Abstract
Phosphorus (P) is essential to life yet constrained by finite reserves, heterogeneous distribution, and strong chemical binding to soil minerals. Pedogenesis progressively alters the availability of P: in ‘young’ soils, P associated with Ca and Mg is relatively labile, while in ‘old’ soils, acidification and leaching deplete base cations, shifting P into organic matter and recalcitrant Al- and Fe-bound pools. Podzolized soils (Spodosols) provide a unique lens for studying this transition because podzolization vertically segregates these dynamics into distinct horizons. Organic cycling dominates the surface horizon, while downward translocation of Al, Fe, and humus creates a spodic horizon that immobilizes P through sorption and co-precipitation in amorphous organometal complexes. This spatial separation establishes two contrasting P pools—biologically dynamic surface P and mineral-stabilized deep P—that may be variably accessible to plants and microbes depending on depth, chemistry, and hydrology. We synthesize mechanisms of spodic P retention and liberation, including redox oscillations, ligand exchange, root exudation, and physical disturbance, and contrast these with strictly mineral-driven or biologically dominated systems. We further propose that podzols serve as natural experimental models for ecosystem aging, allowing researchers to explore how P cycling reorganizes as soils develop, how vertical stratification structures biotic strategies for nutrient acquisition, and how deep legacy P pools may be remobilized under environmental change. By framing podzols as a spatial analogue of long-term weathering, this paper identifies them as critical systems for advancing our understanding of nutrient limitation, biogeochemical cycling, and sustainable management of P in diverse ecosystems.
1. Introduction
Phosphorus (P) is essential to life but is finite, spatially heterogeneous, and chemically recalcitrant [1,2]. There is no way for organisms to readily return a gaseous form of P into the soil, as they might for carbon (C) or nitrogen (N) [1,3,4,5]. All biologically available P originates from mineral sources and, once taken up by organisms and returned to the ecosystem as residue or necromass, is increasingly sequestered into an organic P pool or lost from the system before reaching a point of ecosystem regression (Figure 1). In ‘young’ soils, a greater proportion of calcium (Ca) and magnesium (Mg) is present, soil pH is higher, and most P-containing compounds may be easily weathered via the production of organic acids [6,7]. In these settings, because P is relatively accessible, P may not limit productivity, though in particularly alkaline soils or those with a high fraction of Ca or Mg, P may still have low bioavailability [8,9]. As soils ‘age’, acidification and leaching deplete base cations (Ca, Mg), weathering releases reactive Al and Fe, and biological uptake stores P in organic residues [10,11]. Over centuries to millennia, these processes shift the dominant P pools from relatively labile forms to occluded mineral-bound P and biologically recycled organic phases [8]. Through the rest of this paper, we will use the terms ‘young’ and ‘old’ to refer to soils that are either yet to be acidified and weathered, in which substantial P is associated with Ca and Mg, or to soils that have been highly weathered and are today depleted in base cations, respectively.
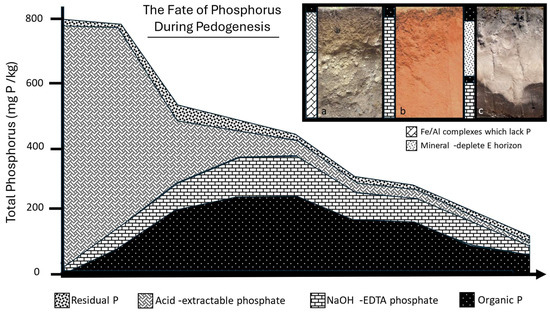
Figure 1.
Evidence demonstrating how P decreases in abundance and shifts to being dominated by more recalcitrant pools over time, taken from [12]. Inset: three different forms of soil, an Inceptisol (a), an oxisol (b), and a podzol (c). In the youngest soil (a), a large fraction of easily dissolved acid-extractable P is available, principally associated with Ca and Mg in soil. As soils age (b), the relative fraction of acid-extractable P decreases and is replaced by more recalcitrant organic and mineral P. In podzols, the formation of a deep soil layer separates the two major late-stage P sources (organic inputs; deeper recalcitrant pools of mineral and organic P) by up to a meter. This separation makes podzolized soils ideal study systems for disentangling late-stage ecosystem controls on soil P.
This turnover from young to old is a consequence of pedogenesis, fundamental to ecosystem succession, and deserving of study (Figure 1) [12]. Understanding how plants access P requires not only tracking total P pools but also disentangling the mechanisms by which pedogenesis shifts plant reliance from one source to another—such as from labile, base-cation-associated P in younger soils to strategies that mobilize P from organic matter or Fe/Al-dominated mineral pools in older, highly weathered systems.
Many highly weathered tropical soils, including Ultisols, Acrisols, Oxisols, and Plinthosols, are rich in Fe or Al, and in these systems P is likely to be complexed with Fe or Al rather than with Ca or Mg [13]. In some of these soils (the Acrisols, the Oxisols), even after weathering Fe and Al remain near surface horizons, where the cycling of P by plants overlaps spatially with other P sources, such as decomposition of new litter inputs. In other soils, like the Plinthosols, biogeochemical processes have transported Fe deeper in the soil profile [14]. However, while P will associate very strongly with this Fe at depth, the metal oxide layers present in Plinthosols may be difficult for roots to access [15], and redox-driven remobilization is limited because drying and re-wetting cycles tend to form stable minerals such as vivianite [16,17].
Podzols (spodosols, USDA, terms used interchangeably henceforth) differ fundamentally from either of these settings. Unlike in the canonical Oxisols, Acrisols, and Ultisols of the tropics, the podzolization necessary to create a podzol implies the translocation of Fe, Al, and organic matter downward, spatially separating recalcitrant P from surface nutrient inputs through the creation of a base-cation-deplete E horizon [18,19] (Figure 1c).
It is not simply the creation of the E horizon that makes a podzol unique, however—a similar process happens in the plinthosols, where re-wetting cycles lead to the precipitation of Fe-oxides at depth and the creation of a nutrient-depleted E horizon, much like in the podzol [14,16]. Importantly, in podzols, more than Fe has been transported to depth—cheluviation also transports humus to depth, creating a layer enriched in amorphous organometal complexes that is both a sink for P and, under the right conditions, partially accessible to roots [20,21]. This unique combination of spatial separation, organic enrichment, and metal complexation effectively creates a “second topsoil horizon”, setting podzols apart from other iron- or aluminum-rich systems and providing a distinctive environment in which plants can access multiple P pools. By creating this spatial decoupling, podzolized soils offer a unique opportunity to study how plants access different P pools and how the strategies for P acquisition shift across pedogenic stages. In a podzolized soil, new inputs of P not taken up or recycled near the surface continue downward until encountering the reactive Fe and Al phases in the spodic horizon [22].
Consequently, plant systems atop podzolized pools (Figure 2)potentially have access to two distinct P pools, which are spatially separated [23,24,25]. Moreover, the depth and composition of the spodic horizon each may vary across podzol systems [26]. In some soils, the P sink is shallow and chemically accessible [27]. In others, the spodic horizon lies well below the rooting zone or is enriched in exchangeable Al, rendering it toxic or inaccessible [20]. Some profiles allow for partial root penetration; others fully exclude biological contact [28]. This variation may allow researchers to probe how ecosystem structure and organismal strategy respond to gradients in P availability. The spatial arrangement imposed by podzolization could be a framework to evaluate how P availability shifts over time, how organisms respond to changing constraints, and how nutrient cycling is reorganized by long-term soil development. This paper focuses on how that structure arises, how P is retained, and what it means for biogeochemical cycling, ecosystem function, and nutrient management under changing environmental conditions.
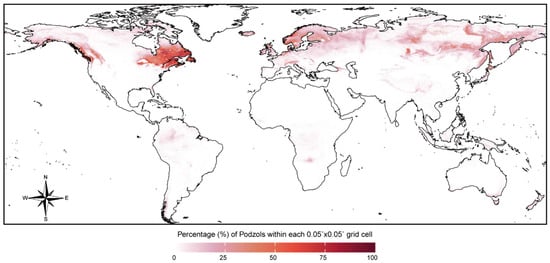
Figure 2.
The global distribution of podzols. Data sourced from the World Soil Information Service [29].
2. Podzolization as a Spatial Analogue of Ecosystem Aging
2.1. Life Begets Acid, Draws Down Parent P
In terrestrial ecosystems, the biological transfer of carbon from the atmosphere into biomass acidifies the soil. In alkaline systems, this acidification is required to mobilize P, which is largely bound in relatively insoluble Ca and Mg phosphates. Plants and microbes accelerate this process by exuding organic acids and protons into the rhizosphere, increasing base cation solubility and disrupting mineral structures [10]. Over time, this acidification promotes a largely unidirectional and irreversible loss of Ca, Mg, and associated P. As these elements are removed or sequestered in biomass, the buffering capacity of the soil declines, Al3+ and Fe3+ begin to dominate the exchange complex, and P released from litter or weathering is more likely to sorb to these reactive metals than remain in solution [25,30].
The shift from Ca and Mg to Al or Fe as the dominant cation pool reflects a fundamental progression in soil development [30]. While phosphate associated with Ca or Mg can also be insoluble, gradual acidification and seasonal weathering allow some P to remain accessible. Once Al- and Fe-bound phosphates form, however, there is no comparable natural process to liberate this P, and losses due to leaching and weathering accumulate [31,32,33,34]. As a result, ‘older’, highly weathered soils—typically those at lower latitudes or in regions with high rainfall and productivity—are doubly P-limited; substantial P has been lost to weathering, and the remainder is bound in increasingly insoluble and recalcitrant phases [10]. In these systems, plants primarily rely on decomposition to access P held in organic matter or on environmental events that temporarily release mineral P [35].
2.2. Podzolization as a Vertical Analogue for the Weathering Process
As soils acidify and base cations are leached (a feature of ecosystem ‘aging’), P progressively binds more and more often with Al and Fe, becoming increasingly recalcitrant [8,36]. In some systems, podzolized soils, this process does not occur uniformly but instead produces a pronounced vertical segregation of soil properties [28] and P dynamics [22]. The downward mobilization and immobilization of Al and Fe, complexed with organic matter, generates a spodic horizon where P is tightly bound to reactive minerals, while the overlying surface horizon is stripped of these metals, leaving only a thin organic layer dominated by biological P cycling through root exudates and litter decomposition. In this way, podzolization creates a natural “model system” in which two contrasting soils—one mineral-rich and metal-mediated, the other biologically dominated—coexist within the same profile, offering a unique framework for studying how pedogenic processes partition P across different soil compartments.
How do such podzolized soils arise? Lundström et al. (2000) defines podzolization as a three-phase process involving (1) mobilization of Al and Fe by complexation with dissolved organic ligands [18,37,38], (2) translocation of these complexes through the E horizon [39,40], and (3) immobilization and accumulation of these metals at depth [41,42,43].
Mobilization occurs primarily through the formation of water-soluble organometallic complexes between Al/Fe and low-molecular-weight organic acids (LMWOAs). These ligands form stable bidentate or tridentate chelates, allowing Al3+ and Fe3+ to remain in solution even at pH values below the solubility threshold for their respective hydroxides. The chelation process is highly pH-sensitive and becomes more energetically favorable as ecosystems age and acidic conditions dominate at the forest floor and upper mineral horizons.
Translocation proceeds as these complexes percolate downward through the E horizon. Chelation of the Al/Fe minerals with LMWOAs allows the complexed metal compounds to move downward, intensely weathering the soil—and in the process, stripping the resulting soil matrix of all but the most resistant minerals (e.g., silica). This generally moves at the pace (and range) of a water table; the downward movement of the water table is what carries the chelated complexes through the soil. The process removes most of the original mineral content, leaving behind the classic ash-white quartz E layer, which gives the podzol its name (the name Podzol is widely thought to be a translation of a Russian expression meaning soil ‘under-ash’, in reference to the ash-like E layer revealed during plowing).
Immobilization occurs when the organometallic complexes destabilize due to changes in the carbon-to-metal ratio or through microbial degradation of the organic ligands necessary to solubilize the metal. Briefly, as Al or Fe accumulates, the ratio of carbon to metal in these mobile organometal complexes declines. Below a critical threshold—typically a C:Al molar ratio of <10—the system becomes supersaturated, leading to the precipitation of amorphous gels or poorly crystalline hydroxides such as proto-imogolite or ferrihydrite [28,42]. The formation of imogolite-type materials can also happen as secondary, amorphous Al-Si products form during weathering and are immobilized in the spodic horizon [44,45]. Likewise, if heterotrophic microbial populations mineralize the organic components of the complex, the released Al3+ and Fe3+ precipitate due to their low solubility at ambient pH [46,47]. This process also results in the co-precipitation or sorption of phosphate, effectively removing it from solution and immobilizing it in the spodic matrix. In both cases, P is retained not by a single dominant mechanism but by a coupled sequence of complexation, transport, and precipitation. P may be either co-precipitated with destabilizing organometallic complexes or secondarily sorbed onto the newly formed Fe/Al (hydr)oxides.
The net result of these processes is the formation of a spodic horizon—an accumulation zone enriched in organic matter, Al, Fe, and P. Its formation requires (1) sufficient organic acid input, (2) a leaching-dominated hydrologic regime, and (3) a parent material low in buffering minerals. These conditions create a stratified soil profile in which reactive P pools are concentrated at depth, spatially separating them from the zone of biological demand. Podzols with a spodic horizon are called Spodosols (Figure 1c). Importantly, this process is distinct from the redistribution of iron oxides present in soil systems like the plinthosols due to the active role of organic compounds as chelating agents, which improve the solubility of metals. The fact that organic matter chelation is necessary to translocate Fe and Al means that the resulting Spodosol maintains a relatively more amorphous organometal layer [48,49,50]. While the pedogenesis of plinthosols also produces a depleted E horizon and consequently a stratified profile where sesquioxides have been depleted from the surface soil and accumulated at depth, the deep soil layer is humus-poor and consequently much less reactive in a Spodosol [29,51]. During the process of plinthization, repeating dry–wet cycles lead to gradual crystallization of sesquioxides and the formation of iron pans that are both (a) inaccessible to roots and (b) redox stable. In a Spodosol, however, the deep soil layer may act as a sink for P, which can still be accessed by roots and from which periodic shifts in redox chemistry may allow for the remobilization of free, dissolved P. The importance of the amorphous nature of the spodic layer is described further below.
3. Biogeochemistry of the Spodic Horizon: P Retention
The spodic horizon functions as a long-term sink for P that escapes biological retention at the surface. Unlike the organic horizon, which is shaped by recycling and active uptake, the spodic horizon is shaped by sorption, co-precipitation, and the physical entrapment of P within amorphous mineral phases. Once P reaches this zone, it is stabilized in forms that resist leaching, microbial access, and enzymatic breakdown.
3.1. Sorption to Fe and Al Oxides
Under the acidic conditions that typify spodic horizons (pH ~4–5), phosphate is retained primarily through ligand exchange reactions with the hydroxylated surfaces of Fe and Al (hydr)oxides [31,52]. This exchange is dominated by sorption to abundant short-range-order iron and aluminum oxides—particularly amorphous ferrihydrite and proto-imogolite-type materials, leached and re-precipitated Fe and Al, which accumulate during podzolization and carry a strong positive surface charge at low pH [53,54]. Phosphate anions form inner-sphere complexes with exposed hydroxyl groups, displacing water or weaker ligands. The surface area and reactivity of these amorphous phases are far greater than those of their crystalline counterparts. As a result, spodic horizons rich in ferrihydrite or Al-humus complexes have substantial P sorption capacity [55,56].
3.2. Co-Precipitation During Collapse of the Organometal Complex
Part of the reason that podzolized soils have a high proportion of amorphous organometals relative to other, similar soils with deep Fe-rich subsoil layers (the plinthosol) is because chelation of Al and Fe complexes by LMWOAs is necessary for the translocation of Fe and Al from the surface through the E horizon to stabilize at depth. The precipitation of Al- and Fe-bearing phases leads to the retention of P either directly (as Fe- or Al-phosphate) or indirectly (via sorption of P to newly formed surfaces). The co-precipitation of phosphate with Al and Fe results in its physical occlusion within amorphous or colloidal matrices. This may include imogolite-like materials or hydroxy-Al silicates formed in the presence of dissolved silicate from weathered parent material [45,47]. These gels can trap phosphate in both adsorbed and structurally integrated forms.
The extent to which phosphate is sorbed versus co-precipitated likely varies by the degree of solution saturation and the timing of P presence during precipitation. As described, the carbon-rich nature of these complexes limits the crystallinity of the resulting minerals [21]. Conventionally, late-stage soils will gradually become dominated by more crystalline minerals, meaning that P in said settings is either freed (and later lost) or more permanently retained within a crystalline mineral. In the spodic horizon, despite the extreme age of some spodic systems, the major phosphorus sink remains predominantly amorphous (due to the large accumulation of organic matter, which keeps minerals in a thick amorphous layer rather than in smaller crystalline chunks, as can be seen in plinthized soils where well-defined mottles of ‘plinthite’ may be identified, or pisoliths may be formed). Importantly, that amorphous layer’s capacity to ‘stick’ P on the surface is more tightly bound to changes in the local environment than in a more crystalline setting [57]. Specifically, while an oxic spodic layer will retain P, that P may be vulnerable to remobilization under certain redox or pH-altering conditions (Section 4). Conversely, in humus-poor subsoils, such as in the Plinthosols, iron is generally more redox stable, and there is less opportunity for shifts in redox chemistry to release P into solution [17].
3.3. Implications for Vertical P Flux
The E horizon in podzolized soils has low cation exchange capacity and limited sorptive potential. As a result, surface inputs (manure, fertilizer, organic residues) leach rapidly through the E horizon and are intercepted only upon reaching the spodic layer [22,56,58]. This explains the deep vertical redistribution of P in Spodosols, particularly in managed systems, and is key to understanding how spodic horizons may act as cryptic reservoirs of legacy P [22,59]. In natural systems, this separation may have evolutionary consequences, as plant communities adapt to select species or microbial consortia capable of accessing deep P pools through fine root proliferation, exudate production, or fungal symbiosis.
While not every spodic layer is necessarily enriched in P [27], in fertilized or manured systems, the nature of podzolization and lack of P-retaining surfaces in the E horizon can result in deep P accumulation beyond the rooting zone (Figure 3), forming a legacy pool that is not easily measured using standard agronomic techniques and may be variably accessible to plants (see Section 7). Importantly, the depth and nature of the Spodosol can vary greatly depending on the local climate, parent soil, and level of biotic activity, meaning that a series of Spodosols spanning a very small geographic area may still provide natural gradients where these two pools are close, separate, or so far vertically stratified as to rarely ever interact. This fact means Spodosols may represent unique study systems, a concept expanded upon in Section 6.
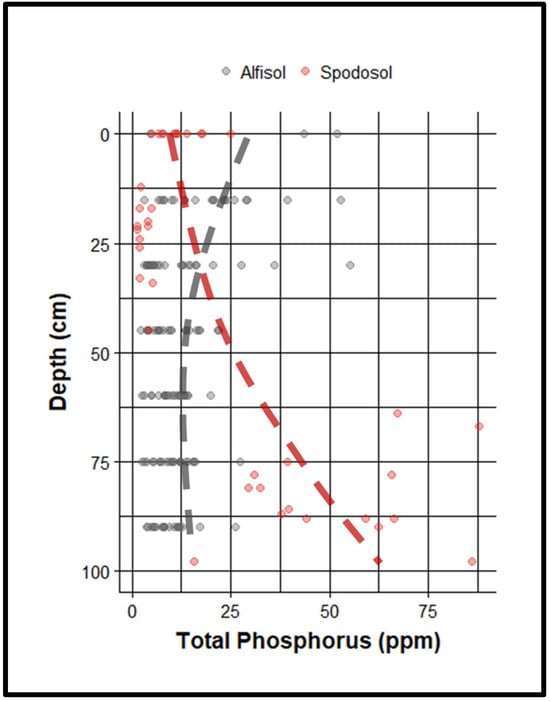
Figure 3.
Depth profile of phosphorus content in two co-occurring soil types, an Alfisol and a Spodosol (a podzol with a pronounced spodic layer). A line of best fit is presented for both, the Alfisol in red and the Spodosol in gray. Note the increase in P with depth in the Spodosol, particularly near the Bh. Comparatively, P is highest in the shallow soils of the Alfisol and drops off precipitously as soil depth increases.
4. Mechanisms of P Liberation from the Spodic Horizon
Considerable discussion above has referenced the remobilization of P from the spodic horizon. These release pathways are often pulse-like and may be triggered by disturbance or episodic environmental change, each described below.
4.1. Redox
Although bonds between P, Al, and Fe are generally irreversible under oxic conditions, redox oscillations or biological mediation may lower the energy necessary to recover P from the ‘kinetic trap’ [60]. The most well-established abiotic mechanism likely to explain P release from spodic horizons is the reductive dissolution of iron oxides [61]. In certain podzols, where the spodic horizon may be completely saturated during water table rise or seasonal flooding, oxygen becomes depleted and redox potential declines [55]. A series of microbes upregulate the use of a succession of electron acceptors for respiration; at an Eh < 100 mV, Fe (III) is reduced to soluble Fe (II), releasing phosphate:
FePO4(s) + 3H+ + e− → Fe2+ + H2PO4−
It has been established that the shift to anaerobic microbial respiration will free P associated with iron oxide when iron is reduced from Fe3+ to Fe2+ [62]. Lab experiments have demonstrated the P retention capacity of some spodic horizons is reduced under anaerobic conditions, suggesting that flooding likely does release P from the spodic horizon into solution in the field [55]. Experiments leveraging suction lysimeters in-field provide evidence that seasonal flooding may release P from the surface of the spodic layer and ‘lift’ this nutrient toward the surface and the rooting zone [63]; however, some podzols remain sinks when flooded or even become stronger sinks for P in some cases [55,62]. The exact mechanism of release in the field remains unclear—the roles of competing cations, changing soil water pH, and other factors are difficult to disentangle.
However, we can make some reasonable assumptions: Bh horizons dominated by amorphous Fe phases or whose capacity to retain dissolved P has been saturated via historical fertilization are probably more likely to release P during shifts in redox. In contrast, in spodic layers with lower iron content, or where P has less capacity to bond to redox-sensitive amorphous surfaces, flooding will do little to lower the energy necessary to escape the ‘trap’. It is also important to consider that the form and stability of the organic matter contained within the spodic layer is likely to influence how easily P can be released during periods of shifting redox conditions. For example, if organic matter is adsorbed onto Fe surfaces, microbial reduction may be slowed, because adsorption “passivates” the mineral surface and prevents microbes from accessing reactive sites. In contrast, when organic matter is co-precipitated with Fe, such as during podzolization, the disruption of crystallinity exposes reactive sites (this is how the amorphous organometal complex is formed and explains its higher reactivity than more crystalline counterparts, as described earlier) [64]. Similarly, forms of organic matter that are more resistant to oxidation are less likely to be degraded during shifts to an anaerobic environment, meaning fewer free electrons to reduce Fe and consequently liberate P. There are complex interactions between Fe, Al, and organic matter content, which drive the relative accessibility of organic matter to be mineralized. Studies on the stability of soil organic carbon would suggest that large amounts of Fe (oxyhydr)oxides with low aluminous clay contents push systems towards more stable carbon reserves, which in this case may also mean P stability in the organometal complex is less sensitive to redox.
Moreover, it is necessary to consider the fate of P once it has been freed, as there is the possibility that P may be intercepted and re-precipitate before reaching the rooting zone and/or being immobilized in organic matter. A high proportion of free Fe (III) surfaces may lead to the immediate re-precipitation of any freed P, a phenomenon sometimes referred to as the ‘iron curtain’ [65]. Liberated phosphate may also subsequently resorb to Al phases or precipitate as vivianite, processes contingent on carbon-to-metal ratios and organic matter complexity [66,67]. Here too, the Spodosol is unique because the E horizon is almost definitionally depleted in these mineral surfaces, meaning freed P may be transported up to the rooting zone with less risk of re-precipitation than may be present in a more homogeneously distributed iron environment, as may be found in an oxisol, Acrisol, or Ultisol.
4.2. Ligand Exchange and Organic Complexation
Biological mechanisms may also liberate P from spodic material. Many plants, particularly in acidic, P-limited environments, exude organic acids such as citrate, malate, or oxalate that chelate Fe and Al, liberating sorbed phosphate via ligand exchange or complexation [68,69,70,71]. Plants may also recruit P-solubilizing microbes that produce siderophores—strong Fe-binding compounds—that can similarly mobilize phosphate by disrupting Fe-P complexes [71,72]. This mechanism is slower than reductive dissolution but may provide gradual continuous P supply—especially in podzols where the spodic horizon lies within reach of the rhizosphere.
Ligand-mediated mobilization is strongly influenced by infiltration dynamics, vegetation type, and the distribution of biological activity. In podzols with shallow or fragmented spodic horizons, roots may access the upper boundary of the P sink and introduce organic ligands directly. In deeper profiles, ligand delivery may be limited to dissolved inputs during storm events or high-flow periods.
4.3. Mycorrhizal and Root Mining of the Spodic Horizon
Rooting into the spodic horizon can be difficult depending on depth and Al content (which can be toxic), but roots are not uncommon. In nutrient-poor systems, particularly those under moisture stress, plant roots may extend into the Bh horizon to access both water and P. Likewise, microbes and roots can each produce enzymes, phosphatases, which hydrolyze organic P compounds and increase the pool of available inorganic phosphate [73,74,75]. Arbuscular mycorrhizal fungi (AMF) extend their hyphae deep into the soil matrix and have been observed colonizing spodic material in sandy, drought-prone regions [76]. However, as spodic horizons become more intensely compacted with time, and the available pore space diminishes, it is possible mycorrhizae and other fungi may become less able to penetrate the spodic, giving way to a more bacteria-dominant microbial community [77].
A combination of root exudates and microbial associations may be necessary to effectively bridge the vertical gap between roots and spodic P, but very little work has been done in this area, and none of it recently [24,78,79]. One interesting area for future research would be if the improved nutrient retention capacity of silvopasture [80] can be explained by a more efficient P-cycling system that includes deep tree roots, which may access the spodic.
4.4. Physical Disruption and Profile Disturbance
P release can also result from structural changes to the soil profile. Root penetration, bioturbation, tillage, or drainage modification may expose spodic material to oxygen, disrupt redox buffering, or alter water flow paths [81]. These changes can shift the chemical conditions within the spodic horizon or increase hydrologic connectivity with surrounding horizons—leading to P export through vertical leaching or lateral flow. Sometimes this happens naturally, such as when trees are torn up and out of the ground in flat, windblown landscapes. Tree throw physically moves the enriched spodic horizon to the surface, where it will begin degrading, restoring P [81]. In this, tree throw is not unlike upwelling, which similarly restores P-saturated reserves to the surface. Likewise, tilling is a regular management recommendation in spodic landscapes, with the intent to recycle inputs from the spodic horizon back into the topsoil [21]. This can be useful for improving soil fertility.
5. Recycling and Competition: Organic P Cycling in the Surface Horizon
In podzolized soils, mineralization, uptake, and immobilization by organisms in the surface horizon operate largely independent of mineral sorption. Reactive Fe and Al phases have been leached downward, leaving the surface horizon decoupled from the geochemical buffering common in other soil types. As a result, P cycling in the organic horizon is dominated by biological processes and tight internal recycling. Any P not retained by biotic demand is subject to vertical loss.
The Potential for a Vertical Stratification of Organic P in Podzols
In older, highly weathered ecosystems, the surface organic horizon takes the form of inositol phosphates (e.g., phytate), nucleic acids, and phospholipids [82]. Organic P can represent over 50% of the total P in tropical forest floor horizons [82]. This organic P pool is cycled through enzymatic mineralization and microbial uptake. As soils age, leaves tend to increase in C:P ratio, leading to slower decomposition meant to increase the efficiency by which plant litter is recycled without loss to mineral pools [83]. As with mineral P, organic P exists in chemically distinct pools that differ in their biological availability and in their affinity for soil minerals. These differences likely govern the extent to which each compound class is retained near the surface, leached through the profile, or sequestered at depth. One theoretical consideration introduced by the concepts described above is that podzolization likely induces separation of the forms of organic P much the same as it does soil minerals and total organic matter. Studies of organic P compounds in other systems provide a loose framework that may describe how organic P could become vertically stratified.
For example, phosphate monoesters—including nucleotide monophosphates and sugar phosphates—are weakly sorbed and readily hydrolyzed. These compounds dominate the biologically available organic P pool and are likely to be rapidly cycled in the surface organic horizon. Due to their weak association with soil minerals and relatively fast enzymatic turnover, such monoesters are unlikely to be stabilized at depth and likely do not accumulate in spodic horizons.
Phosphate diesters, such as nucleic acids and phospholipids, degrade more slowly and often undergo partial stabilization within microbial necromass or humic substances. Diesters may increase in relative abundance in older soils because they become protected within soil organic matrices [12]. In podzols, where the E horizon is highly leached and microbial activity is low, these compounds may bypass the surface enzymatic filter and enter deeper horizons in dissolved or colloidal form. Once in the spodic horizon, they may be retained physically (e.g., through encapsulation) or slowly mineralized.
Inositol phosphates, particularly myo-inositol hexaphosphate (phytate), exhibit the strongest interaction with mineral phases. These compounds bind readily to Fe and Al (hydr)oxides and accumulate in soils with high oxide content. Phytate abundance tends to peak in soils with abundant amorphous Fe and Al phases and declines in older soils as these phases recrystallize. In podzolized soils, the spodic horizon is the only part of the profile with sufficient reactive mineral surfaces to stabilize phytate. It follows that inositol phosphates may undergo preferential downward migration through the E horizon, accumulating at depth because their high sorption affinity causes them to be selectively removed from solution upon encountering the Fe- and Al-rich spodic matrix.
This compound-specific sorting is likely to result in vertical partitioning of various organic P pools, which may be useful for the design of experiments. Labile monoesters may be rapidly mineralized and incorporated into allochthonous biomass. Diesters exist in a transitional state—partially decomposed or occluded—and may be retained near the surface or slowly lost. Inositol phosphates, by contrast, are not decomposed near the surface and instead accumulate at depth, where they are stabilized by mineral sorption. As a consequence, the organic P pool may itself be stratified, with each class of compound occupying a characteristic depth range defined by its chemical properties and interactions with soil mineralogy and enzymatic activity. Such a vertical profile has not yet been characterized, but the podzol system would provide a novel experimental opportunity for such work.
Importantly, the surface horizon is also an important opportunity to ‘catch’ new P inputs or to retain dissolved P in rainfall. In ecosystems with a well-developed O horizon, the retention and recycling of P within this layer can significantly reduce P leaching to deeper horizons [84,85]. However, the E horizon below has zero capacity to retain migrating P, so when this system is overwhelmed because P inputs exceed plant uptake capacity, the remainder of labile P is immediately leached [86]. P is made inaccessible to the plant without either abiotic shifts in redox or substantial secondary metabolic investment (increased root depth, root production of exudates, symbioses with microbes).
Field evidence suggests the distribution of inorganic and organic P through the podzol can be strongly mediated by the structure of preferential flow paths. In podzolized till soils, stone-surface flow paths tended to flush more labile forms of inorganic and organic P (pyrophosphate, diesters, and non-phytate monoesters), while coarse-grain and root-associated flow paths retained or accumulated more stable forms, including phytate [87]. Thus, the heterogeneity of the surface horizon—whether water moves along stones, through coarse material, or along root channels—directly governs the composition of P transported to depth and the extent to which it is subsequently stabilized in spodic horizons.
6. Podzols as Useful Experimental Models Where Surface P Cycles Independently from Mineral Al/Fe Reserves
What makes podzolized systems particularly analytically powerful is their variability. Across a landscape or along a chronosequence, the depth, continuity, and accessibility of the spodic horizon markedly differ. In some settings, roots may extend into the upper margin of the spodic horizon, allowing partial access to mineral-bound P. In others, the spodic is deep, compacted, or seasonally saturated, effectively excluding biological inputs. This variability creates a natural gradient that can be leveraged as an experimental framework.
At the same time, this dichotomy is not absolute. Minerals persist in surface horizons, and biological activity is detectable in spodic layers. For example, oxic surface soils often host microbial siderophore producers capable of liberating P from Fe(III), even in the absence of strong mineral sinks. However, these horizons rarely experience prolonged anoxia, meaning that large redox-driven shifts in Fe and P availability are uncommon compared to deeper horizons. Likewise, spodic horizons are almost certainly inhabited by microbial communities, but biological cycling there is constrained by the recalcitrant nature of the organic matter and the strong organometal associations that dominate. As a result, the stability and mobility of P at depth are more likely governed by environmental fluctuations (e.g., hydrology, redox, saturation) than by more biotic processes.
Taken together, a series of podzols offers a natural experiment for understanding how P acquisition strategies respond to variation in subsoil proximity. Across this gradient, trade-offs emerge between recycling and uptake. Organisms must weigh sustained biological effort in the surface horizon against reliance on sporadic environmental events to access recalcitrant P retained at depth. This variation allows for controlled comparisons: How do the structure and function of the rhizosphere shift when the geochemical sink is near versus when it is out of reach? How do plants and microbes adjust their investment in phosphatase production, root exudates, or mycorrhizal associations depending on their access to the deeper pool?
Podzols therefore offer opportunities for controlled comparisons. For example:
Biological investment vs. geochemical access: How do plants and microbes balance the costs of sustained recycling in the surface horizon with episodic opportunities to access recalcitrant P at depth?
Trait-based responses: Do phosphatase activity, root exudation, or mycorrhizal associations increase when the spodic horizon is inaccessible and diminish when that more mineral subsidy is shallower or otherwise nearer to the rhizosphere?
Comparative sampling designs: Studies could explicitly contrast sites where spodic horizons are shallow versus deep or employ chronosequences where spodic development intensifies with time.
Analytical tools: Potential approaches include enzyme assays, isotopic tracers (e.g., 33P as short-term labels, or potentially the use of labeled oxygen in phosphate), lysimeter-based monitoring of P leaching, and non-invasive methods (e.g., geophysical imaging) to constrain horizon depth and structure.
By framing podzols not just as “unique soils,” but as natural experiments in which biological and mineral P cycles are spatially decoupled, we highlight their value as tractable systems for addressing fundamental questions in phosphorus biogeochemistry.
7. Considerations on Podzols as Reservoirs of Soil Fertility or Sources of Pollutant P
The spodic horizon acts as a terminus for P that escapes surface recycling, whether from natural inputs or anthropogenic sources. The capacity of a deep spodic horizon to immobilize P through sorption, co-precipitation, and organometal complexation makes it an effective long-term sink. In a world where legacy reservoirs of historic P inputs are increasingly relied upon for agriculture [88], consideration of deep spodic pools may be an under-considered asset [89,90]. Moreover, the redox-sensitive nature of this sink suggests spodic horizons should be considered in landscape nutrient budgets, as circumstances exist where the P-sink of the spodic becomes a source of nutrient pollution.
7.1. Natural P Reserves
In natural systems, the spodic horizon represents a slow-forming P sink. The 9m thick podzols of Northern Carolina and the ‘giant’ podzols of the Amazon must have taken millennia to accrue naturally and represent substantial stocks of C and possibly P [21,91]. Because of the poor retention capacity of the O and E horizons, P from surface inputs that bypass the root zone are likely to accumulate at depth [56,92]. Consequently, podzols with P-saturated spodic horizons could represent cryptic ‘islands of fertility’ in well-weathered landscapes. Clever hydrological or biological solutions that leverage existing P on-site would greatly alleviate the P cost of agriculture at low latitudes [89,90]. Pools of recalcitrant organic matter that sequester and retain P could provide substantial improvements to agriculture in these ecosystems. The ability to access the reservoir of resources associated with a ‘loaded’ Spodosol would improve fertility much as indigenous people leverage terra preta (Amazonian dark earth) or as we may today use biochar [93].
Moreover, further research into nutrient dynamics within tropical forests atop Spodosols may provide insight into how P is regulated at scale in the most nutrient-limited and highly productive ecosystems in the world. As described, the spodic horizon provides a unique natural laboratory to separate the role of litter recycling near the surface from deeper soil, microbe, mineral, and nutrient interactions at depth.
7.2. Cryptic Contribution to Global Climate Crises
Because podzols are definitionally older, more heavily weathered, and less fertile soils, podzols converted to agriculture are often fertilized with inorganic phosphorus to improve the fertility of the surface soil [94]. Given the low retention capacity of the surface soil, the efficiency of fertilizer applications is extremely low—and many ecosystems around the globe have received literal decades of annual fertilizer input [95,96]. These fertilizer loads are likely to increase the amount of P stored in spodic horizons with time. Consistent with this, Graetz and Nair found high cattle density areas had higher levels of water-soluble phosphorus (WSP) concentrations in podzols compared to landscapes unimpacted by manure [55,56,59]. Further, even dairy landscapes abandoned for 18 years retained a legacy of manure phosphorus at depth, suggesting historically impacted podzols may remain long-term P sources [59]. Obour et al. 2011, replicated this work in fertilized beef pastures and found that historic fertilizer applications could load deep soil horizons with phosphorus much the same as manure might [92]. Obour and collaborators found soil P in the Bh was 10-fold that of the soil surface and three times greater than beneath the spodic horizon. The same project also noted Al and Fe concentrations in the Bh horizon were 14- and 2.5-fold of the surface soil, respectively—evidence of the podzolization process we describe throughout this paper [92].
Importantly, as we have described, while P retained in the spodic horizon is generally stable under oxic conditions, it is not immune to release. Changes in redox potential—especially under saturation or anoxia—can trigger mobilization. As outlined in Section 4, the reduction of Fe(III) to Fe(II) under anoxic conditions dissolves Fe-oxide surfaces, releasing co-precipitated phosphate. Similarly, microbial degradation of organometal complexes under fluctuating redox can liberate previously stabilized organic P. These processes are especially relevant in poorly drained Spodosols or systems experiencing rising water tables, altered drainage, or hydrologic manipulation. Such conditions are increasingly common in agricultural landscapes, where land-use change and soil disturbance—such as conversion of forest to pasture or pasture to cropland—frequently involve tillage, compaction, and artificial drainage. These interventions can disrupt subsurface oxygen dynamics and infiltration patterns, enhancing the risk of phosphorus release from spodic horizons. Once mobilized, this legacy phosphorus can be transported into nearby water bodies, contributing to eutrophication by elevating nutrient loads that fuel algal blooms and aquatic degradation. Clearly, the addition of phosphorus to the spodic layer from manure and fertilizers can lead to the accumulation of phosphorus at depth, posing a threat of legacy phosphorus remobilization during flooding events. This exact phenomenon, the remobilization of legacy P from a flooded spodic horizon, has been observed in Florida [92,97], where high levels of legacy phosphorus have accumulated in seasonally flooded spodic horizons (data from a Florida pasture site is presented in Figure 3).
The Anthropocene has already seen fundamentally altered global and regional precipitation [98] and temperature [99,100] patterns, with significant implications for Spodosol dynamics. Increased frequency and intensity of flooding events can shift the balance of source–sink dynamics in podzols, leading to greater methane emissions [Box 1] and phosphorus release [101,102]. As climate change leads to more frequent and intense flooding events, the methane emissions from podzols are likely to increase [103,104].
Box 1. Spodic horizons also represent significant carbon reservoirs.
Spodic carbon stocks exceed those in the surface horizon by two- to three-fold [105,106]. Under anaerobic conditions, this carbon becomes available for microbial respiration, and methane is produced [107]. Seasonal bursts of methane in subtropical pasture, boreal upland forests, or Arctic areas could all reasonably be explained by deep spodic sources, which are typically ignored [103,108,109].
While much of the organic matter associated with the spodic is definitionally heavily complexed with Al and Fe [28,110] and thus relatively unavailable for decomposition even during periods of flooding, the presence of the spodic horizon leads to the accumulation of relatively ‘newer’ DOC [106,111]. Further, several plant species send roots down to the spodic horizon, providing additional inputs of labile organic carbon, which may either maintain methanogenesis during periods of anoxia or provide valuable catalytic elements during periods of flooding [20,107,112].
In fact, there may be interesting feedbacks whereby plants seeking to acquire phosphate either directly contribute labile carbon for methanogenesis [62,78] or inadvertently provide routes for methane transport to the surface in aerenchyma [113]. It has been well established that P-stress increases the production of root exudates, which in turn may increase the emission of methane [69,114,115].
7.3. Challenges of Detection and Management
One of the core challenges in managing P in podzolized systems is the disconnect between observed surface P concentrations and total landscape P content. Standard agronomic sampling protocols target the topsoil (typically 0–15 cm), ignoring P retained at depth. As a result, P stored in spodic horizons can accumulate silently, contributing nothing to yield but representing a latent risk for downstream nutrient export. This disconnect has practical implications. Agricultural management strategies that focus solely on surface application rates fail to account for legacy loading and vertical migration.
7.4. Toward Subsurface-Inclusive P Budgets
Managing P in podzolized systems requires expanding the conceptual model of nutrient retention to include subsurface storage and delayed release. P budgets must incorporate vertical fluxes, storage compartments, and compound-specific retention mechanisms—not just annual application rates and topsoil concentrations. Landscape-scale P assessments should include subsurface sampling, especially in areas with known spodic development, high rainfall, or a history of fertilization. Policy frameworks that prioritize surface indicators of P saturation may fail to anticipate delayed P release from spodic horizons. Effective P management in Spodosol-dominated systems must account for the dual role of the spodic horizon as both a sink and a source—a buried legacy of past inputs and a potential driver of future eutrophication.
Deep sampling poses logistical and financial challenges. However, tools such as suction lysimeters [63], ground-penetrating radar [Petticord, in prep.], and coupled geophysical-modeling approaches provide feasible pathways to incorporate subsurface P into nutrient budgets.
8. Conclusions
P increasingly limits primary productivity as ecosystems develop, not only because P is lost, but also because P is stabilized in forms that resist biological access. In highly weathered systems, this constraint emerges through a combination of leaching, acidification, and the gradual transformation of P into organic or mineral-bound pools that are difficult to recover. Podzolization accelerates and spatially organizes this transformation, producing soils in which the biological and geochemical phases of P cycling are physically distinct.
This separation provides a natural laboratory for exploring how plants adjust to shifting resource constraints during ecosystem succession. Most highly weathered soils see plants seeking P from a surface layer in which mineral sorption, enzymatic mineralization, and root uptake overlap. In podzols, these processes are stratified. P cycling in the organic surface horizon is governed by biological retention, changes in the physical structure of the topsoil, and recycling. P that escapes this zone moves through the E horizon until reaching the spodic horizon, where it either sorbs or co-precipitates with Fe and Al. The vertical structure imposed by podzolization allows researchers to track how P moves through the system, where it accumulates, and how its availability changes with distance from biological demand. This structure also permits comparative work. Spodic depth, morphology, and accessibility vary across landscapes, creating a gradient of P availability that shapes root strategy, microbial function, and recycling efficiency. Where the spodic horizon is shallow, organisms may exploit both surface and subsoil pools. Where it is deep or chemically hostile, P acquisition must be sustained entirely through surface turnover. We suggest these gradients may provide researchers the opportunity to test how the form, location, and accessibility of P influence trait evolution, biogeochemical partitioning, and the balance between uptake and retention.
Moreover, as nutrient management shifts toward long-term sustainability, spodic P demands greater attention. Legacy loading and saturation have created subsurface P stores that are largely invisible to standard monitoring. Redox shifts, ligand inputs, and structural disturbance can remobilize P long thought to be stabilized. Understanding these triggers and predicting where and when P release may occur will require field-based monitoring that captures chemical transitions at depth.
Author Contributions
Conceptualization, D.F.P.; methodology, D.F.P., B.T.U.; investigation, D.F.P.; resources, E.H.B., J.P.S.; data curation, D.F.P., B.T.U.; writing—original draft preparation, D.F.P.; writing—review and editing, D.F.P., B.T.U., E.H.B., B.D.S., J.P.S.; visualization, D.F.P., B.T.U.; supervision, J.P.S.; project administration, D.F.P.; funding acquisition, D.F.P., E.H.B., J.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Everglades Foundation; OSP 180036.
Data Availability Statement
A de-minimis dataset necessary for the creation of Figure 3 was uploaded at the time of submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Leist, M.; Single, B.; Castoldi, A.F.; Kühnle, S.; Nicotera, P. Intracellular Adenosine Triphosphate (ATP) Concentration: A Switch in the Decision Between Apoptosis and Necrosis. J. Exp. Med. 1997, 185, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. Models of Photosynthesis. Plant Physiol. 2001, 125, 42–45. [Google Scholar] [CrossRef]
- Howard, J.B.; Rees, D.C. Structural Basis of Biological Nitrogen Fixation. Chem. Rev. 1996, 96, 2965–2982. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Lal, R. Sustainable phosphorus management in calcareous soils: Problems and prospects. J. Plant Nutr. 2025, 48, 2179–2200. [Google Scholar] [CrossRef]
- Hopkins, B.; Ellsworth, J. Phosphorus availability with alkaline/calcareous soil. In Proceedings of the Western Nutrient Management Conference, Reno, NV, USA, 9–11 March 2005; University of Idaho: Idaho Falls, ID, USA, 2005; pp. 83–93. Available online: https://www.spring-lake.net/pdfs/calcareous/phosp-availability-calcareous.pdf (accessed on 8 September 2025).
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- VITOUSEK, P.M.; FARRINGTON, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Derry, L.A.; Vitousek, P.M.; Huebert, B.J.; Hedin, L.O. Changing sources of nutrients during four million years of ecosystem development. Nature 1999, 397, 491–497. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Condron, L.M.; Richardson, S.J.; Peltzer, D.A.; Allison, V.J. Soil Organic Phosphorus Transformations During Pedogenesis. Ecosystems 2007, 10, 1166–1181. [Google Scholar] [CrossRef]
- Reed, S.C.; Wood, T.E. Soil phosphorus cycling in tropical soils: An ultisol and oxisol perspective. In Soil phosphorus; CRC Press: Boca Raton, FL, USA, 2016; pp. 247–284. Available online: https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.1201/9781315372327-11&type=chapterpdf (accessed on 8 September 2025).
- Santos, D.P.; Santos, G.G.; de Oliveira, V.Á.; da Silva, G.C.; Flores, R.A.; Azevedo, A.C.; de Souza Júnior, V.S.; Pereira, M.G. Chemical and mineralogical constitution of redoximorphic features and mechanism of formation of Plinthosols from the Araguaia River plain, Brazil. Rev. Bras. Ciênc. Solo 2024, 48, e0230115. [Google Scholar] [CrossRef]
- Takenaka, K.; Koala, J.; Yamadera, Y. Characteristics of the root system of four tree species planted on Plinthosols in Burkina Faso: Application of the soil-briquette method. J. Arid Land Stud. 2024, 34, 17–33. [Google Scholar] [CrossRef]
- Eze, P.; Udeigwe, T.; Meadows, M. Plinthite and Its Associated Evolutionary Forms in Soils and Landscapes: A Review. Pedosphere 2014, 24, 153–166. [Google Scholar] [CrossRef]
- Martins, A.P.B.; Santos, G.G.; de Oliveira, V.Á.; Maranhão, D.D.C.; Collier, L.S. Hardening and Stability of Plinthic Materials of the Araguaia River Floodplain under Different Drying Treatments. Rev. Bras. Ciênc. Solo 2018, 42, e0170190. [Google Scholar] [CrossRef]
- Bloomfield, C. A Study of Podzolization. J. Soil Sci. 1954, 5, 39–45. [Google Scholar] [CrossRef]
- Lundström, U.S.; van Breemen, N.; Bain, D. The podzolization process. A review. Geoderma 2000, 94, 91–107. [Google Scholar] [CrossRef]
- Alva, A.K.; Huang, B.; Paramasivam, S.; Sajwan, K.S. Evaluation of Root Growth Limiting Factors in Spodic Horizons of Spodosols. J. Plant Nutr. 2002, 25, 2001–2014. [Google Scholar] [CrossRef]
- McKeague, J.A.; DeConinck, F.; Franzmeier, D.P. Chapter 6 Spodosols1. In Developments in Soil Science; Wilding, L.P., Smeck, N.E., Hall, G.F., Eds.; Pedogenesis and Soil Taxonomy; Elsevier: Amsterdam, The Netherlands, 1983; Volume 11, pp. 217–252. [Google Scholar]
- Graetz, D.A.; Nair, V.D. Fate of phosphorus in Florida Spodosols contaminated with cattle manure. Ecol. Eng. 1995, 5, 163–181. [Google Scholar] [CrossRef]
- Gerke, J.; Meyer, U. Phosphate aquisition by red clover and black mustard on a humic podzol. J. Plant Nutr. 1995, 18, 2409–2429. [Google Scholar] [CrossRef]
- Gerke, J. Humic (Organic Matter)-Al(Fe)-Phosphate Complexes: An Underestimated Phosphate Form in Soils and Source of Plant-Available Phosphate. Soil Sci. 2010, 175, 417. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Cole, C.V. Pathways of Phosphorus Transformations in Soils of Differing Pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Schaetzl, R.J.; Kasmerchak, C.; Samonil, P.; Baish, C.; Hadden, M.; Rothstein, D. Acidification and weathering associated with deep tongues in sandy Spodosols, Michigan, USA. Geoderma Reg. 2020, 23, e00332. [Google Scholar] [CrossRef]
- Chakraborty, D.; Nair, V.D.; Chrysostome, M.; Harris, W.G. Soil phosphorus storage capacity in manure-impacted Alaquods: Implications for water table management. Agric. Ecosyst. Environ. 2011, 142, 167–175. [Google Scholar] [CrossRef]
- De Coninck, F. Major mechanisms in formation of spodic horizons. Geoderma 1980, 24, 101–128. [Google Scholar] [CrossRef]
- Mantel, S.; Dondeyne, S.; Deckers, S. World Reference Base for Soil Resources (WRB). Encycl. Soils Environ. 2023, 4, 206–217. [Google Scholar]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Bloom, P.R. Phosphorus Adsorption by an Aluminum-Peat Complex. Soil Sci. Soc. Am. J. 1981, 45, 267–272. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Jdrgensen, S.S.; Moberg, J.P.; Raben-Lange, B. Influence of organic matter on phosphate adsorption by aluminium and iron oxides in sandy soils. J. Soil Sci. 1990, 41, 443–449. [Google Scholar] [CrossRef]
- Peña, F.; Torrent, J. Predicting phosphate sorption in soils of mediterranean regions. Fertil. Res. 1990, 23, 173–179. [Google Scholar] [CrossRef]
- Singh, B.; Gilkes, R. Phosphorus sorption in relation to soil properties for the major soil types of South-Western Australia. Soil Res. 1991, 29, 603. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Sanford, R.L. Nutrient Cycling in Moist Tropical Forest. Annu. Rev. Ecol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Turner, B.L.; Laliberté, E. Soil Development and Nutrient Availability Along a 2 Million-Year Coastal Dune Chronosequence Under Species-Rich Mediterranean Shrubland in Southwestern Australia. Ecosystems 2015, 18, 287–309. [Google Scholar] [CrossRef]
- Fox, T.R.; Comerford, N.B. Low-Molecular-Weight Organic Acids in Selected Forest Soils of the Southeastern USA. Soil Sci. Soc. Am. J. 1990, 54, 1139–1144. [Google Scholar] [CrossRef]
- Pohlman, A.A.; McColl, J.G. Soluble Organics from Forest Litter and their Role in Metal Dissolution. Soil Sci. Soc. Am. J. 1988, 52, 265–271. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.J.; Verstraten, J.M. Mobilization of dissolved organic matter, aluminium and iron in podzol eluvial horizons as affected by formation of metal-organic complexes and interactions with solid soil material. Eur. J. Soil Sci. 2004, 55, 287–297. [Google Scholar] [CrossRef]
- Lundström, U.; Öhman, L.-O. Dissolution of feldspars in the presence of natural, organic solutes. J. Soil Sci. 1990, 41, 359–369. [Google Scholar] [CrossRef]
- McKeague, J.A.; Brydon, J.E.; Miles, N.M. Differentiation of Forms of Extractable Iron and Aluminum in Soils. Soil Sci. Soc. Am. J. 1971, 35, 33–38. [Google Scholar] [CrossRef]
- Schnitzer, M.; Desjardins, J.G. Molecular and Equivalent Weights of the Organic Matter of a Podzol. Soil Sci. Soc. Am. J. 1962, 26, 362–365. [Google Scholar] [CrossRef]
- Schnitzer, M.; Skinner, S.I.M. Organo-metallic interactions in soils. Soil Sci. 1963, 96, 86–93. [Google Scholar] [CrossRef]
- Anderson, H.A.; Berrow, M.L.; Farmer, V.C.; Hepburn, A.; Russell, J.D.; Walker, A.D. A reassessment of podzol formation processes. J. Soil Sci. 1982, 33, 125–136. [Google Scholar] [CrossRef]
- McKeague, J.A.; Kodama, H. Imogolite in cemented horizons of some British Columbia soils. Geoderma 1981, 25, 189–197. [Google Scholar] [CrossRef]
- Boudot, J.P.; Bel Hadj^Brahim, A.; Steiman, R.; Seigle-Murandi, F. Biodegradation of synthetic organo-metallic complexes of iron and aluminium with selected metal to carbon ratios. Soil Biol. Biochem. 1989, 21, 961–966. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Bhattacharya, P.; Bain, D.C.; Fraser, A.R.; McHardy, W.J. Podzolisation mechanisms and the synthesis of imogolite in northern Scandinavia. Geoderma 1995, 66, 167–184. [Google Scholar] [CrossRef]
- Bazilevskaya, E.; Archibald, D.D.; Martínez, C.E. Mineral colloids mediate organic carbon accumulation in a temperate forest Spodosol: Depth-wise changes in pore water chemistry. Biogeochemistry 2018, 141, 75–94. [Google Scholar] [CrossRef]
- Prasetyo, B.H. Mineralogical and chemical characteristics of Spodosols in Toba highland, North Sumatra. Indones. J. Agric. Sci. 2016, 10, 54–64. [Google Scholar] [CrossRef]
- Van Ranst, E.; Wilson, M.A.; Righi, D. Chapter 22—Spodic Materials. In Interpretation of Micromorphological Features of Soils and Regoliths, 2nd ed.; Stoops, G., Marcelino, V., Mees, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 633–662. ISBN 978-0-444-63522-8. [Google Scholar]
- dos Santos, C.C.; Souza de Lima Ferraz Junior, A.; Oliveira Sá, S.; Andrés Muñoz Gutiérrez, J.; Braun, H.; Sarrazin, M.; Brossard, M.; Desjardins, T. Soil carbon stock and Plinthosol fertility in smallholder land-use systems in the eastern Amazon, Brazil. Carbon Manag. 2018, 9, 655–664. [Google Scholar] [CrossRef]
- Mezenner, N.Y.; Bensmaili, A. Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem. Eng. J. 2009, 147, 87–96. [Google Scholar] [CrossRef]
- Parfitt, R.L. Phosphate reactions with natural allophane, ferrihydrite and goethite. J. Soil Sci. 1989, 40, 359–369. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Tang, Y.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci. Nano 2017, 4, 2193–2204. [Google Scholar] [CrossRef]
- Nair, V.D.; Villapando, R.R.; Graetz, D.A. Phosphorus Retention Capacity of the Spodic Horizon under Varying Environmental Conditions. J. Environ. Qual. 1999, 28, 1308–1313. [Google Scholar] [CrossRef]
- Graetz, D.A.; Nair, V.D.; Portier, K.M.; Voss, R.L. Phosphorus accumulation in manure-impacted Spodosols of Florida. Agric. Ecosyst. Environ. 1999, 75, 31–40. [Google Scholar] [CrossRef]
- Li, X.; Sheng, A.; Ding, Y.; Liu, J. A model towards understanding stabilities and crystallization pathways of iron (oxyhydr)oxides in redox-dynamic environments. Geochim. Cosmochim. Acta 2022, 336, 92–103. [Google Scholar] [CrossRef]
- Villapando, R.R.; Graetz, D.A. Phosphorus Sorption and Desorption Properties of the Spodic Horizon from Selected Florida Spodosols. Soil Sci. Soc. Am. J. 2001, 65, 331–339. [Google Scholar] [CrossRef]
- Nair, V.D.; Graetz, D.A.; Reddy, K.R. Dairy Manure Influences on Phosphorus Retention Capacity of Spodosols. J. Environ. Qual. 1998, 27, 522–527. [Google Scholar] [CrossRef]
- Shenker, M.; Seitelbach, S.; Brand, S.; Haim, A.; Litaor, M.I. Redox reactions and phosphorus release in re-flooded soils of an altered wetland. Eur. J. Soil Sci. 2005, 56, 515–525. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.-J.; van Loosdrecht, M.C.M. The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Fox, T.R.; Comerford, N.B.; McFee, W.W. Phosphorus and Aluminum Release from a Spodic Horizon Mediated by Organic Acids. Soil Sci. Soc. Am. J. 1990, 54, 1763–1767. [Google Scholar] [CrossRef]
- Obour, A.K.; Silveira, M.L.; Vendramini, J.M.B.; Sollenberger, L.E.; O’Connor, G.A. Fluctuating water table effect on phosphorus release and availability from a Florida Spodosol. Nutr. Cycl. Agroecosyst. 2011, 91, 207–217. [Google Scholar] [CrossRef]
- Fritzsche, A.; Bosch, J.; Sander, M.; Schröder, C.; Byrne, J.M.; Ritschel, T.; Joshi, P.; Maisch, M.; Meckenstock, R.U.; Kappler, A.; et al. Organic Matter from Redoximorphic Soils Accelerates and Sustains Microbial Fe(III) Reduction. Environ. Sci. Technol. 2021, 55, 10821–10831. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.M.; Odum, W.E. Porewater oxidation, dissolved phosphate and the iron curtain. Biogeochemistry 1990, 10, 37–52. [Google Scholar] [CrossRef]
- Dijkstra, N.; Hagens, M.; Egger, M.; Slomp, C.P. Post-depositional formation of vivianite-type minerals alters sediment phosphorus records. Biogeosciences 2018, 15, 861–883. [Google Scholar] [CrossRef]
- Heiberg, L.; Koch, C.B.; Kjaergaard, C.; Jensen, H.S.; Hans Christian, B.H. Vivianite precipitation and phosphate sorption following iron reduction in anoxic soils. J. Environ. Qual. 2012, 41, 938–949. [Google Scholar] [CrossRef]
- Ligaba, A.; Shen, H.; Shibata, K.; Yamamoto, Y.; Tanakamaru, S.; Matsumoto, H. The role of phosphorus in aluminium-induced citrate and malate exudation from rape (Brassica napus). Physiol. Plant. 2004, 120, 575–584. [Google Scholar] [CrossRef]
- Lipton, D.S.; Blanchar, R.W.; Blevins, D.G. Citrate, Malate, and Succinate Concentration in Exudates from P-Sufficient and P-Stressed Medicago sativa L. Seedlings. Plant Physiol. 1987, 85, 315–317. [Google Scholar] [CrossRef]
- López-Bucio, J.; de la Vega, O.M.; Guevara-García, A.; Herrera-Estrella, L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000, 18, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, Z.; Shenker, M.; Romheld, V.; Marschner, H.; Hadar, Y.; Chen, Y. The Role of Ligand Exchange in the Uptake of Iron from Microbial Siderophores by Gramineous Plants. Plant Physiol. 1996, 112, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Xu, T.; Chen, J.; Yang, H.; Liu, X.; Zhuo, R.; Peng, Y.; Tang, W.; Wang, R.; Chen, L.; et al. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. CoT10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 2022, 367, 133110. [Google Scholar] [CrossRef]
- Hayes, J.E.; Richardson, A.E.; Simpson, R.J. Components of organic phosphorus in soil extracts that are hydrolysed by phytase and acid phosphatase. Biol. Fertil. Soils 2000, 32, 279–286. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Claassen, N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol. Fertil. Soils 1988, 5, 308–312. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Jungk, A. Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biol. Fertil. Soils 1987, 3, 199–204. [Google Scholar] [CrossRef]
- Phillips, D.H.; FitzPatrick, E.A. Biological influences on the morphology and micromorphology of selected Podzols (Spodosols) and Cambisols (Inceptisols) from the eastern United States and north-east Scotland. Geoderma 1999, 90, 327–364. [Google Scholar] [CrossRef]
- Vermeire, M.-L.; Cornélis, J.-T.; Van Ranst, E.; Bonneville, S.; Doetterl, S.; Delvaux, B. Soil Microbial Populations Shift as Processes Protecting Organic Matter Change During Podzolization. Front. Environ. Sci. 2018, 6, 70. [Google Scholar] [CrossRef]
- Gerke, J.; Römer, W.; Jungk, A. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. Z. Für Pflanzenernähr. Bodenkd. 1994, 157, 289–294. [Google Scholar] [CrossRef]
- Lan, M.; Comerford, N.B.; Fox, T.R. Organic Anions’ Effect on Phosphorus Release from Spodic Horizons. Soil Sci. Soc. Am. J. 1995, 59, 1745–1749. [Google Scholar] [CrossRef]
- Nair, V.D.; Nair, P.K.R.; Kalmbacher, R.S.; Ezenwa, I.V. Reducing nutrient loss from farms through silvopastoral practices in coarse-textured soils of Florida, USA. Ecol. Eng. 2007, 29, 192–199. [Google Scholar] [CrossRef]
- Schaetzl, R.J. Effects of treethrow microtopography on the characteristics and genesis of Spodosols, Michigan, USA. CATENA 1990, 17, 111–126. [Google Scholar] [CrossRef]
- Turner, B.L.; Engelbrecht, B.M.J. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 2011, 103, 297–315. [Google Scholar] [CrossRef]
- Vitousek, P.M. Litterfall, Nutrient Cycling, and Nutrient Limitation in Tropical Forests. Ecology 1984, 65, 285–298. [Google Scholar] [CrossRef]
- Dunbabin, V.; Diggle, A.; Rengel, Z. Is there an optimal root architecture for nitrate capture in leaching environments? Plant Cell Environ. 2003, 26, 835–844. [Google Scholar] [CrossRef]
- Ehdaie, B.; Merhaut, D.J.; Ahmadian, S.; Hoops, A.C.; Khuong, T.; Layne, A.P.; Waines, J.G. Root System Size Influences Water-Nutrient Uptake and Nitrate Leaching Potential in Wheat. J. Agron. Crop Sci. 2010, 196, 455–466. [Google Scholar] [CrossRef]
- Abdala, D.B.; Ghosh, A.K.; da Silva, I.R.; de Novais, R.F.; Alvarez Venegas, V.H. Phosphorus saturation of a tropical soil and related P leaching caused by poultry litter addition. Agric. Ecosyst. Environ. 2012, 162, 15–23. [Google Scholar] [CrossRef]
- Backnäs, S.; Laine-Kaulio, H.; Kløve, B. Phosphorus Forms and Related Soil Chemistry in Preferential Flowpaths and the Soil Matrix of a Forested Podzolic Till Soil Profile. Geoderma 2012, 189–190, 50–64. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Cherubin, M.R.; Soltangheisi, A.; Rocha, G.C.; Chadwick, D.R.; Jones, D.L. Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci. Rep. 2020, 10, 15615. [Google Scholar] [CrossRef] [PubMed]
- Pavinato, P.S.; Gotz, L.F.; Teles, A.P.B.; Arruda, B.; Herrera, W.B.; Chadwick, D.R.; Jones, D.L.; Withers, P.J.A. Legacy Soil Phosphorus Bioavailability in Tropical and Temperate Soils: Implications for Sustainable Crop Production. Soil Tillage Res. 2024, 244, 106228. [Google Scholar] [CrossRef]
- Daniels, R.B.; Gamble, E.E.; Holzhey, C.S. Thick Bh Horizons in the North Carolina Coastal Plain: I. Morphology and Relation to Texture and Soil Ground Water. Soil Sci. Soc. Am. J. 1975, 39, 1177–1181. [Google Scholar] [CrossRef]
- Obour, A.K.; Silveira, M.L.; Vendramini, J.M.B.; Jawitz, J.; O’Connor, G.A.; Sollenberger, L.E. A Phosphorus Budget for Bahiagrass Pastures Growing on a Typical Florida Spodosol. Agron. J. 2011, 103, 611–616. [Google Scholar] [CrossRef]
- Woods, W.I.; Lehmann, J.; Rebellato, L.; Steiner, C.; Teixeira, W.G.; WinklerPrins, A. (Eds.) Amazonian Dark Earths: Wim Sombroek’s Vision; Springer: Berlin/Hamburg, Germany, 2009; ISBN 978-1-4020-9030-1. [Google Scholar]
- Zielinski, R.A.; Orem, W.H.; Simmons, K.R.; Bohlen, P.J. Fertilizer-Derived Uranium and Sulfur in Rangeland Soil and Runoff: A Case Study in Central Florida. Water. Air. Soil Pollut. 2006, 176, 163–183. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; Li, J. A 1961–2010 record of fertilizer use, pesticide application and cereal yields: A review. Agron. Sustain. Dev. 2015, 35, 83–93. [Google Scholar] [CrossRef]
- Sharpley, A.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus Legacy: Overcoming the Effects of Past Management Practices to Mitigate Future Water Quality Impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef]
- Zhi, R.; Boughton, E.H.; Li, H.; Petticord, D.F.; Saha, A.; Sparks, J.P.; Reddy, K.R.; Qiu, J. Soil legacy phosphorus and loss risk in subtropical grasslands. J. Environ. Manag. 2024, 366, 121656. [Google Scholar] [CrossRef]
- Zhang, X.; Zwiers, F.W.; Hegerl, G.C.; Lambert, F.H.; Gillett, N.P.; Solomon, S.; Stott, P.A.; Nozawa, T. Detection of human influence on twentieth-century precipitation trends. Nature 2007, 448, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2019; Volume 1, pp. 93–174. [Google Scholar]
- IPCC Core Writing Team; Lee, H.; Romero, J. (Eds.) Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023; Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 23 September 2025).
- Lelieveld, J.; Crutzen, P.J. Indirect chemical effects of methane on climate warming. Nature 1992, 355, 339–342. [Google Scholar] [CrossRef]
- Zhang, Z.; Zimmermann, N.E.; Stenke, A.; Li, X.; Hodson, E.L.; Zhu, G.; Huang, C.; Poulter, B. Emerging role of wetland methane emissions in driving 21st century climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 9647–9652. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.D.; Gomez-Casanovas, N.; Walter, M.T.; Boughton, E.H.; Bernacchi, C.J.; DeLucia, E.H.; Groffman, P.M.; Keel, E.W.; Sparks, J.P. Influence of transient flooding on methane fluxes from subtropical pastures. J. Geophys. Res. Biogeosci. 2016, 121, 965–977. [Google Scholar] [CrossRef]
- Min, S.-K.; Zhang, X.; Zwiers, F. Human-Induced Arctic Moistening. Science 2008, 320, 518–520. [Google Scholar] [CrossRef]
- Grand, S.; Lavkulich, L.M. Effects of Forest Harvest on Soil Carbon and Related Variables in Canadian Spodosols. Soil Sci. Soc. Am. J. 2012, 76, 1816–1827. [Google Scholar] [CrossRef]
- Stone, E.L.; Harris, W.G.; Brown, R.B.; Kuehl, R.J. Carbon Storage in Florida Spodosols. Soil Sci. Soc. Am. J. 1993, 57, 179–182. [Google Scholar] [CrossRef]
- Bernal, B.; Megonigal, J.P.; Mozdzer, T.J. An Invasive Wetland Grass Primes Deep Soil Carbon Pools. Glob. Change Biol. 2017, 23, 2104–2116. [Google Scholar] [CrossRef]
- Yavitt, J.B.; Fahey, T.J.; Simmons, J.A. Methane and Carbon Dioxide Dynamics in a Northern Hardwood Ecosystem. Soil Sci. Soc. Am. J. 1995, 59, 796–804. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Romero, A.J.B.; Jørgensen, N.O.G.; Jørgensen, C.J.; Berg, L.K.; Elberling, B. Methane Fluxes and the Functional Groups of Methanotrophs and Methanogens in a Young Arctic Landscape on Disko Island, West Greenland. Biogeochemistry 2015, 122, 15–33. [Google Scholar] [CrossRef]
- Lee, F.Y.; Yuan, T.L.; Carlisle, V.W. Nature of Cementing Materials in Ortstein Horizons of Selected Florida Spodosols: II. Soil Properties and Chemical Form(s) of Aluminum. Soil Sci. Soc. Am. J. 1988, 52, 1796–1801. [Google Scholar] [CrossRef]
- Dai, K.H.; David, M.B.; Vance, G.F. Characterization of solid and dissolved carbon in a spruce-fir Spodosol. Biogeochem. 1996, 35, 339–365. [Google Scholar] [CrossRef]
- Bernal, B.; McKinley, D.C.; Hungate, B.A.; White, P.M.; Mozdzer, T.J.; Megonigal, J.P. Limits to soil carbon stability; Deep, ancient soil carbon decomposition stimulated by new labile organic inputs. Soil Biol. Biochem. 2016, 98, 85–94. [Google Scholar] [CrossRef]
- Kludze, H.K.; DeLaune, R.D.; Patrick, W.H. Aerenchyma Formation and Methane and Oxygen Exchange in Rice. Soil Sci. Soc. Am. J. 1993, 57, 386–391. [Google Scholar] [CrossRef]
- Hoffland, E.; Findenegg, G.R.; Nelemans, J.A. Solubilization of rock phosphate by rape. Plant Soil. 1989, 113, 161–165. [Google Scholar] [CrossRef]
- Lu, Y.; Wassmann, R.; Neue, H.U.; Huang, C. Impact of phosphorus supply on root exudation, aerenchyma formation and methane emission of rice plants. Biogeochem. 1999, 47, 203–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).