Redline, S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2020, 75, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ghinaglia, N.; Fernandez-Mendoza, J. Sleep variability and regularity as contributors to obesity and cardiometabolic health in adolescence. Obesity 2023, 31, 597–614. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Makarem, N.; Redline, S.; Aggarwal, B.; Jelic, S.; St-Onge, M.-P. Sleep Regularity and Cardiometabolic Heath: Is Variability in Sleep Patterns a Risk Factor for Excess Adiposity and Glycemic Dysregulation? Curr. Diab. Rep. 2020, 20, 38. [Google Scholar] [CrossRef]
- Windred, D.P.; Burns, A.C.; Lane, J.M.; Saxena, R.; Rutter, M.K.; Cain, S.W.; Phillips, A.J.K. Sleep regularity is a stronger predictor of mortality risk than sleep duration: A prospective cohort study. Sleep 2024, 47, zsad253. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Ellenbogen, J.M.; Bianchi, M.T.; Czeisler, C.A. Sleep deficiency and motor vehicle crash risk in the general population: A prospective cohort study. BMC Med. 2018, 16, 44. [Google Scholar] [CrossRef]
- Sletten, T.L.; Weaver, M.D.; Foster, R.G.; Gozal, D.; Klerman, E.B.; Rajaratnam, S.M.W.; Roenneberg, T.; Takahashi, J.S.; Turek, F.W.; Vitiello, M.V.; et al. The importance of sleep regularity: A consensus statement of the National Sleep Foundation sleep timing and variability panel. Sleep Health 2023, 9, 801–820. [Google Scholar] [CrossRef]
- Manber, R.; Bootzin, R.R.; Acebo, C.; Carskadon, M.A. The effects of regularizing sleep-wake schedules on daytime sleepiness. Sleep 1996, 19, 432–441. [Google Scholar] [CrossRef]
- Okuda, M.; Noda, A.; Iwamoto, K.; Nakashima, H.; Takeda, K.; Miyata, S.; Yasuma, F.; Ozaki, N.; Shimouchi, A. Effects of long sleep time and irregular sleep–wake rhythm on cognitive function in older people. Sci. Rep. 2021, 11, 7039. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.K.; Clerx, W.M.; O’Brien, C.S.; Sano, A.; Barger, L.K.; Picard, R.W.; Lockley, S.W.; Klerman, E.B.; Czeisler, C.A. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 2017, 7, 3216. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Kaczmarzyk, J.R.; Dave, N.; Gabrieli, J.D.E.; Grossman, J.C. Sleep quality, duration, and consistency are associated with better academic performance in college students. NPJ Sci. Learn. 2019, 4, 16. [Google Scholar] [CrossRef]
- Diem, S.J.; Blackwell, T.L.; Stone, K.L.; Yaffe, K.; Tranah, G.; Cauley, J.A.; Ancoli-Israel, S.; Redline, S.; Spira, A.P.; Hillier, T.A.; et al. Measures of Sleep–Wake Patterns and Risk of Mild Cognitive Impairment or Dementia in Older Women. Am. J. Geriatr. Psychiatry 2016, 24, 248–258. [Google Scholar] [CrossRef]
- Fenton, L.; Isenberg, A.L.; Aslanyan, V.; Albrecht, D.; Contreras, J.A.; Stradford, J.; Monreal, T.; Pa, J. Variability in objective sleep is associated with Alzheimer’s pathology and cognition. Brain Commun. 2023, 5, fcad031. [Google Scholar] [CrossRef]
- Qin, S.; Chee, M. The Emerging Importance of Sleep Regularity on Cardiovascular Health and Cognitive Impairment in Older Adults: A Review of the Literature. Nat. Sci. Sleep 2024, 16, 585–597. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Tomadesso, C.; De Flores, R.; Branger, P.; Rehel, S.; Mézenge, F.; Landeau, B.; De La Sayette, V.; Eustache, F.; Chételat, G.; et al. Brain and cognitive correlates of sleep fragmentation in elderly subjects with and without cognitive deficits. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 142–150. [Google Scholar] [CrossRef]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef]
- Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005, 25, 117–129. [Google Scholar] [CrossRef]
- Fischer, D.; McHill, A.W.; Sano, A.; Picard, R.W.; Barger, L.K.; Czeisler, C.A.; Klerman, E.B.; Phillips, A.J.K. Irregular sleep and event schedules are associated with poorer self-reported well-being in US college students. Sleep 2020, 43, zsz300. [Google Scholar] [CrossRef]
- Duncan, M.J.; Kline, C.E.; Rebar, A.L.; Vandelanotte, C.; Short, C.E. Greater bed- and wake-time variability is associated with less healthy lifestyle behaviors: A cross-sectional study. J. Public Health 2016, 24, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Kim, S.J. Instability in daily life and depression: The impact of sleep variance between weekday and weekend in South Korean workers. Health Soc. Care Community 2020, 28, 874–882. [Google Scholar] [CrossRef]
- Hysing, M.; Sivertsen, B.; Stormark, K.M.; O’Connor, R.C. Sleep problems and self-harm in adolescence. Br. J. Psychiatry 2015, 207, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.M.; Dautovich, N.D.; Dzierzewski, J.M. The Rhythm is Gonna Get You: Social Rhythms, Sleep, Depressive, and Anxiety Symptoms. J. Affect. Disord. 2021, 286, 197–203. [Google Scholar] [CrossRef]
- Bernert, R.A.; Hom, M.A.; Iwata, N.G.; Joiner, T.E. Objectively Assessed Sleep Variability as an Acute Warning Sign of Suicidal Ideation in a Longitudinal Evaluation of Young Adults at High Suicide Risk. J. Clin. Psychiatry 2017, 78, e678–e687. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.M.; Goldschmied, J.R.; Kelly, M.R.; Perkins, S.; Gehrman, P.R.; Haynes, P.L. Social rhythm regularity moderates the relationship between sleep disruption and depressive symptoms in veterans with post-traumatic stress disorder and major depressive disorder. Chronobiol. Int. 2019, 36, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Menon, V. 20 years of the default mode network: A review and synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef]
- Wu, J.; Dong, D.; Jackson, T.; Wang, Y.; Huang, J.; Chen, H. The Neural Correlates of Optimistic and Depressive Tendencies of Self-Evaluations and Resting-State Default Mode Network. Front. Hum. Neurosci. 2015, 9, 618. [Google Scholar] [CrossRef]
- Chou, T.; Deckersbach, T.; Dougherty, D.D.; Hooley, J.M. The default mode network and rumination in individuals at risk for depression. Soc. Cogn. Affect. Neurosci. 2023, 18, nsad032. [Google Scholar] [CrossRef]
- Berman, M.G.; Peltier, S.; Nee, D.E.; Kross, E.; Deldin, P.J.; Jonides, J. Depression, rumination and the default network. Soc. Cogn. Affect. Neurosci. 2011, 6, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Jankowski, S.; Henderson-Arredondo, K.; Lucas, D.A.; Patel, S.I.; Hildebrand, L.L.; Huskey, A.; Dailey, N.S. Functional connectivity of the default mode network predicts subsequent polysomnographically measured sleep in people with symptoms of insomnia. Neuroreport 2023, 34, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Qin, H.; Wu, T.; Hu, H.; Liao, K.; Cheng, F.; Gao, D.; Lei, X. Rest but busy: Aberrant resting-state functional connectivity of triple network model in insomnia. Brain Behav. 2018, 8, e00876. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Harris, A.L.; Falco, A.; Edinger, J.D. The relation between insomnia symptoms, mood, and rumination about insomnia symptoms. J. Clin. Sleep Med. JCSM 2013, 9, 567–575. [Google Scholar] [CrossRef]
- Morin, C.M.; Drake, C.L.; Harvey, A.G.; Krystal, A.D.; Manber, R.; Riemann, D.; Spiegelhalder, K. Insomnia disorder. Nat. Rev. Dis. Primer 2015, 1, 15026. [Google Scholar] [CrossRef]
- Lunsford-Avery, J.R.; Damme, K.S.F.; Engelhard, M.M.; Kollins, S.H.; Mittal, V.A. Sleep/Wake Regularity Associated with Default Mode Network Structure among Healthy Adolescents and Young Adults. Sci. Rep. 2020, 10, 509. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Du, J.; Wei, D.; Qiu, J.; Dai, D.; Zhou, Q.; Xie, P.; Feng, J. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc. Cogn. Affect. Neurosci. 2020, 15, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef]
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
- Maithani, T.; Prabhu, S.; Pant, S. Daytime-sleepiness, factors affecting sleep and sleep-quality among professional college students of South India—A correlative study. Clin. Epidemiol. Glob. Health 2024, 26, 101534. [Google Scholar] [CrossRef]
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the self in the brain’s default mode network. NeuroImage 2016, 132, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain J. Neurol. 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klerman, E.B.; Phillips, A.J.K. Measuring sleep regularity: Theoretical properties and practical usage of existing metrics. Sleep 2021, 44, zsab103. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Wright, K.P.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef]
- Gonnissen, H.K.J.; Mazuy, C.; Rutters, F.; Martens, E.A.P.; Adam, T.C.; Westerterp-Plantenga, M.S. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PLoS ONE 2013, 8, e72877. [Google Scholar] [CrossRef][Green Version]
- Hasan, S.; Foster, R.G.; Vyazovskiy, V.V.; Peirson, S.N. Effects of circadian misalignment on sleep in mice. Sci. Rep. 2018, 8, 15343. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Zimmerman, J.C.; Ronda, J.M.; Moore-Ede, M.C.; Weitzman, E.D. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep 1980, 2, 329–346. [Google Scholar]
- Wurts, S.W.; Edgar, D.M. Circadian and homeostatic control of rapid eye movement (REM) sleep: Promotion of REM tendency by the suprachiasmatic nucleus. J. Neurosci. 2000, 20, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Tempesta, D.; Socci, V.; De Gennaro, L.; Ferrara, M. Sleep and emotional processing. Sleep Med. Rev. 2018, 40, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Glosemeyer, R.W.; Diekelmann, S.; Cassel, W.; Kesper, K.; Koehler, U.; Westermann, S.; Steffen, A.; Borgwardt, S.; Wilhelm, I.; Müller-Pinzler, L.; et al. Selective suppression of rapid eye movement sleep increases next-day negative affect and amygdala responses to social exclusion. Sci. Rep. 2020, 10, 17325. [Google Scholar] [CrossRef]
- Rosales-Lagarde, A.; Armony, J.L.; Del Río-Portilla, Y.; Trejo-Martínez, D.; Conde, R.; Corsi-Cabrera, M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: An fMRI study. Front. Behav. Neurosci. 2012, 6, 23353. [Google Scholar] [CrossRef]
- Markam, P.S.; Bourguignon, C.; Zhu, L.; Ward, B.; Darvas, M.; Sabatini, P.V.; Kokoeva, M.V.; Giros, B.; Storch, K.-F. Mesolimbic dopamine neurons drive infradian rhythms in sleep-wake and heightened activity state. Sci. Adv. 2025, 11, eado9965. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, F.; Lucassen, E.A.; Houben, T.; vanderLeest, H.T.; Antle, M.C.; Meijer, J.H. Amplitude of the SCN clock enhanced by the behavioral activity rhythm. PLoS ONE 2012, 7, e39693. [Google Scholar] [CrossRef]
- Menon, V.; Adleman, N.E.; White, C.D.; Glover, G.H.; Reiss, A.L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 2001, 12, 131–143. [Google Scholar] [CrossRef]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage 2010, 50, 1313–1319. [Google Scholar] [CrossRef]
- Swick, D.; Ashley, V.; Turken, A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008, 9, 102. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.L.; Eichenbaum, H. Hippocampus as a memory map: Synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus 1999, 9, 365–384. [Google Scholar] [CrossRef]
- Chase, H.W.; Moses-Kolko, E.L.; Zevallos, C.; Wisner, K.L.; Phillips, M.L. Disrupted posterior cingulate-amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc. Cogn. Affect. Neurosci. 2014, 9, 1069–1075. [Google Scholar] [CrossRef]
- Ehlers, C.L. Social Zeitgebers and Biological Rhythms: A Unified Approach to Understanding the Etiology of Depression. Arch. Gen. Psychiatry 1988, 45, 948. [Google Scholar] [CrossRef] [PubMed]
- Grandin, L.D.; Alloy, L.B.; Abramson, L.Y. The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clin. Psychol. Rev. 2006, 26, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Yi, H.; Shin, K.; Shin, C. Development of the sleep quality scale. J. Sleep Res. 2006, 15, 309–316. [Google Scholar] [CrossRef]
- Akerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; ISBN 978-0-578-64400-4. [Google Scholar]
- Nieto-Castanon, A. Preparing fMRI Data for Statistical Analysis [Internet]. arXiv 2022, arXiv:2210.13564. [Google Scholar]
- Nieto-Castanon, A.; Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox: RRID SCR_009550, Release 22, 22nd ed.; Hilbert Press: Boston, MA, USA, 2022; Available online: https://www.hilbertpress.org/link-nieto-castanon2022 (accessed on 14 September 2024).
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.R.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K. Modeling Geometric Deformations in EPI Time Series. NeuroImage 2001, 13, 903–919. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A.; Ghosh, S. Artifact Detection Tools (ART), Version 7:11; NeuroImaging Tools and Resources Collaboratory: Cambridge, MA, USA, 2011.
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.-B.; Heather, J.D.; Frackowiak, R.S.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Wager, T.D.; Krishnan, A.; Rosch, K.S.; Seymour, K.E.; Nebel, M.B.; Mostofsky, S.H.; Nyalakanai, P.; Kiehl, K. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum. Brain Mapp. 2017, 38, 5331–5342. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Chai, X.J.; Castañón, A.N.; Öngür, D.; Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. NeuroImage 2012, 59, 1420–1428. [Google Scholar] [CrossRef]
- Worsley, K.J.; Marrett, S.; Neelin, P.; Vandal, A.C.; Friston, K.J.; Evans, A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996, 4, 58–73. [Google Scholar] [CrossRef]
| Demographics | |
|---|---|
| Age | Mean = 23, range = [18,19,20,21,22,23,24,25,26,27,28,29,30,31] |
| Biological Sex | n = 18, female = 11 |
| Race/ethnicity | White = 8 Hispanic = 8 African American = 1 Other = 1 |
| Education | High school graduate = 3 Some college = 8 Associate’s degree = 2 Bachelor’s degree = 4 Master’s degree = 1 |
| Variable | Mean (σ) |
|---|---|
| Sleep Onset | 11:10 PM (33.44 min) |
| Wake Onset | 06:56 AM (45.17 min) |
| Sleep Duration | 7 h 43 min (35.11 min) |
| Sleep Efficiency | 93.36% (8.59%) |
| Sleep Quality (SQS) | 23.28 (8.16) |
| Positive Affect (P.A.N.A.S.) | 28.22 (7.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negelspach, D.; Kennedy, K.E.R.; Huskey, A.; Cha, J.; Alkozei, A.; Killgore, W.D.S. Mapping the Neural Basis of Wake Onset Regularity and Its Effects on Sleep Quality and Positive Affect. Clocks & Sleep 2025, 7, 15. https://doi.org/10.3390/clockssleep7010015
Negelspach D, Kennedy KER, Huskey A, Cha J, Alkozei A, Killgore WDS. Mapping the Neural Basis of Wake Onset Regularity and Its Effects on Sleep Quality and Positive Affect. Clocks & Sleep. 2025; 7(1):15. https://doi.org/10.3390/clockssleep7010015
Chicago/Turabian StyleNegelspach, David, Kathryn E. R. Kennedy, Alisa Huskey, Jungwon Cha, Anna Alkozei, and William D. S. Killgore. 2025. "Mapping the Neural Basis of Wake Onset Regularity and Its Effects on Sleep Quality and Positive Affect" Clocks & Sleep 7, no. 1: 15. https://doi.org/10.3390/clockssleep7010015
APA StyleNegelspach, D., Kennedy, K. E. R., Huskey, A., Cha, J., Alkozei, A., & Killgore, W. D. S. (2025). Mapping the Neural Basis of Wake Onset Regularity and Its Effects on Sleep Quality and Positive Affect. Clocks & Sleep, 7(1), 15. https://doi.org/10.3390/clockssleep7010015






