Microbiota Composition and Probiotics Supplementations on Sleep Quality—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
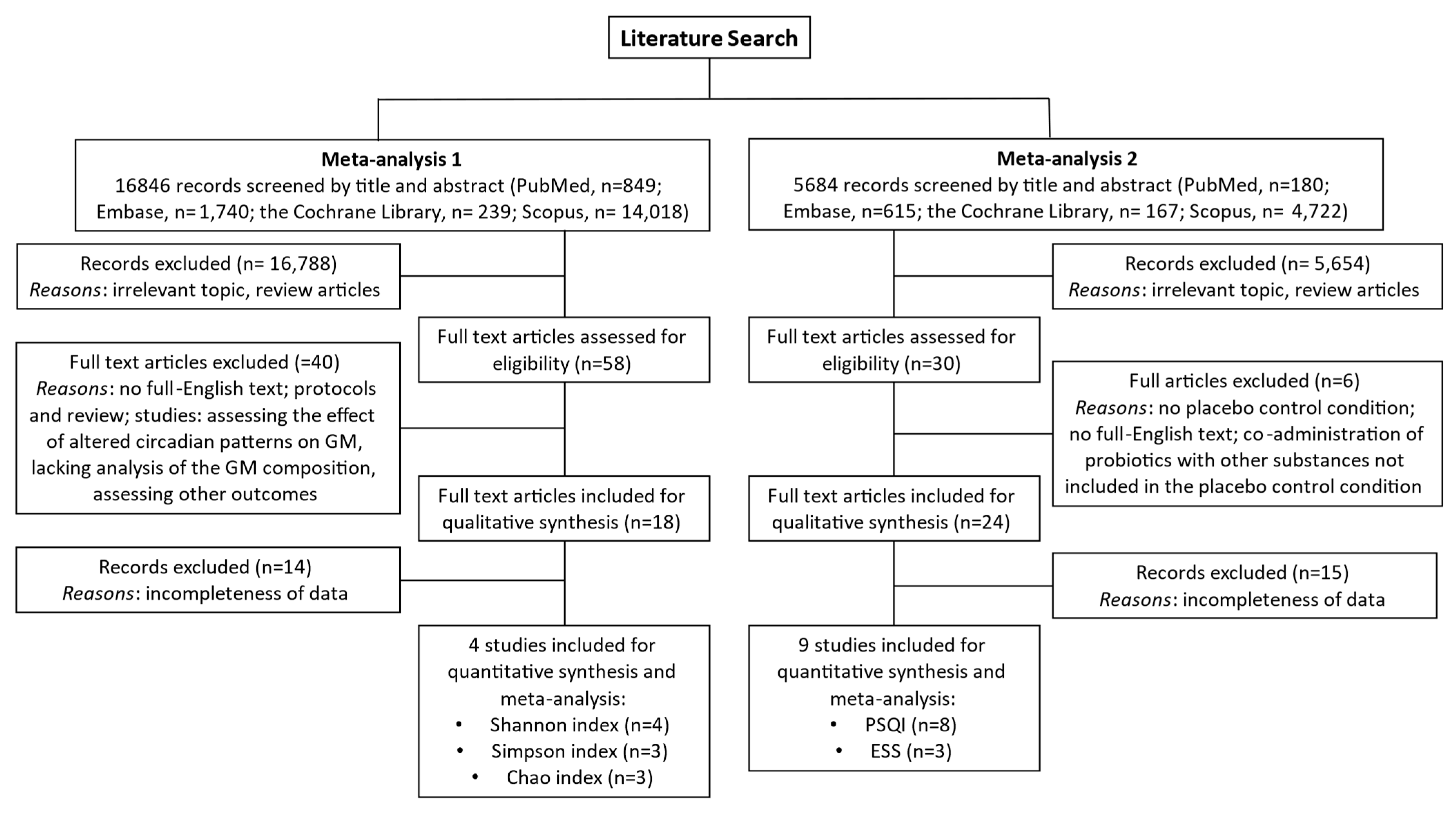
2. Materials and Methods
2.1. Study Selection and Inclusion Criteria
2.2. Data Extraction and Analysis
3. Results
3.1. Overview of Included Studies
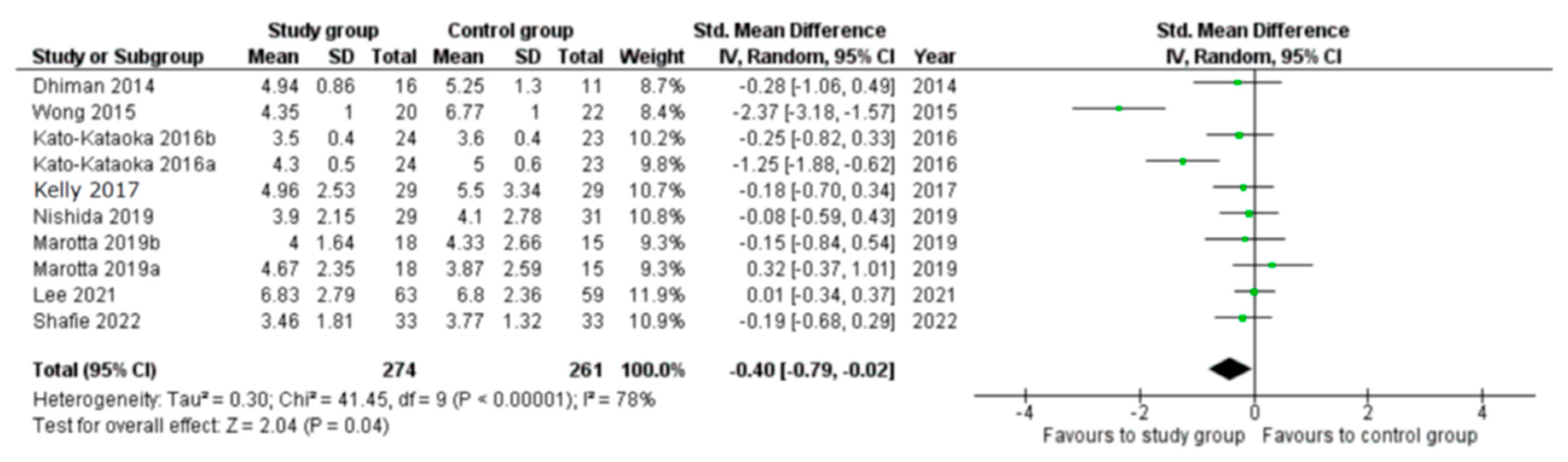
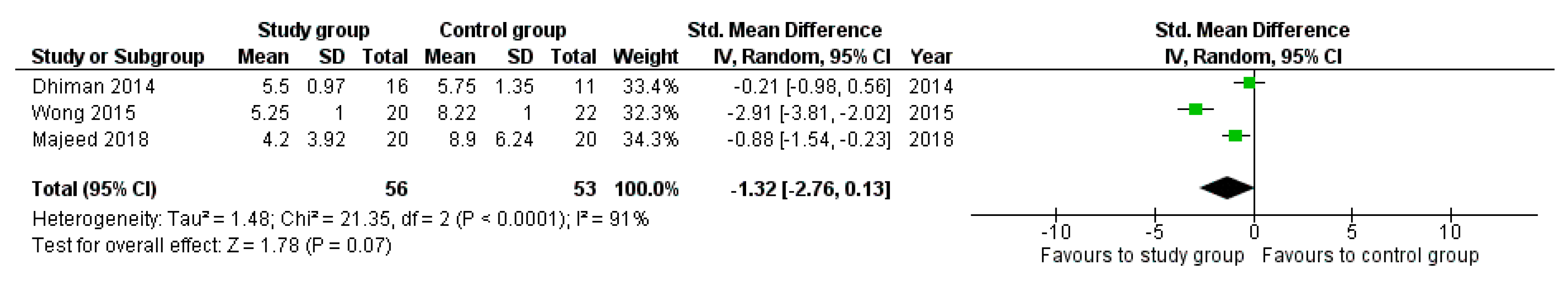
3.2. Meta-Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. (Landmark Ed.) 2021, 26, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, F.; Chen, J.; Chen, F.; Wu, Z.; Li, L.; Hou, K. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes: A systematic review. Front. Cell. Infect. Microbiol. 2022, 12, 1075201. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Eren, A.M.; Maignien, L.; Sul, W.J.; Murphy, L.G.; Grim, S.L.; Morrison, H.G.; Sogin, M.L. Oligotyping: Differentiating between closely related microbial taxa using 16S rRNA gene data. Methods Ecol. Evol. 2013, 4, 1111–1119. [Google Scholar] [CrossRef]
- Acinas, S.G.; Marcelino, L.A.; Klepac-Ceraj, V.; Polz, M.F. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J. Bacteriol. 2004, 186, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Marsh, T.L.; Cheng, H.; Forney, L.J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.R.; Stahl, D.A.; Lane, D.J.; Olsen, G.J. The Analysis of Natural Microbial Populations by Ribosomal RNA Sequences. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1986; Volume 9. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Tan, J.; Chen, Y.X. Dietary and Lifestyle Factors Associated with Colorectal Cancer Risk and Interactions with Microbiota: Fiber, Red or Processed Meat and Alcoholic Drinks. Gastrointest. Tumors 2016, 3, 17–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, C.; Zhang, A. Gut microbiota in acute leukemia: Current evidence and future directions. Front. Microbiol. 2022, 13, 1045497. [Google Scholar] [CrossRef] [PubMed]
- Matzaras, R.; Nikopoulou, A.; Protonotariou, E.; Christaki, E. Gut Microbiota Modulation and Prevention of Dysbiosis as an Alternative Approach to Antimicrobial Resistance: A Narrative Review. Yale J. Biol. Med. 2022, 95, 479–494. [Google Scholar]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Neroni, B.; Evangelisti, M.; Radocchia, G.; Di Nardo, G.; Pantanella, F.; Villa, M.P.; Schippa, S. Relationship between sleep disorders and gut dysbiosis: What affects what? Sleep Med. 2021, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. Iii. Sleep assessment methods. Monogr. Soc. Res. Child Dev. 2015, 80, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Markun, L.C.; Sampat, A. Clinician-Focused Overview and Developments in Polysomnography. Curr. Sleep Med. Rep. 2020, 6, 309–321. [Google Scholar] [CrossRef]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.X. Objective and subjective measures for sleep disorders. Neurosci. Bull. 2007, 23, 236–240. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Lok, R.; Zeitzer, J.M. Physiological correlates of the Epworth Sleepiness Scale reveal different dimensions of daytime sleepiness. Sleep Adv. 2021, 2, zpab008. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yuan, S.; Zhang, J. The interplay between sleep and gut microbiota. Brain Res. Bull. 2022, 180, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.; Romeo, M.; Casarini, L.; Granata, A.R.M.; Simoni, M.; Santi, D. Human fertility and sleep disturbances: A narrative review. Sleep Med. 2022, 98, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Riemann, B.L.; Flatt, A.A.; Valentino, T.; Lustgarten, M.S. Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: A pilot study. Sleep Med. 2020, 73, 76–81. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, October 2001. Available online: https://www.iqb.es/digestivo/pdfs/probioticos.pdf (accessed on 4 December 2023).
- Spaggiari, G.; Brigante, G.; De Vincentis, S.; Cattini, U.; Roli, L.; De Santis, M.C.; Baraldi, E.; Tagliavini, S.; Varani, M.; Trenti, T.; et al. Probiotics Ingestion Does Not Directly Affect Thyroid Hormonal Parameters in Hypothyroid Patients on Levothyroxine Treatment. Front. Endocrinol. 2017, 8, 316. [Google Scholar] [CrossRef]
- McKean, J.; Naug, H.; Nikbakht, E.; Amiet, B.; Colson, N. Probiotics and Subclinical Psychological Symptoms in Healthy Participants: A Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 2017, 23, 249–258. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Senok, A.C.; Ismaeel, A.Y.; Botta, G.A. Probiotics: Facts and myths. Clin. Microbiol. Infect. 2005, 11, 958–966. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Liu, Q.Q.; Su, H.Z.; Zhang, H.P.; Fan, J.M.; Yang, J.H.; Hu, A.K.; Liu, Y.Q.; Chou, D.; Zeng, Y.M. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: Disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 2019, 133, 905–917. [Google Scholar] [CrossRef]
- Collado, M.C.; Katila, M.K.; Vuorela, N.M.; Saarenpää-Heikkilä, O.; Salminen, S.; Isolauri, E. Dysbiosis in Snoring Children: An Interlink to Comorbidities? J. Pediatr. Gastroenterol. Nutr. 2019, 68, 272–277. [Google Scholar] [CrossRef]
- Zhang, Q.; Yun, Y.; An, H.; Zhao, W.; Ma, T.; Wang, Z.; Yang, F. Gut Microbiome Composition Associated with Major Depressive Disorder and Sleep Quality. Front. Psychiatry 2021, 12, 645045. [Google Scholar] [CrossRef]
- Fei, N.; Choo-Kang, C.; Reutrakul, S.; Crowley, S.J.; Rae, D.; Bedu-Addo, K.; Plange-Rhule, J.; Forrester, T.E.; Lambert, E.V.; Bovet, P.; et al. Gut microbiota alterations in response to sleep length among African-origin adults. PLoS ONE 2021, 16, e0255323. [Google Scholar] [CrossRef]
- Valentini, F.; Evangelisti, M.; Arpinelli, M.; Di Nardo, G.; Borro, M.; Simmaco, M.; Villa, M.P. Gut microbiota composition in children with obstructive sleep apnoea syndrome: A pilot study. Sleep Med. 2020, 76, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, S.; Wang, S.; Zhang, J.; Bai, Y.; He, S.; Zhao, P.; Zhang, H. Gut Microbiota in Patients with Type 1 Narcolepsy. Nat. Sci. Sleep 2021, 13, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- González-Mercado, V.J.; Lim, J.; Yu, G.; Penedo, F.; Pedro, E.; Bernabe, R.; Tirado-Gómez, M.; Aouizerat, B. Co-Occurrence of Symptoms and Gut Microbiota Composition Before Neoadjuvant Chemotherapy and Radiation Therapy for Rectal Cancer: A Proof of Concept. Biol. Res. Nurs. 2021, 23, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Ajami, N.J.; Malhotra, S.; Chen, L.; White, D.L.; Sharafkhaneh, A.; Hoffman, K.L.; Graham, D.Y.; El-Serag, H.B.; Petrosino, J.F.; et al. Habitual Sleep Duration and the Colonic Mucosa-Associated Gut Microbiota in Humans-A Pilot Study. Clocks Sleep 2021, 3, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The Gut Microbiota and Associated Metabolites Are Altered in Sleep Disorder of Children with Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.T.; Niu, J.Y.; He, C. Associations of OSAHS complicated by cerebral infarction with intestinal flora, inflammatory factors, homocysteine and adiponectin expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12993–12999. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Koga, N.; Hattori, K.; Ota, M.; Kunugi, H. Bifidobacterium and Lactobacillus Counts in the Gut Microbiota of Patients with Bipolar Disorder and Healthy Controls. Front. Psychiatry 2019, 18, 730. [Google Scholar] [CrossRef]
- Tang, S.S.; Liang, C.H.; Liu, Y.L.; Wei, W.; Deng, X.R.; Shi, X.Y.; Wang, L.M.; Zhang, L.J.; Yuan, H.J. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 2022, 28, 2320–2333. [Google Scholar] [CrossRef]
- Masyutina, A.A.; Gumenyuk, L.N.; Fatovenko, Y.V.; Sorokina, L.E.; Bayramova, S.S.; Alekseenko, A.I.; Shavrov, Y.V.; Romanova, A.A.; Seydametova, D.I. Changes in gut microbiota composition and their associations with cortisol, melatonin and interleukin 6 in patients with chronic insomnia. Bull. RSMU 2021, 2, 18–24. [Google Scholar] [CrossRef]
- Bikov, A.; Szabo, H.; Piroska, M.; Kunos, L.; Szily, M.; Ligeti, B.; Makra, N.; Szabo, D.; Tarnoki, D.L.; Tarnoki, A.D. Gut Microbiome in Patients with Obstructive Sleep Apnoea. J Appl. Sci. 2022, 12, 2007. [Google Scholar] [CrossRef]
- Cai, H.; Wang, C.; Qian, Y.; Zhang, S.; Zhang, C.; Zhao, W.; Zhang, T.; Zhang, B.; Chen, J.; Liu, S.; et al. Large-scale functional network connectivity mediate the associations of gut microbiota with sleep quality and executive functions. Hum. Brain Mapp. 2021, 42, 3088–3101. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.A.; Burke, L.A.; Calik, M.W.; Watanabe-Chailland, M.; Sweeney, D.; Romick-Rosendale, L.E.; Green, S.J.; Fink, A.M. Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats. Physiol. Genom. 2020, 52, 280–292. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- West, N.P.; Hughes, L.; Ramsey, R.; Zhang, P.; Martoni, C.J.; Leyer, G.J.; Cripps, A.W.; Cox, A.J. Probiotics, Anticipation Stress, and the Acute Immune Response to Night Shift. Front. Immunol. 2020, 11, 599547. [Google Scholar] [CrossRef]
- Marotta, A.; Sarno, E.; Del Casale, A.; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of Probiotics on Cognitive Reactivity, Mood, and Sleep Quality. Front. Psychiatry 2019, 10, 164. [Google Scholar] [CrossRef]
- Harnett, J.E.; Pyne, D.B.; McKune, A.J.; Penm, J.; Pumpa, K.L. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J. Sci. Med. Sport 2021, 24, 195–199. [Google Scholar] [CrossRef]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomized, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017, 123, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Moloney, G.M.; Long-Smith, C.M.; Murphy, A.; Dorland, D.; Hojabri, S.F.; Ramirez, L.O.; Marin, D.C.; Bastiaanssen, T.F.S.; Cusack, A.M.; Berding, K.; et al. Improvements in sleep indices during exam stress due to consumption of a. Brain Behav. Immun. Health 2021, 10, 100174. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Griffin, S.M.; Ibarra, A.; Ellsiepen, E.; Hellhammer, J. Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: A randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol. Stress 2020, 13, 100277. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes. 2017, 8, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Baião, R.; Capitão, L.P.; Higgins, C.; Browning, M.; Harmer, C.J.; Burnet, P.W.J. Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: A randomised, double-blind, placebo-controlled study. Psychol. Med. 2023, 53, 3437–3447. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented Milk Containing Lactobacillus casei Strain Shirota Preserves the Diversity of the Gut Microbiota and Relieves Abdominal Dysfunction in Healthy Medical Students Exposed to Academic Stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef]
- Nakakita, Y.; Tsuchimoto, N.; Takata, Y.; Nakamura, T. Effect of dietary heat-killed Lactobacillus brevis SBC8803 (SBL88™) on sleep: A non-randomised, double blind, placebo-controlled, and crossover pilot study. Benef. Microbes 2016, 7, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Daisuke, S.; Tomoko, K.; Kensei, N.; Yuki, K.; Shigeru, F.; Kazuhito, R. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J. Funct. Food 2017, 31, 188–197. [Google Scholar] [CrossRef]
- Yamamura, S.; Morishima, H.; Kumano-go, T.; Suganuma, N.; Matsumoto, H.; Adachi, H.; Sigedo, Y.; Mikami, A.; Kai, T.; Masuyama, A.; et al. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur. J. Clin. Nutr. 2009, 63, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Calandre, E.P.; Hidalgo-Tallon, J.; Molina-Barea, R.; Rico-Villademoros, F.; Molina-Hidalgo, C.; Garcia-Leiva, J.M.; Carrillo-Izquierdo, M.D.; Slim, M. The Probiotic VSL#3 Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2021, 14, 1063. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K.; Rana, B.; Agrawal, S.; Garg, A.; Chopra, M.; Thumburu, K.K.; Khattri, A.; Malhotra, S.; Duseja, A.; Chawla, Y.K. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: A randomized, controlled trial. Gastroenterology 2014, 147, 1327–1337.e1323. [Google Scholar] [CrossRef]
- Wong, R.K.; Yang, C.; Song, G.H.; Wong, J.; Ho, K.Y. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: A randomized double-blinded placebo study. Dig. Dis. Sci. 2015, 60, 186–194. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Shafie, M.; Homayouni Rad, A.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M. The effect of probiotics on mood and sleep quality in postmenopausal women: A triple-blind randomized controlled trial. Clin. Nutr. ESPEN 2022, 50, 15–23. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.K.; Kim, D.H.; Jang, S.W.; Han, S.W.; Yoon, I.Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef]
- Gil-Hernández, E.; Ruiz-González, C.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Rueda-Ruzafa, L.; Sánchez-Labraca, N.; Roman, P. Effect of gut microbiota modulation on sleep: A systematic review and meta-analysis of clinical trials. Nutr. Rev. 2023, 81, 1556–1570. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; Vitiello, M.V.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Sen, P.; Molinero-Perez, A.; O’Riordan, K.J.; McCafferty, C.P.; O’Halloran, K.D.; Cryan, J.F. Microbiota and sleep: Awakening the gut feeling. Trends Mol. Med. 2021, 27, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Guo, P.; Li, Y.; Liu, L.; Yan, R.; Liu, S.; Wang, S.; Xue, F.; Zhou, X.; Yuan, Z. Role of the Gut-Brain Axis in the Shared Genetic Etiology between Gastrointestinal Tract Diseases and Psychiatric Disorders: A Genome-Wide Pleiotropic Analysis. JAMA Psychiatry 2023, 80, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Raison, C.L.; Lowry, C.A.; Rook, G.A. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry 2010, 67, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Kinnucan, J.A.; Rubin, D.T.; Ali, T. Sleep and inflammatory bowel disease: Exploring the relationship between sleep disturbances and inflammation. Gastroenterol. Hepatol. 2013, 9, 718–727. [Google Scholar]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [PubMed]
- Haarhuis, J.E.; Kardinaal, A.; Kortman, G.A.M. Probiotics, prebiotics and postbiotics for better sleep quality: A narrative review. Benef. Microbes 2022, 13, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Irwin, C.; McCartney, D.; Desbrow, B.; Khalesi, S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1536–1549. [Google Scholar] [CrossRef]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
- Troncoso, C.; Petermann-Rocha, F.; Brown, R.; Leiva, A.M.; Martinez, M.A.; Diaz-Martinez, X.; Garrido-Mendez, A.; Poblete-Valderrama, F.; Iturra-Gonzalez, J.A.; Villagran, M.; et al. Patterns of healthy lifestyle behaviours in older adults: Findings from the Chilean National Health Survey 2009–2010. Exp. Gerontol. 2018, 113, 180–185. [Google Scholar] [CrossRef]
- Grandner, M.; Mullington, J.M.; Hashmi, S.D.; Redeker, N.S.; Watson, N.F.; Morgenthaler, T.I. Sleep Duration and Hypertension: Analysis of >700,000 Adults by Age and Sex. J. Clin. Sleep Med. 2018, 14, 1031–1039. [Google Scholar] [CrossRef]

| Common Features | Study Group (Altered) | Control Group (Healthy) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Type of Study | Microbiota Profiling Method | Microbiota Detected | Method (Sleep) | Inclusion Criteria | Number | Age (Years ± SD) | Relative Abundance | Number | Age (Years ± SD) | Relative Abundance |
| Evans | 2017 [49] | CCS | 16S rRNA amplicon sequencing (V4 region) using Illumina MiSeq | Phyla Bacteroidetes. Firmicutes. Verrucomicrobia. Actinobacteria. Genera Bacteroides. Facealibacterium. Prevotella. Roswburia. Akkermansia. Alistipes. Bifidobacterium. Parabacteroides. Blautia. Phascolarctobacterium. Alistipes | PSQI | BD | 115 | 50.2 ± 12.8 | Phylum: Faecalibacterium 5.1 ± 4.3% unclassified Firmicutes 0.6 ± 1% | 64 | 48.6 ± 16.6 | Phylum: Faecalibacterium 7.7 ± 5.0%; unclassified Firmicutes 1.1 ± 1.2% |
| Ko | 2019 [50] | CCS | 16S rRNA pyrosequencing (V3–V4 regions) using Illumina Miseq | Genera Bacteroides. Ruminococcus. Prevotella | PSG | AHI score > 5 health controls | 52 | NA | NA | 61 | NA | NA |
| Collado | 2019 [51] | CCS | 16S rRNA amplicon sequencing (V3–V4 region) using MiSeqIllumina protocols | Phylum Tenericutes. Firmicutes. TM7. Lentisphaerae. Fusobacteria. Proteobacteria. Verrucomicrobacteria. Actinobacteria. Bacteroidetes. Porphyromonadaceae. Peptospreptococcaceae and other clostriales | Snoring assessed by interview | Snoring frequency (< or ≥3/week) | 27 | 2.0 ± 0.0 | Proteobacteria 1.1% | 16 | 2.0 ± 0.0 | Proteobacteria 0.4% |
| Zhang | 2021 [52] | CCS | 16S rRNA amplicon sequencing (V4–V5 region) using qIllumina | Phyla Bacteroidetes. Firmicutes Orders Pasteurellales and Actinomycetes Families Bacteroidaceae. Prevotellaceae. Porphyromonadaceae. Rikenellaceae Genera Bacteroides. Prevotella. Parabacteroides. Escherichia. Flavonifractora. Alloprevotella. Parabacteroides. Hungatella | PSQI | MDD diagnosis | 36 | 36.81 ± 13.5 | GENUS: Bacteroides 40.0% Prevotella 5.9% Parabacteroides 2.8% Escherichia 2.5% Alistipes 2.2% Alloprevotella 0.5% Tyzzerella 0.3% Paraprevotella 0.2% Haemophilus 0.1% Flavonifractor 0.2% Anaerotruncus 0.1% | 45 | 39.29 ± 11.44 | GENUS: Bacteroides 25.0% Prevotella 24.3% Parabacteroides 1.7% Escherichia 0.7% Alistipes 0.8%. Alloprevotella 0.18% Tyzzerella 0.2%. Paraprevotella 0.2% Haemophilus 0.2% Flavonifractor 0.1% Anaerotruncus 0.1% Weissella 0.02. Eisenbergiella 0.01 |
| Fei | 2021 [53] | CCS | 16S rRNA pyrosequencing (V4 regions) using Illumina Miseq | Family Ruminococcaceae Erysipelotrichaceae Genera Bacteroides. Oscillospira. Catenibacterium. Prevotella. Dialister | Questionnaire | Sleep lenght (short ≤ 7 h. normal 7–9 h long ≥ 9 h) | Short 154 Long 248 | Short 35.6 ± 6.2 Long 33.6 ± 6.3 | GENUS: Streptococcus 0.7% Coprococcus 1.0% Dorea 0.3 Bamasiella 0.9% Intestinibacter 0.1% SPECIES: Blautia_obeum 0.7% Streptococcus_salivarius 0.7% Clostridium_sp 0.1% Dorea_formicigenerans 0.2% Coprococcus_sp 0.2% Ruminococcus_lactaris 0.7% | 250 | 35.7 ± 6.4 | NA |
| Valentini | 2020 [54] | CCS | 16S rRNA amplicon sequencing | Phyla Bacteroidetes Actinobacteria Firmicutes Bacteroidetes Proteobacteria Families Clostridiaceae Lactobacillaceae Lachnospiraceae Oscillospiraceae Erysipelotrichaceae Coriobacteriaceae Desulfovibrionaceae Enterobacteriaceae Erwiniaceae Enterobacteriaceae. Erwiniaceae Bacteroidaceae Prevotellaceae Lactobacillaceae Prevotellaceae Sutterellaceae Flavobacteriaceae Genera Colinsella. Eubacterium. Faecalibacterium Colinsella Escherichia Klebsiella Clostridium Lactobacillus Oscillobacter Clostridium Ruminococcus Oscillospira Veillonella Klebsiella | Sleep Clinical Record PSG | OSAHS | 7 | 5.0 ± 1.9 | NA | 8 | 8.7 ± 3.6 | NA |
| Zhang | 2021 [55] | CCS | 16S rRNA amplicon sequencing (V3–V4 region) using Illumina | Order Coriobacteriales Class Coriobacteria Family Barnesiellaceae. Genera Klebsiella. Barnesiella. Ruminiclostridium. Phocea. Blautia. Lactococcus. Bilophila | PSG ESS MSLT | NT1 | 20 | 19.0 | NA | 16 | 26.0 | LDA score: More abundant in HC individuals Class Coriobacteriia 2.26%. Order Coriobacteriales 2.26%. Family Barnesiellaceae 2.52%. Genus Lactococcus 2.37%. Genus Phocea 2.39%. Genus Ruminiclostridium 2.00%. Genus Barnesiella 2.46%. Genus Blautia 3.25%. Genus Bilophila 2.00% |
| Mercado | 2021 [56] | CCS | 16s rDNA amplificon sequencing (V3–V4 region) using the MiSeq Illumina | Ezakiella. Clostridium sensu stricto. Porphyromonas and Barnesiella (family Porphyromonadaceae). Coriobacteriales Incertae Sedis. Synergistaceae/Synergistales/Synergistia/Synergistestes. Escherichia-Shigella. Turicibacter | PROMIS-SD | PROMIS-SD. T-score > 55 | 19 high-occurring symptoms | 60.9 ± 16.0 | LDA score: More abundant in NT1 individuals Genus Klebsiella 3.19 | 22 low-occurring symptoms | 56.4 ± 7.9 | NA |
| Agrawal | 2021 [57] | CCS | 16S rRNA amplicon sequencing (V4 region) using Illumina MiSeq | Phyla Firmicutes. Bacteroidetes Order Rhodospirillales Families Acidaminococcaceae. Rikenellaceae. Sutterellaceae.Acidaminococcaceae. Rikenellaceae. Alcaligenaceae.Desulfovibrionaceae. Pseudomonadaceae. Pasteurellaceae Genera Lachnoclostridium. Sutterella. Bilophila. Phascolarctobacterium. Alistipes. Pseudomonas | Sleep length (self-reported) | Sleep length < 6 h short sleepers, 6–8 normal sleepers | 16 | 59.4 ± 7.5 | NA | 47 | 62.7 ± 5.8 | FIRMICUTES 40% BACTEROIDOTA 36% Lachnoclostridium 1.5% Sutterella 1.25% Alistipes 1.3% Bilophila 0.61% Phascolarctobacterium 0.5% UBA1819 0.13% Paraprevotella 0. 29% Pseudomonas 0.06% Eubacterium_siraeum 0.006% |
| Hua | 2020 [58] | CCS | 16S rRNA amplicon sequencing using Illumina MiSeq | Phyla Firmicutes. Actinobacteria. Bacteroidetes. Proteobacteria.Verrucomicrobia Genera Faecalibacterium. Agathobacter | CSHQ | CSHQ < 41 | 60 | 4.0 ± 0.2 | FIRMICUTES 34% BACTEROIDOTA 39%. Lachnoclostridium 0.40% Sutterella 0.38% Alistipes 0.48% Bilophila 0.25%. Phascolarctobacterium 0.20% UBA1819 0.03% Paraprevotella 0.11% Pseudomonas 0.08% Eubacterium_siraeum 0.13% | 60 | 3.9 ± 0.1 | Predominant phyla: Firmicutes 43.3%. Actinobacteria 28.3%. Bacteroidetes 20.7%. Proteobacteria 5.6%. Verrucomicrobia 1.3% |
| Buschart | 2018 [59] | CCS | 16S and 18S rRNA amplicon sequencing (V4 regions) using Illumina HiSeq | Families Corynobacteriaceae. Lachnospiraceae. Rumnococcaceae. Bacteroidaceae. Prevotellaceae. Porphyromonadaceae. Enterobacteriaceae. Phylobacteriaceae. Streptococcaceae. Comamonadaceae. Moraxellaceae. | ESS PSG | PD or iRBD | 97 (76 PD and 21 iRBD) | PD: 68.0 ± 9.7 iRBD: 66.1 ± 7.9 | Predominant phyla: Firmicutes 43.15% Actinobacteria 25.88% Bacteroidetes 22.57% Proteobacteria 6.34% Verrucomicrobia1.62% | 78 | 68.4 ± 6.7 | |
| Zhang | 2020 [60] | CCS | 16S rRNA gene sequencing (V4 region) | Phyla Actinobacteria. Proteobacteria. Firmicutes. Bacteroidetes Orders Coriobacteriales. Sphingobacteriales Genera Vagococcus. Adlercreutzia. Bifidobacterium. Parascardovia. Metascardovia. Ruminococcus Species Anaerostipes caccae | OSHAS | OSHAS/OSHAS + cerebral infarction diagnosis | Cerebral infarction group: 28 OSAHS + cerebral infarction group: 28 | NA | NA | 30 | NA | NA |
| Aizawa | 2019 [61] | CCS | 16S or 23S rRNA-targeted RT-qPCR | Genera Bifidobacterium. Lactobacillus | HAM-D subscale | BD | 39 | 40.3 ± 9.2 | NA | 58 | 43.1 ± 12.9 | NP |
| Tang | 2022 [62] | CCS | 16S rRNA gene sequencing (V3–V4 region) | Phyla Firmicutes. Proteobacteria Genera Escherichia-Shigella. Faecalibacterium. Streptococcus. Haemophilus. Phascolarctobacterium. Oscillibacter | AHI | OSHAS + T2DM | 27 | 47.6 ± 5.2 | NP | 26 | 45.6 ± 8.8 | NA |
| Masyutina | 2021 [63] | CCS | 16S rRNA gene sequencing | Phyla Actinobacteria Genera Faecalibacterium. Prevotella 9. Lachnospira. Blautia. Faecalibacterium. Lachnospira Species Eubacterium hallii | PSQI ISI | CI diagnosis | 55 | 31.6 ± 7.4 | NA | 50 | 33.2 ± 6.6 | NA |
| Grosicki | 2020 [38] | CCS | 16S rRNA gene sequencing (V3–V4 region) | Phyla Firmicutes. Bacteroidetes. Proteobacteria Classes Clostridia. Clostridia. Negativicutes Orders Clostridiales. Bacteroidales Families Bacteroidales. Lachnospiraceae. Ruminococcaceae Genera Blautia. Prevotella. Faecalibacterium. Ruminococcus. Bacteroides | PSQI | PSQI > 5 | 9 | 28.8 ± 10 | Euryachaeota 2.41 × 104 Actinobacteria 9.06 × 103 Bacteriodetes 3.16 × 101 Chloroflexi 1.47 × 106 Cyanobacteria 1.84 × 103 Elusimicrobia 1.66 × 104 Firmicutes 5.99 × 101 Fusobacteria 1.36 × 104 Lentisphaerae 6.21 × 105 Proteobacteria 4.19 × 102 Spirochaetes 7.35 × 107 Synergistetes 6.25 × 105 TM7 1.23 × 105 Tenericutes 9.26 × 104 Verrucomicrobia 3.10 × 102 Thermi 2.20 × 106 | 19 | 30.3 ± 10.8 | Phylum Firmicutes 38.0 ± 10.3 Bacteroidetes 34.6 ± 11.8 Proteobacteria 2.8 ± 1.8 Class Clostridia 32.0 ± 9.9 Bacteroidia 34.6 ± 11.8 Negativicutes 2.2 ± 1.5 Order Clostridiales 32.0 ± 9.9 Bacteroidales 34.6 ± 11.8 Family Bacteroidaceae 13.9 ± 9.3 Lachnospiraceae 9.6 ± 4.2 Ruminococcaceae 12.2 ± 5.5 Genus Blautia 2.2 ± 1.1 Prevotella 16.0 ± 19.1 Faecalibacterium 8.7 ± 4.2 Bacteroides 13.9 ± 9.3 Ruminococcus 2.3 ± 2.6 |
| Bikov | 2022 [64] | CCS | 16S rRNA gene sequencing (V3–V4 region) | Phyla Actinobacteria. Proteobacteria Class Gammaproteobacteria Families Prevotellaceae. Lactobacillae Genera Porphyromonas. Lachnosporaceae. Lactobacillus. Roseburia | PSG | OSAHS | 19 | 55 ± 12 | Phylum Firmicutes 38.0 ± 10.3 Bacteroidetes 34.6 ± 11.8 Proteobacteria 2.8 ± 1.8 Class Clostridia 32.0 ± 9.9 Bacteroidia 34.6 ± 11.8 Negativicutes 2.2 ± 1.5 Order Clostridiales 32.0 ± 9.9 Bacteroidales 34.6 ± 11.8 Family Bacteroidaceae 13.9 ± 9.3 Lachnospiraceae 9.6 ± 4.2 Ruminococcaceae 12.2 ± 5.5 Genus Blautia 2.2 ± 1.1 Prevotella 16.0 ± 19.1 Faecalibacterium 8.7 ± 4.2 Bacteroides 13.9 ± 9.3 Ruminococcus 2.3 ± 2.6 | 20 | 43 ± 16 | NA |
| Cai | 2021 [65] | rRNA gene sequencing | PSQI | Healthy controls | 157 | 22.3 ± 2.4 | NP | |||||
| Common Features | Study Group (Probiotic) | Control Group (Placebo) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Study Design | Microbiota Profiling Method | Sleep Assessment Method | Inclusion Criteria | Treatment Duration | Participants (n) | Age (y ± SD) | Intervention Treatment | Probiotic Bacteria | CFU | Participants (n) | Age (y ± SD) | Placebo Treatment |
| Nishida et al. [81] | 2017 | R/DB/PC/P | NP | PSQI EEG | Healthy 6th-year Japanese medical students | 12 weeks | 34 | 25.1 ± 2.37 | 200 mL Fermented Milk/Day | Heat—inactivated L. gasseri CP2305 | 1 × 1010 | 35 | 25.1 ± 2.4 | Milk: high-fructose corn syrup, powdered skim milk, lactic acid, soybean polysaccharide, pectin, sodium citrate, flavors, sweeteners |
| Kato-Kataoka et al. [82] | 2016 | R/DB/PC/P | NP | PSQI | Healthy 4th-year medical students undertaking an examination for promotion | 6 or 8 weeks | 24 | 23.0 ± 0.4 | 100 mL Fermented Milk/Day | L. casei strain Shirota | 1 × 109/mL | 23 | 22.7 ± 0.4 | Milk: similar composition with the addition of lactic acid |
| Nakakita et al. [83] | 2016 | NR/DB/PC/CO | NP | EEG, AIS, Sleep journals | Healthy males suffering from sleep challenges (AIS ≥ 6) | 10 days | 6 | 53.9 ± 8.8 | 1 × Capsule/Day | Heat-killed L. brevis SBC8803 | NS | 8 | 53.9 ± 8.8 | Capsules: caramel pigment, finely powdered silica, calcium stearate, starch, cellulose |
| Sawada et al. [84] | 2017 | R/DB/PC/CO | Gene expression analysis | PSQI | Male medical students undertaking the cadaver dissection course | 4 weeks | NS | 24 | 1 × Bag/Day | L. gasseri CP2305 | 1 × 1010 | NS | 24 | Lyophilized powder: skim milk (20%), yeast extract (0.50%) |
| Yamamura et al. [85] | 2009 | R/DB/PC/CO | NP | Actigraphy, SHRI | Healthy subjects | 3 weeks | 14 | 72.1 ± 21.2 | 100 g Fermented Milk/Day | L. helveticus | NS | 15 | 70.7 ± 21.9 | Artificially acidified milk added with L-lactic acid |
| Calandre et al. [86] | 2021 | R/DB/PC/P | NP | ISI | Fibromyalgia patients | 12 weeks | 28 | 56.0 ± 7.5 | 4 × Sachets/Day | S. thermophilus BT01 B. breve BB02 B. animalis subsp. lactis BL03 B. animalis subsp. lactis BI04 L. acidophilus BA05 L. plantarum BP06 L. paracasei BP07 L. helveticus BD08 | 4.5 × 1014 | 35 | 55.5 ± 8.6 | Sachets: maltose, cornstarch, silicon dioxide |
| Dhiman et al. [87] | 2014 | R/DB/PC/P | NP | PSQI, ESS | Cirrhosis patients | 24 weeks | 16 | 48.0 ± 1.4 | 1 × VSL#3 Sachet/Day | L. paracasei DSM 24733 L. plantarum DSM 24730 L acidophilus DSM 24735, L. delbrueckii bulgaricus DSM 24734) B. longum DSM 24736 B. infantis DSM 24737 B. breve DSM 24732 Streptococcus thermophilus DSM 24731 | 9 × 1011 | 11 | 50.1 ± 9.8 | Sachets: corn flour |
| Wong et al. [88] | 2015 | R/DB/PC/P | NP | PSQI, ESS | IBS | 6 weeks | 20 | 53.3 ± 18.6 | 8 Capsules/Day | L. acidophilus L. casei L. delbrueckii bulgaricus L. plantarum B. longum B. infantis B. breve Streptococcus salivarius thermophilus | 1.125 × 1011 | 22 | 40.9 ± 16.5 | Capsules: NS |
| Majeed et al. [89] | 2018 | R/DB/PC/P | NP | mESS | MDD and IBS | 90 days | 20 | 40.4 ± 10.3 | 1 × Tablet/Day | B. coagulans MTCC 5856 | 2 × 109 | 20 | 43.9 ± 9.8 | Tablets: microcrystalline cellulose, starch, sodium starch glycolate, magnesium stearate |
| Shafie et al. [90] | 2022 | R/DB/PC/P | NP | PSQI | Post-menopausal women | 6 weeks | 33 | 51.8 ± 2.3 | 100 g Yogurt/Day | B. lactis L. acidophilus | 1 × 108 C CFU/g | 33 | 52.4 ± 2.4 | Yogurt: containing L. bulgaricus and Streptococcus thermophilus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, D.; Debbi, V.; Costantino, F.; Spaggiari, G.; Simoni, M.; Greco, C.; Casarini, L. Microbiota Composition and Probiotics Supplementations on Sleep Quality—A Systematic Review and Meta-Analysis. Clocks & Sleep 2023, 5, 770-792. https://doi.org/10.3390/clockssleep5040050
Santi D, Debbi V, Costantino F, Spaggiari G, Simoni M, Greco C, Casarini L. Microbiota Composition and Probiotics Supplementations on Sleep Quality—A Systematic Review and Meta-Analysis. Clocks & Sleep. 2023; 5(4):770-792. https://doi.org/10.3390/clockssleep5040050
Chicago/Turabian StyleSanti, Daniele, Valentina Debbi, Francesco Costantino, Giorgia Spaggiari, Manuela Simoni, Carla Greco, and Livio Casarini. 2023. "Microbiota Composition and Probiotics Supplementations on Sleep Quality—A Systematic Review and Meta-Analysis" Clocks & Sleep 5, no. 4: 770-792. https://doi.org/10.3390/clockssleep5040050
APA StyleSanti, D., Debbi, V., Costantino, F., Spaggiari, G., Simoni, M., Greco, C., & Casarini, L. (2023). Microbiota Composition and Probiotics Supplementations on Sleep Quality—A Systematic Review and Meta-Analysis. Clocks & Sleep, 5(4), 770-792. https://doi.org/10.3390/clockssleep5040050








