Experimental Warming of Typically Acidic and Nutrient-Poor Boreal Soils Does Not Affect Leaf-Litter Decomposition of Temperate Deciduous Tree Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Litterbags
2.4. Other Environmental Variables
2.5. Data Analysis
3. Results and Discussion
3.1. Litter Type
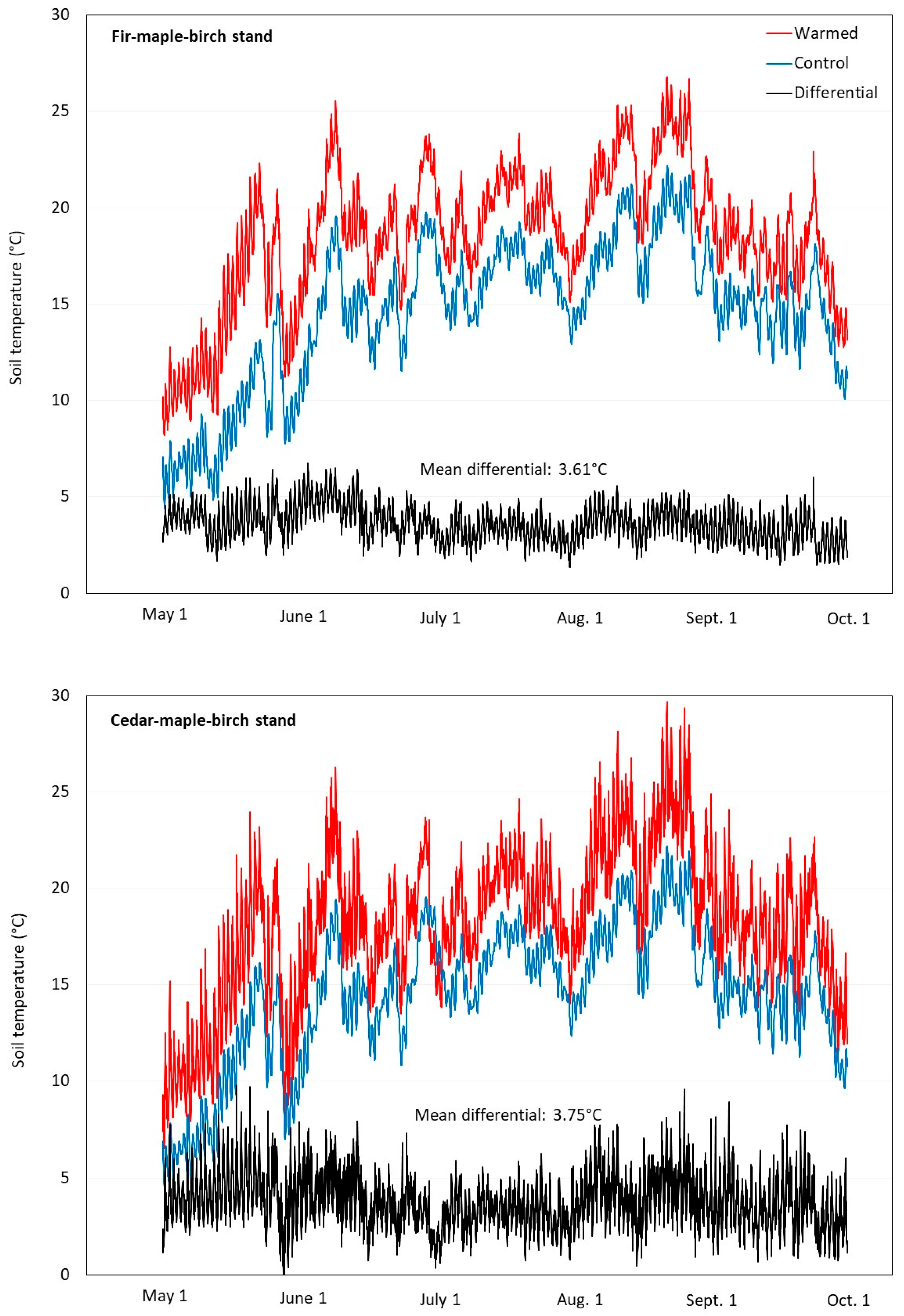
3.2. Artificial Heating
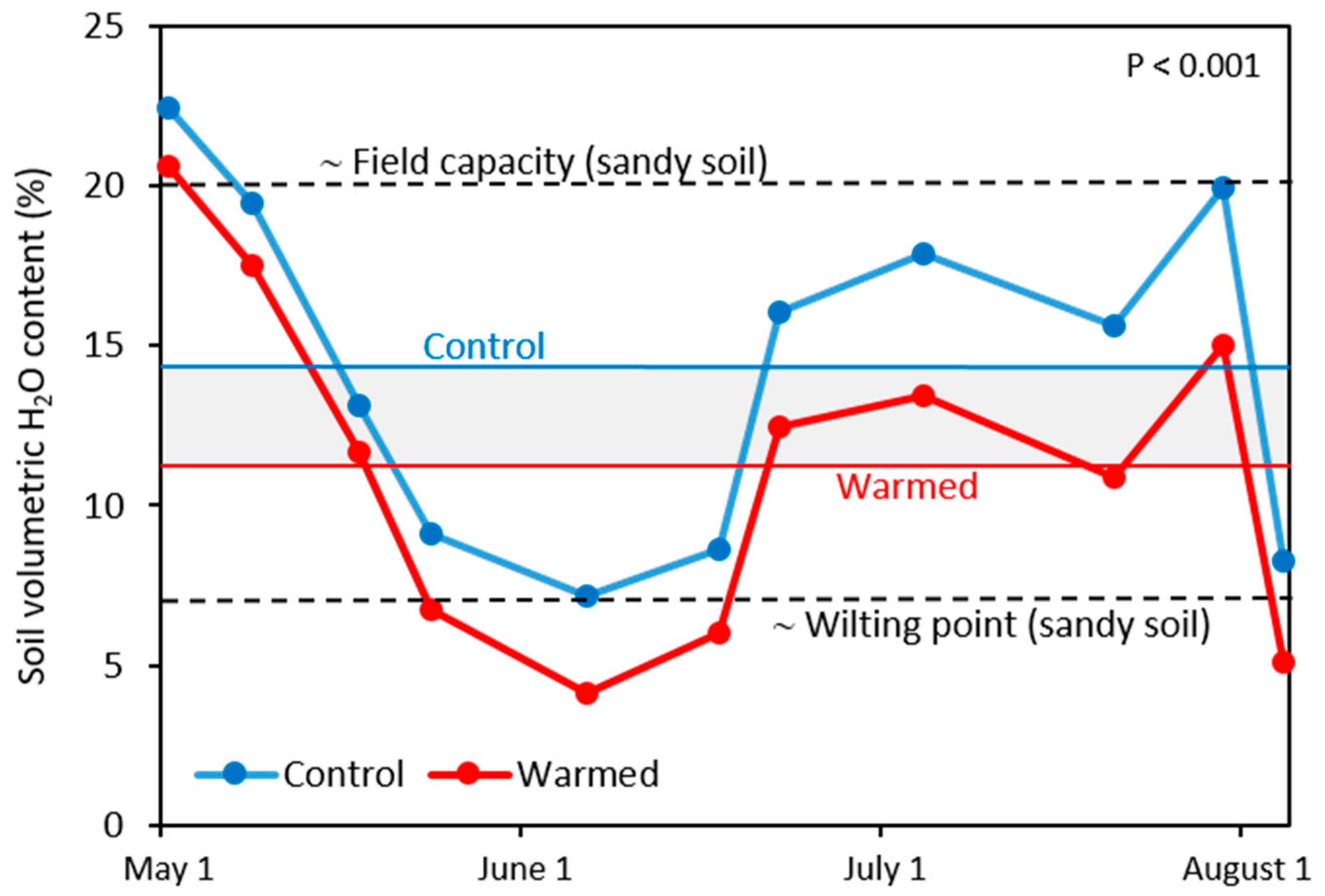
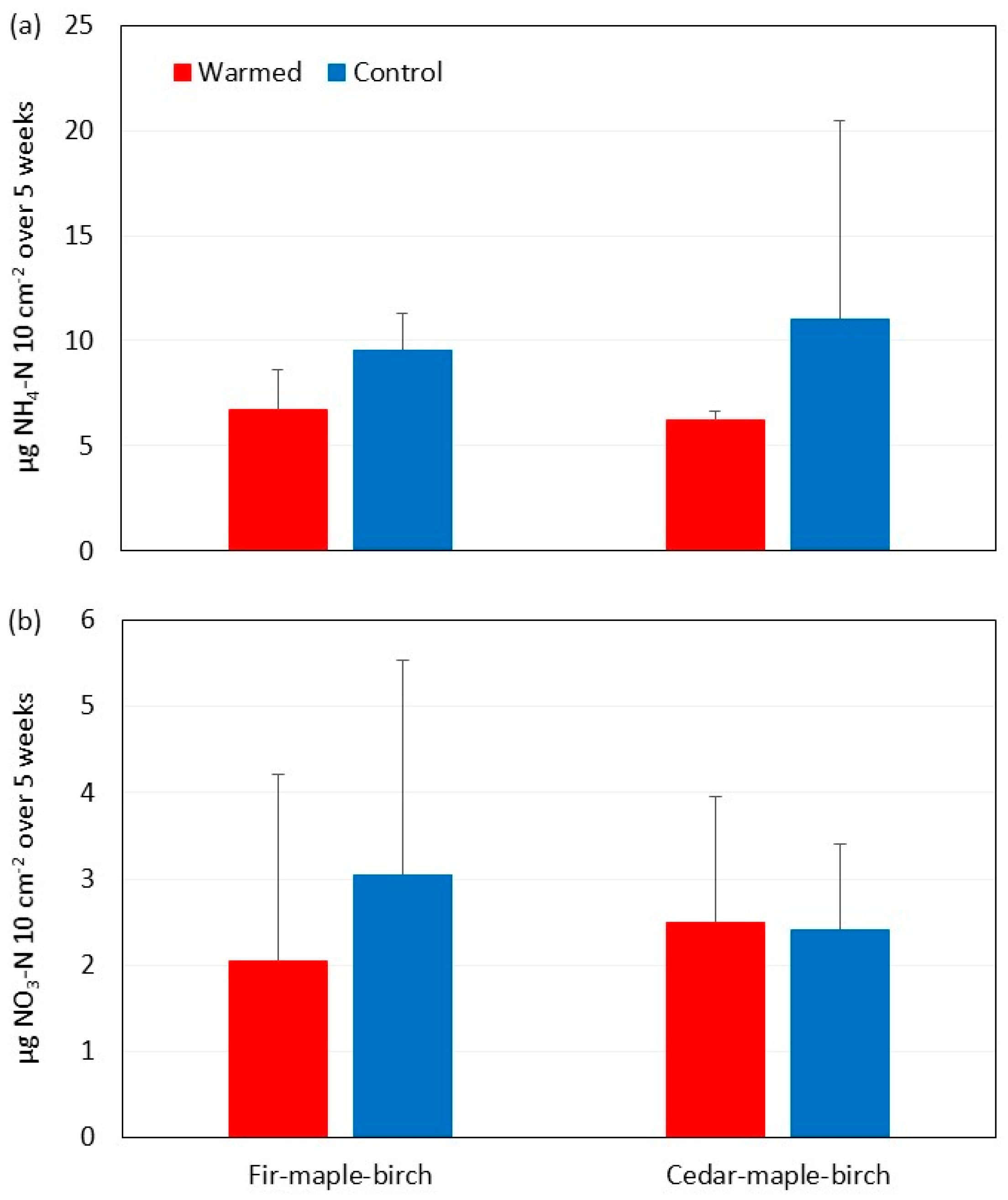
3.3. Soil Moisture and N Availability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2015, 259, 660–684. [Google Scholar] [CrossRef]
- Salomón, R.L.; Peters, R.L.; Zweifel, R.; Sass-Klaassen, U.G.W.; Stegehuis, A.I.; Smiljanic, M.; Poyatos, R.; Babst, F.; Cienciala, E.; Fonti, P.; et al. The 2018 European heatwave led to stem dehydration but not to consistent growth reductions in forests. Nat. Commun. 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.E.; Kooijmans, L.M.J.; Koren, G.; van Schaik, E.; van der Woude, A.M.; Wanders, N.; Ramonet, M.; Xueref-Remy, I.; Siebicke, L.; Manca, G.; et al. Spring enhancement and summer reduction in carbon uptake during the 2018 drought in northwestern Europe. Philos. Trans. R. Soc. B 2020, 375, 20190509. [Google Scholar] [CrossRef]
- Beckage, B.; Osborne, B.; Gavin, D.G.; Perkins, T. A rapid upward shift of a forest ecotone due to warming. Proc. Natl. Acad. Sci. USA 2008, 105, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Boisvert-Marsh, L.; Périé, C.; De Blois, S. Shifting with climate? Evidence for recent changes in tree species distribution. Ecosphere 2014, 5, 83. [Google Scholar] [CrossRef]
- Sittaro, F.; Paquette, A.; Messier, C.; Nock, C.A. Tree range expansion in eastern North America fails to keep pace with climate warming at northern range limits. Glob. Chang. Biol. 2017, 23, 3292–3301. [Google Scholar] [CrossRef]
- Clark, J.S.; Bell, D.M.; Kwit, M.C.; Zhu, K. Competition-interaction landscapes for the joint response of forests to climate change. Glob. Chang. Biol. 2014, 20, 1979–1991. [Google Scholar] [CrossRef]
- Wilson, J.B.; Agnew, A.D.Q. Positive-feedback switches in plant communities. Adv. Ecol. Res. 1992, 23, 263–336. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Ettinger, A.K.; Hille Ris Lambers, J. Climate isn’t everything: Competitive interactions and variation by life stage will also affect range shifts under warming. Am. J. Bot. 2013, 100, 1344–1355. [Google Scholar] [CrossRef]
- Lafleur, B.; Paré, D.; Munson, A.D.; Bergeron, Y. Response of forests to climate change: Will soil conditions constrain tree species migration? Environ. Rev. 2010, 18, 279–289. [Google Scholar] [CrossRef]
- Collin, A.; Messier, C.; Bélanger, N. Conifer presence may negatively affect sugar maple’s ability to migrate into the boreal forest through reduced foliar nutritional status. Ecosystems 2017, 20, 701–716. [Google Scholar] [CrossRef]
- Lin, D.; Xia, J.; Wan, S. Climate warming and biomass accumulation of terrestrial plants: A meta-analysis. New Phytol. 2010, 188, 187–198. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.; Xu, W.; Li, W.; Dai, W.; Jiang, P. A meta-analysis of experimental warming effects on terrestrial N pools/dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; Yang, Q.; Li, H.; Luo, Y.; Fang, C.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem C cycle to experimental warming: A meta-analysis. Ecology 2013, 94, 726–738. [Google Scholar] [CrossRef]
- Carey, J.; Tang, J.; Templer, P.H.; Kroeger, K.D.; Crowther, T.W.; Burton, A.J.; Dukes, J.S.; Emmett, B.; Frey, S.D.; Heskel, M.A.; et al. Temperature response of Rs largely unaltered with experimental warming. Proc. Natl. Acad. Sci. USA 2016, 113, 13797–13802. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Wu, Q.; Yue, K.; Wang, X.; Ma, Y.; Li, Y. Differential responses of litter decomposition to warming, elevated CO2, and changed precipitation regime. Plant Soil 2020, 455, 155–169. [Google Scholar] [CrossRef]
- Savage, C. Recolonisation Forestière dans les Basses Laurentides au sud du Domaine Climacique de L’érablière à Bouleau Jaune. Master’s Thesis, Université de Montréal, Montréal, QC, Canada, 2001. [Google Scholar]
- Bélanger, N.; Collin, A.; Khlifa, R.; Lebel-Desrosiers, S. Balsam fir and American beech influence soil respiration rates in opposite directions in a sugar maple forest near its northern range limit. Front. For. Glob. Chang. 2021, 4, 664584. [Google Scholar] [CrossRef]
- Bélanger, N.; Holmden, C.; Courchesne, F.; Côté, B.; Hendershot, W.H. Constraining soil mineral weathering 87Sr/86Sr for calcium apportionment studies of a deciduous forest growing on soils developed from granitoid igneous rocks. Geoderma 2012, 185, 84–96. [Google Scholar] [CrossRef]
- Soil Classification Working Group. The Canadian System of Soil Classification, 3rd ed.; Publication 1646; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1998.
- Collin, A.; Messier, C.; Kembel, S.W.; Bélanger, N. Can sugar maple establish into the boreal forest? Insights from seedlings. Ecosphere 2018, 9, e02022. [Google Scholar] [CrossRef]
- Sanders-DeMott, R.; Templer, P.H. What about winter? Integrating the missing season into climate change experiments in seasonally snow covered ecosystems. Methods Ecol. Evol. 2017, 8, 1183–1191. [Google Scholar] [CrossRef]
- Topp, G.C.; Davis, J.L.; Annan, A.P. Electromagnetic determination of soil water content: Measurement in coaxial transmission lines. Water Resour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef]
- Bilodeau-Gauthier, S.; Paré, D.; Messier, C.; Bélanger, N. Root production of hybrid poplars and nitrogen mineralization improve following mounding of boreal Podzols. Can. J. For. Res. 2013, 43, 1092–1103. [Google Scholar] [CrossRef]
- Gosz, J.R.; Likens, G.E.; Bormann, F. Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire. Ecol. Monogr. 1973, 43, 173–191. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Côté, B.; Fyles, J.W. Leaf litter disappearance of hardwood species of southern Quebec: Interaction between litter quality and stand type. Ecoscience 1994, 1, 322–328. [Google Scholar] [CrossRef]
- Moore, T.R.; Trofymow, J.A.; Taylor, B.; Prescott, C.; Camiré, C.; Duchesne, L.; Fyles, J.; Kozak, L.; Kranabetter, M.; Morrison, I.; et al. Rates of litter decomposition in Canadian forests. Glob. Chang. Biol. 1999, 5, 75–82. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how we can alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Marley, A.R.G.; Smeaton, C.; Austin, W.E.N. An assessment of the tea bag index method as a proxy for organic matter decomposition in intertidal environments. J. Geophys. Res. Biogeosci. 2019, 124, 2991–3004. [Google Scholar] [CrossRef]
- Blume-Werry, G.; Di Maurizio, V.; Beil, I.; Lett, S.; Schwieger, S.; Kreyling, J. Don’t drink it, bury it: Comparing decomposition rates with the tea bag index is possible without prior leaching. Plant Soil 2021, 465, 613–621. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- McHale, P.J.; Mitchell, M.J. Soil warming in a northern hardwood forest: Trace gas fluxes and leaf litter decomposition. Can. J. For. Res. 1998, 28, 1365–1372. [Google Scholar] [CrossRef]
- Rustad, L.E.; Fernandez, I.J. Soil warming: Consequences for foliar litter decay in a spruce-fir forest in Maine, USA. Soil Sci. Soc. Amer. J. 1998, 62, 1072–1080. [Google Scholar] [CrossRef]
- Xu, Z.; Pu, X.Z.; Yin, H.; Zhao, C.; Liu, Q.; Wu, F. Warming effects on the early decomposition of three litter types, Eastern Tibetan Plateau, China. Eur. J. Soil Sci. 2012, 63, 360–367. [Google Scholar] [CrossRef]
- Berbeco, M.R.; Melillo, J.M.; Orians, C.M. Soil warming accelerates decomposition of fine woody debris. Plant Soil 2012, 356, 405–417. [Google Scholar] [CrossRef]
- Kasurinen, A.; Silfver, T.; Rousi, M.; Mikola, J. Warming and ozone exposure effects on silver birch (Betula pendula Roth) leaf litter quality, microbial growth and decomposition. Plant Soil 2017, 414, 127–142. [Google Scholar] [CrossRef]
- Romero-Olivares, A.L.; Allison, S.D.; Treseder, K.K. Decomposition of recalcitrant carbon under experimental warming in boreal forest. PLoS ONE 2017, 12, e0179674. [Google Scholar] [CrossRef]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea Bag Index: A novel approach to collect unifoirm decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Djukic, I.; Guerra, C.A.; Maestre, F.T.; Hagedorn, F.; Oggioni, A.; Bergami, C.; Magagna, B.; Kwon, T.; Shibata, H.; Eisenhauer, N.; et al. The TeaComposition Initiative: Unleashing the power of international collaboration to understand litter decomposition. Soil Org. 2021, 93, 73–78. [Google Scholar] [CrossRef]
- Duddigan, S.; Shaw, L.J.; Alexander, P.D.; Collins, C.D. Chemical underpinning of the tea bag index: An examination of the decomposition of tea leaves. Appl. Environ. Soil Sci. 2020, 2020, 6085180. [Google Scholar] [CrossRef]
- Mori, T.; Nakamura, R.; Aoyagi, R. Risk of misinterpreting the tea bag index: Field observations and a random simulation. Ecol. Res. 2022, 37, 381–389. [Google Scholar] [CrossRef]
- Didion, M.; Repo, A.; Liski, J.; Forsius, M.; Bierbaumer, M.; Djukic, I. Towards harmonizing leaf litter decomposition studies using standard tea bags—A field study and model application. Forests 2016, 7, 167. [Google Scholar] [CrossRef]
- Butzen, V.; Seeger, M.; Marruedo, A.; de Jonge, L.; Wengel, R.; Ries, J.B.; Casper, M.C. Water repellency under coniferous and deciduous forest—Experimental assessment and impact on overland flow. Catena 2015, 133, 255–265. [Google Scholar] [CrossRef]
- Datta, S.; Taghvaeian, S.; Stivers, J. Understandiung Soil Water Content and Thresholds for Irrigation Management. Oklahoma Cooperative Extension Service, Report BAE-1573; Division of Agriculture Sciences and Natural resources, Oklahoma State University: Stillwater, OK, USA, 2017. [Google Scholar]
- Coble, A.P.; Vadeboncoeur, M.A.; Carter Berry, Z.; Jennings, K.A.; McIntire, C.D.; Campbell, J.L.; Rustad, L.E.; Templer, P.H.; Asbjornsen, H. Are Northeastern, U.S. forests vulnerable to extreme drought? Ecol. Process. 2017, 6, 34. [Google Scholar] [CrossRef]
- Allison, S.D.; Hanson, C.A.; Treseder, K.K. Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems. Soil Biol. Biochem. 2007, 39, 1878–1887. [Google Scholar] [CrossRef]
- Kroes, J.G.; Wesseling, J.G.; Van Dam, J.C. Integrated modelling of the soil-water-atmosphere-plant system using the model SWAP 2.0. An overview of theory and an application. Hydrol. Process. 2000, 14, 1993–2002. [Google Scholar] [CrossRef]
- Massmann, A.; Gentine, P.; Lin, C. When does vapor pressure deficit drive or reduce evapotranspiration? J. Adv. Model. Earth Syst. 2019, 11, 3305–3320. [Google Scholar] [CrossRef]
- Zhang, Q.; Manzoni, S.; Katul, G.; Porporato, A.; Yang, D. The hysteretic evapotranspiration—Vapor pressure deficit relation. J. Geophys. Res. Biogeosci. 2014, 119, 125–140. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Li, M.; Zhao, C.; Yu, K.; Hagedorn, F. Responses of soil nitrogen and phosphorus cycling to drying and rewetting cycles: A meta-analysis. Soil Biol. Biochem. 2020, 148, 107896. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soil. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]



| % of Basal Area | |||
|---|---|---|---|
| Total Basal Area (m2 ha−1) | Conifers | Deciduous | |
| Fir-maple-birch | 77.2 | 33.3 | 66.7 |
| Cedar-maple-birch | 85.8 | 45.4 | 54.6 |
| Maple spp. | Birch spp. | Large-Tooth Aspen | American Beech | White Cedar | Needles | |
|---|---|---|---|---|---|---|
| Fir-maple-birch | 48.8 (22.4) | 38.8 (23.0) | 4.14 (8.63) | 0.00 (0.00) | 0.00 (0.00) | 8.21 (4.12) |
| Cedar-maple-birch | 45.1 (11.5) | 14.8 (10.1) | 1.15 (1.24) | 0.09 (0.20) | 38.8 (7.08) | 0.00 (0.00) |
| pH | C | N | C:N | PO4-P | Ca | Al | ||
|---|---|---|---|---|---|---|---|---|
| ______ % ______ | ______ mg Per kg of Soil ______ | |||||||
| Forest floor | Fir-maple-birch | 4.88 (0.13) | 37.9 (7.16) | 1.86 (0.32) | 20.4 (2.00) | 77.7 (59.8) | 3589 (1217) | 995 (696) |
| Cedar-maple-birch | 4.48 (0.33) | 44.4 (1.41) | 2.12 (0.05) | 21.0 (0.93) | 88.8 (35.2) | 3779 (547) | 329 (64.7) | |
| Upper B horizon | Fir-maple-birch | 5.25 (0.22) | 9.36 (4.08) | 0.47 (0.12) | 19.3 (3.28) | 0.37 (0.18) | 250 (74.3) | 2105 (187) |
| Cedar-maple-birch | 4.73 (0.29) | 10.1 (2.82) | 0.49 (0.11) | 20.5 (2.27) | 0.46 (0.29) | 374 (160) | 1863 (325) | |
| Warmed | Control | |
|---|---|---|
| Sugar maple | 0.00315 (0.00055) d | 0.00330 (0.00060) d |
| Green tea | 0.00226 (0.00031) c | 0.00212 (0.00025) c |
| Red maple | 0.00144 (0.00040) b | 0.00144 (0.00038) b |
| American beech | 0.00107 (0.00042) ab | 0.00094 (0.00024) ab |
| Large-tooth aspen | 0.00070 (0.00020) a | 0.00075 (0.00022) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bélanger, N.; Chaput-Richard, C. Experimental Warming of Typically Acidic and Nutrient-Poor Boreal Soils Does Not Affect Leaf-Litter Decomposition of Temperate Deciduous Tree Species. Soil Syst. 2023, 7, 14. https://doi.org/10.3390/soilsystems7010014
Bélanger N, Chaput-Richard C. Experimental Warming of Typically Acidic and Nutrient-Poor Boreal Soils Does Not Affect Leaf-Litter Decomposition of Temperate Deciduous Tree Species. Soil Systems. 2023; 7(1):14. https://doi.org/10.3390/soilsystems7010014
Chicago/Turabian StyleBélanger, Nicolas, and Clémence Chaput-Richard. 2023. "Experimental Warming of Typically Acidic and Nutrient-Poor Boreal Soils Does Not Affect Leaf-Litter Decomposition of Temperate Deciduous Tree Species" Soil Systems 7, no. 1: 14. https://doi.org/10.3390/soilsystems7010014
APA StyleBélanger, N., & Chaput-Richard, C. (2023). Experimental Warming of Typically Acidic and Nutrient-Poor Boreal Soils Does Not Affect Leaf-Litter Decomposition of Temperate Deciduous Tree Species. Soil Systems, 7(1), 14. https://doi.org/10.3390/soilsystems7010014






