Autogenous Eutrophication, Anthropogenic Eutrophication, and Climate Change: Insights from the Antrift Reservoir (Hesse, Germany)
Abstract
1. Introduction
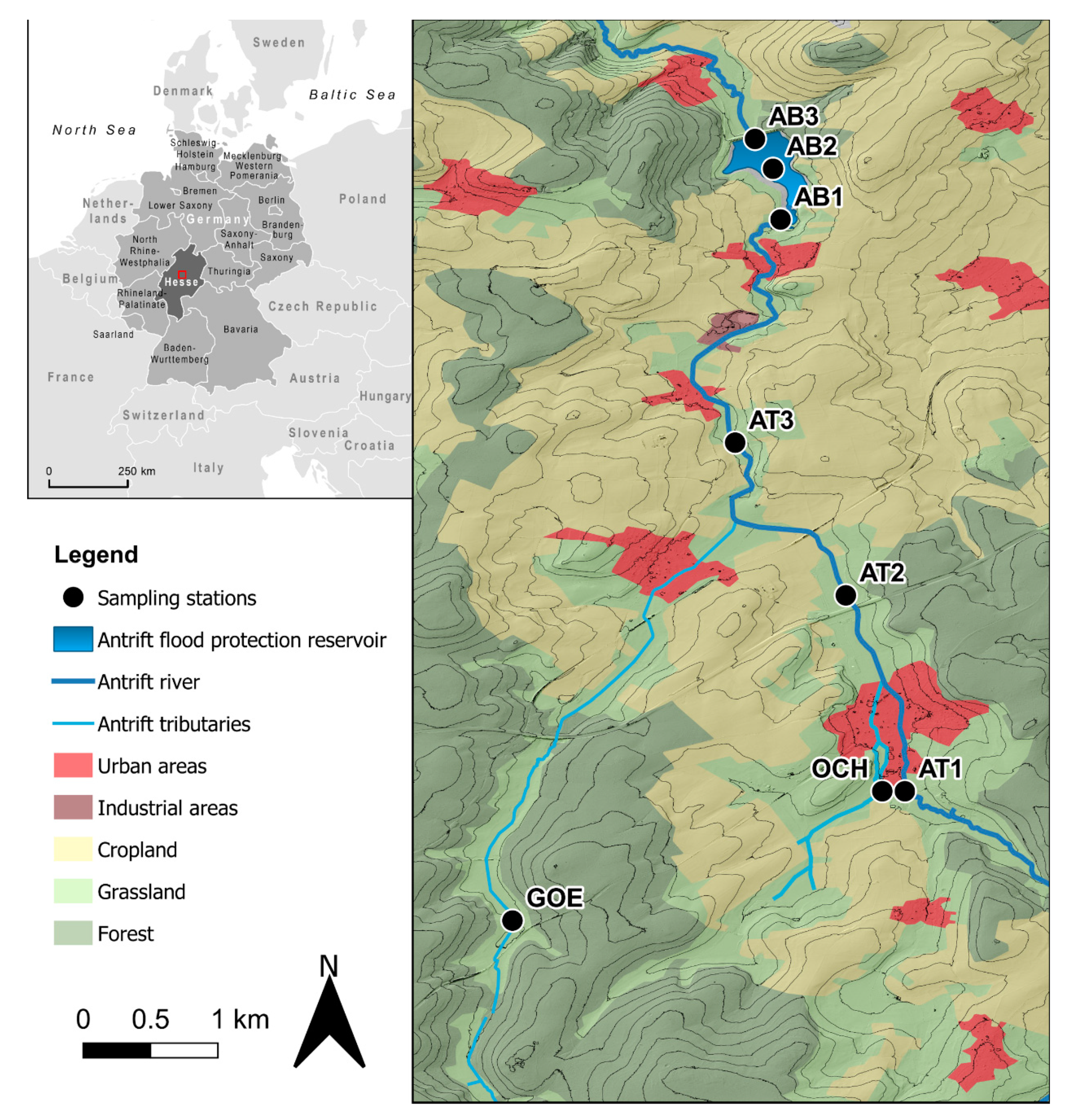
2. Study Area
3. Material and Methods
3.1. Water Sampling and Water Quality Analyses in the Field
3.2. Determination of Total Phosphorus in Water Samples
3.3. Sediment Analyses
3.4. Discharge and Precipitation Datasets
3.5. Statistical Analyses
4. Results
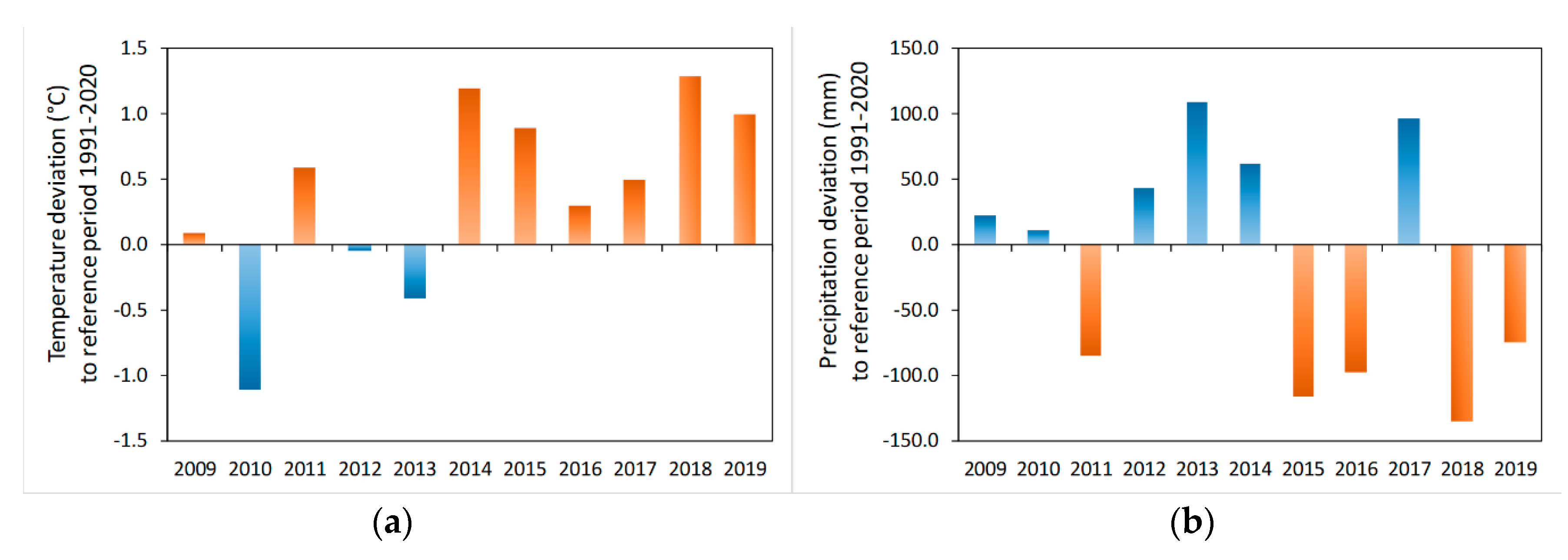
4.1. Evaluation of Climate Data: Discharge and Precipitation
4.2. Water Quality Assessment
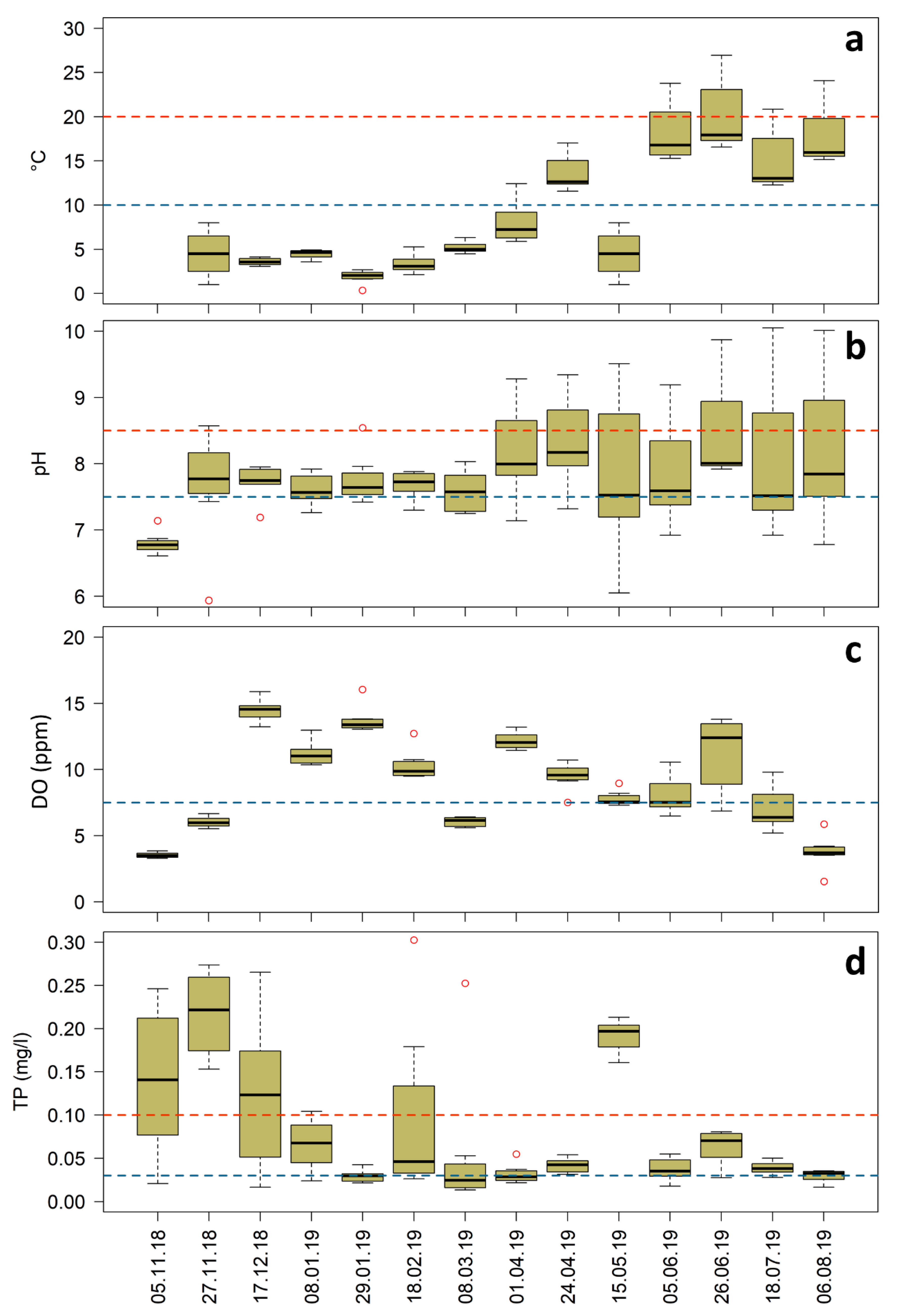
4.2.1. Water Temperature
4.2.2. pH
4.2.3. Dissolved Oxygen
4.2.4. Total Phosphorus
4.3. Correlation of Total Phosphorus Concentrations and Precipitation Events
4.4. Sediment Analyses
4.4.1. General Sediment Features
4.4.2. Sediment Phosphorus Contents
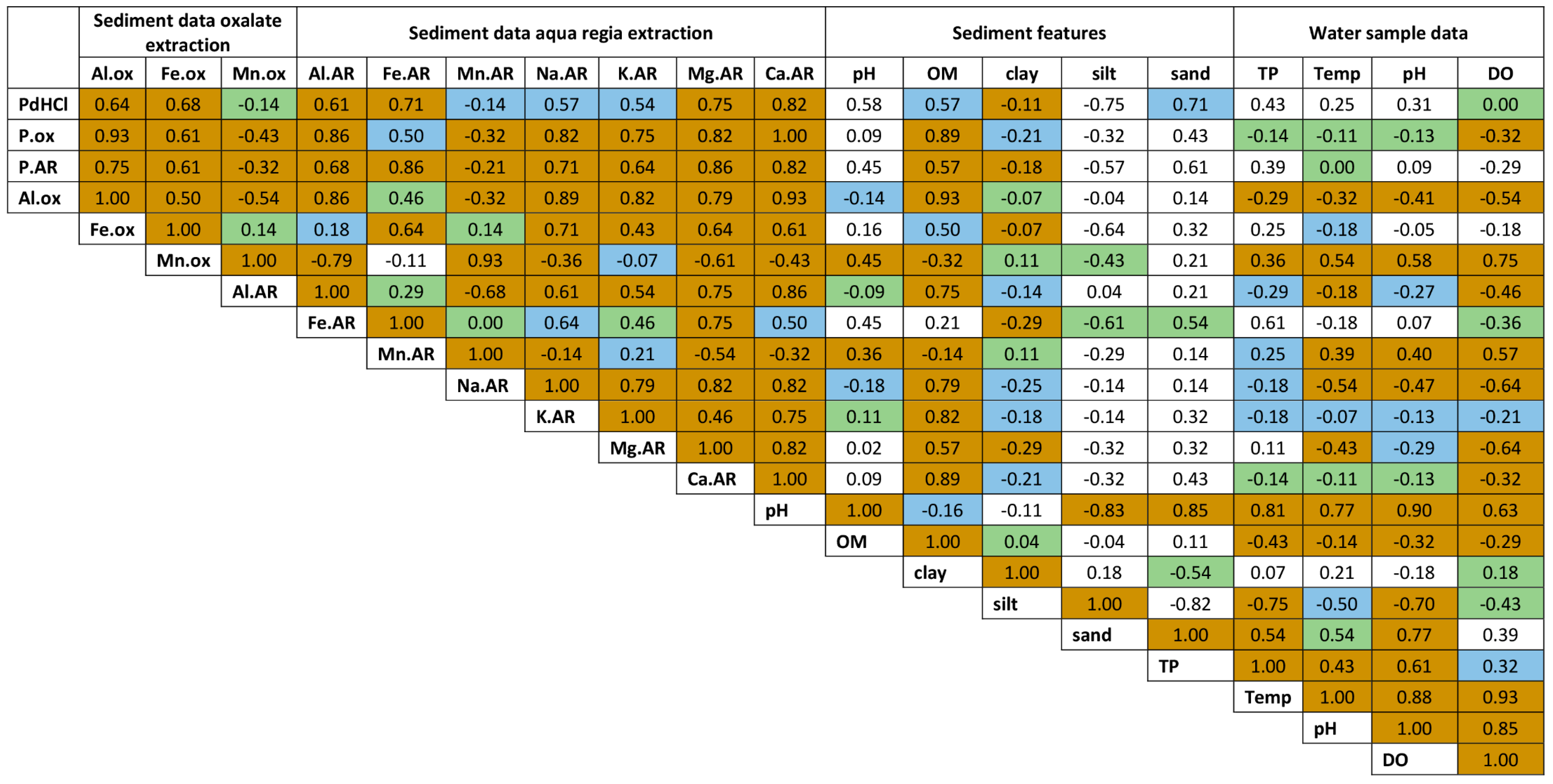
4.4.3. Correlation Analyses
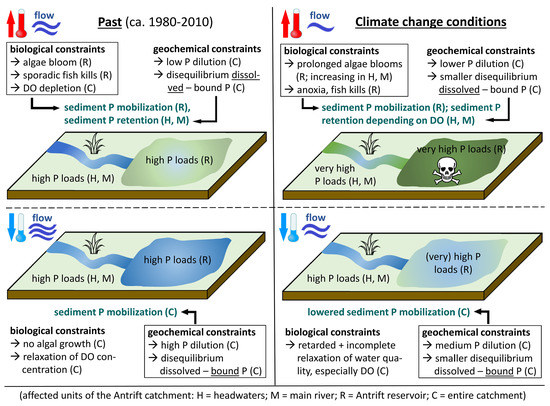
5. Discussion
5.1. Evaluation of Climate Data: Discharge and Precipitation
5.2. Water Quality Assessment
5.2.1. Water Temperature
5.2.2. pH
5.2.3. Dissolved Oxygen
5.2.4. Total Phosphorus
5.3. Phosphorus Sources in the Antrift Catchment
5.4. Sediment Analyses
5.4.1. General Sediment Features
5.4.2. Sediment Phosphorus Contents
5.5. Spearman Correlation Analyses of Sediments and Water Samples
5.5.1. Correlations with Acid and Base Metal Cations
5.5.2. Correlations with Sediment Organic Matter
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef]
- Nazari-Sharabian, M.; Ahmad, S.; Karakouzian, M. Climate Change and Eutrophication: A Short Review. Engineering. Technol. Appl. Sci. Res. 2018, 8, 3668–3672. [Google Scholar]
- Bowes, M.; Davison, P.; Hutchins, M.; McCall, S.; Prudhomme, C.; Sadowsko, J.; Soley, R.; Wells, R.; Willets, S. Climate Change and Eutrophication Risk in English Rivers; Technical Report No. SC140013/R; Environment Agency: Bristol, UK, 2016.
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of US Freshwaters: Analysis of Potential Economic Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef]
- Charlton, M.B.; Bowes, M.J.; Hutchins, M.G.; Orr, H.G.; Soley, R.; Davison, P. Mapping eutrophication risk from climate change: Future phosphorus concentrations in English rivers. Sci. Total Environ. 2018, 613, 1510–1526. [Google Scholar] [CrossRef]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Fisher, M. Subsoil Phosphorus Loss: A complex problem with no easy solutions. CSA News 2015, 4–9. [Google Scholar] [CrossRef]
- Breuning-Madsen, H.; Bloch Ehlers, C.; Borggaard, O.K. The impact of perennial cormorant colonies on soil phosphorus state. Geoderma 2008, 148, 51–54. [Google Scholar] [CrossRef]
- Sharpley, A.; Rekolainen, S. Phosphorus in agriculture and its environmental implications. In Phosphorus Loss from Soil to Water; Tunney, H., Carton, O.T., Brookes, P.C., Johnston, A.E., Eds.; CAB International: Wallingford, Ireland, 1997; pp. 1–53. ISBN 0851991564. [Google Scholar]
- Lal, R. Phosphorus and the environment. In Soil Phosphorus; Lal, R., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 209–223. ISBN 9781482257847. [Google Scholar]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Baker, L.A. Can urban P conservation help to prevent the brown devolution? Chemosphere 2011, 84, 779–784. [Google Scholar] [CrossRef]
- Jeppesen, E.; Moss, B.; Bennion, H.; Carvalho, L.; DeMeester, L.; Feuchtmayr, H.; Friberg, N.; Gessner, M.O.; Hefting, M.; Lauridsen, T.L.; et al. Interaction of climate change and eutrophication. In Climate Change Impacts on Freshwater Ecosystems; Kernan, M.R., Battarbee, R.W., Moss, B., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 119–151. ISBN 9781405179133. [Google Scholar]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; de Meester, L.; et al. Allied attack: Climate change and eutrophication. IW 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Nickus, U.; Bishop, K.; Erlandsson, M.; Evans, C.D.; Forsius, M.; Laudon, H.; Livingstone, D.M.; Monteith, D.; Thies, H. Direct impacts of climate change on freshwater ecosystems. In Climate Change Impacts on Freshwater Ecosystems; Kernan, M.R., Battarbee, R.W., Moss, B., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 38–64. ISBN 9781405179133. [Google Scholar]
- Whitehead, P.G.; Wilby, R.L.; Battarbee, R.W.; Kernan, M.; Wade, A.J. A review of the potential impacts of climate change on surface water quality. Hydrol. Sci. J. 2009, 101–123. [Google Scholar] [CrossRef]
- Hering, D.; Haidekker, A.; Schmidt-Kloiber, A.; Barker, T.; Buisson, L.; Graf, W.; Grenouillet, G.; Lorenz, A.; Sandin, L.; Stendera, S. Monitoring the responses of freshwater ecosystems to climate change. In Climate Change Impacts on Freshwater Ecosystems; Kernan, M.R., Battarbee, R.W., Moss, B., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 84–118. ISBN 9781405179133. [Google Scholar]
- Verdonschot, P.F.M.; Hering, D.; Murphy, J.; Jähnig, S.C.; Rose, N.L.; Graf, W.; Brabec, K.; Sandin, L. Climate change and the hydrology and morphology of freshwater ecosystems. In Climate Change Impacts on Freshwater Ecosystems; Kernan, M.R., Battarbee, R.W., Moss, B., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 65–83. ISBN 9781405179133. [Google Scholar]
- Quinton, J.N.; Catt, J.A.; Hess, T.M. The selective removal of phosphorus from soil: Is event size important? J. Environ. Qual. 2001, 30, 538–545. [Google Scholar] [CrossRef]
- Hessisches Ministerium für Umwelt, Klimaschutz, Landwirtschaft und Verbraucherschutz. Bewirtschaftungsplan 2015–2021; HMUKLV: Wiesbaden, Germany, 2015.
- Hessisches Ministerium für Umwelt, Klimaschutz, Landwirtschaft und Verbraucherschutz. Maßnahmenprogramm Hessen 2015–2021; HMUKLV: Wiesbaden, Germany, 2015.
- Hessisches Landesamt für Naturschutz, Umwelt und Geologie. Umweltatlas Hessen—Die Naturräume Hessens. Available online: http://atlas.umwelt.hessen.de/atlas/ (accessed on 10 October 2019).
- Strahler, A.H. Introducing Physical Geography, 5th ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470418116. [Google Scholar]
- Climate-Data. Klima Romrod. Available online: https://de.climate-data.org/europa/deutschland/hessen/romrod-22186/ (accessed on 13 December 2019).
- Global Warming of 1.5 °C. In An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change, Ed.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.E.A. IPCC, 2018: Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change, Ed.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Hessisches Statistisches Landesamt. Hessische Gemeindestatistik 2019 (Hessian Municipal Statistics 2019); Hessisches Statistisches Landesamt (Hessian State Statistical Office): Wiesbaden, Germany, 2019. [Google Scholar]
- Friedrich, K.; Sabel, K.-J. Standorttypisierung für Biotopenentwicklung, Maßstab 1:50.000, Blatt L 5320 Alsfeld; Hessisches Landesamt für Naturschutz, Umwelt und Geologie: Wiesbaden, Germany, 2002.
- Rosenberger, W.; Sabel, K.-J. Geologische Karte von Hessen, Maßstab 1:50.000, Blatt L 5320 Alsfeld; Hessisches Landesamt für Naturschutz, Umwelt und Geologie (HLNUG): Wiesbaden, Germany, 2002.
- Lotz, K. Einführung in die Geologie des Landes Hessen; Hitzeroth: Marburg, Germany, 1995. [Google Scholar]
- Meschede, M.; Warr, L.N. The Geology of Germany: A Process-Oriented Approach; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Diehl, O. Geologische Karte von Hessen, Maßstab 1:25.000, Blatt 5221 Alsfeld; Hessisches Landesamt für Naturschutz, Umwelt und Geologie (HLNUG): Darmstadt, Germany, 1926.
- Nesbor, H.-D. Geologische Karte von Hessen, Maßstab 1:25.000, Blatt 5321 Storndorf; Hessisches Landesamt für Naturschutz, Umwelt und Geologie (HLNUG): Wiesbaden, Germany, 2009.
- Frede, H.-G. Nährstoffeinträge im Einzugsgebiet der Antrifttalsperre—Abschätzung der Anteile aus Diffusen und Punktuellen Quellen. Gutachten im Auftrag des Wasserverbandes Antrifttal; unpublished assessment on behalf of the Wasserverband Antrifttal: Giessen, Germany, 1994. [Google Scholar]
- Hessisches Landesamt für Naturschutz, Umwelt und Geologie. Gütebewertung Seen 2015 Bereich Regierungspräsidium Kassel; Hessisches Landesamt für Naturschutz, Umwelt und Geologie (HLNUG): Wiesbaden, Germany, 2015.
- Hessisches Landesamt für Naturschutz, Umwelt und Geologie. Umsetzung der Wasserrahmenrichtlinie in Hessen—Bewirtschaftungsplan Hessen 2009–2015, 1. Aufl.; Hessisches Ministerium für Umwelt, Energie, Landwirtschaft und Verbraucherschutz: Wiesbaden, Germany, 2009; ISBN 9783892742890.
- Landesbetrieb Landwirtschaft Hessen. Pilotprojekt—Umstellung der Landwirtschaftlichen Bewirtschaftung zur Verminderung des Erosiven Nährstoffeintrages in den Antrift-Stausee; Landesbetrieb Landwirtschaft Hessen: Alsfeld, Germany, 2007. [Google Scholar]
- Tunney, H.; Coulter, B.; Daly, K.; Kurz, I.; Coxon, C.; Jeffrey, D.; Mills, P.; Kiely, G.; Morgan, G. Quantification of Phosphorus Loss from Soil to Water: End of Project Report, ARMIS 4365; Teagasc (Agriculture and Food Development Authority): Dublin, Ireland, 2000. [Google Scholar]
- Bergström, L.; Kirchmann, H.; Djodjic, F.; Kyllmar, K.; Ulén, B.; Liu, J.; Andersson, H.; Aronsson, H.; Börjesson, G.; Kynkäänniemi, P.; et al. Turnover and losses of phosphorus in Swedish agricultural soils: Long-term changes, leaching trends, and mitigation measures. J. Environ. Qual. 2015, 44, 512–523. [Google Scholar] [CrossRef]
- Andersson, H.; Bergström, L.; Djodjic, F.; Ulén, B.; Kirchmann, H. Topsoil and subsoil properties influence phosphorus leaching from agricultural soils. J. Environ. Qual. 2013, 455–463. [Google Scholar] [CrossRef]
- Delgado, A.; Scalenghe, R. Aspects of phosphorus transfer from soils in Europe. J. Plant Nutr. Soil Sci. 2008, 171, 552–575. [Google Scholar] [CrossRef]
- Grimm, M. Ermittlung des Erosiven P-Eintrages in den Antrift-Stausee und Maßnahmen zu ihrer Reduzierung; Gesellschaft für Boden- und Gewässerschutz: Giessen, Germany, 2000. [Google Scholar]
- Sharpley, A.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus legacy: Overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef]
- Borchardt, D.; Fischer, J. Untersuchungen zum P-Rücklösungspotential der Sedimente der Antrifttalsperre; Institut für Gewässerforschung und Gewässerschutz (IAG) und Universität Kassel: Kassel, Germany, 2001. [Google Scholar]
- Cornel, P.; Schaum, C.; Lutze, R. Gutachten zur Rücklösung von Phosphor im Gewässer aus Feststoffen von Kläranlagenabläufen; unpublished Assessment on behalf of the Hessian Agency of Nature Conservation, Environment and Geology (HLNUG); Hessisches Landesamt für Naturschutz, Umwelt und Geologie (HLNUG): Wiesbaden, Germany, 2015.
- Bundesministeriums der Justiz und für Verbraucherschutz. Oberflächengewässerverordnung (National Implementation of the EU Water Framework Directive); OGewV: Berlin, Germany, 2016. [Google Scholar]
- Kianpoor Kalkhajeh, Y.; Jabbarian Amiri, B.; Huang, B.; Henareh Khalyani, A.; Hu, W.; Gao, H.; Thompson, M.L. Methods for Sample Collection, Storage, and Analysis of Freshwater Phosphorus. Water 2019, 11, 1889. [Google Scholar] [CrossRef]
- Hanna Instruments Inc. HI9829 Unstruction Manual; Hanna Instruments Inc.: Woonsocket, RI, USA, 2016. [Google Scholar]
- DIN EN ISO 6878: 2004-09. Water quality—Determination of phosphorus—Ammonium molybdate spectrometric method (ISO 6878:2004); German version EN ISO 6878:2004. In Handbuch der Bodenuntersuchung. Terminologie, Verfahrensvorschriften und Datenblätter; Physikalische, Chemische, Biologische Untersuchungsverfahren; Gesetzliche Regelwerke; Deutsches Institut für Normung, Ed.; Wiley: Berlin, Germany, 2004. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 293–306. [Google Scholar]
- Telgheder, U. Leitfaden zur Auswertung Analytischer Ergebnisse. Available online: https://www.uni-due.de/imperia/md/content/water-science/ws1314/1351_2661_ws1314_leitfaden_ausertung.pdf (accessed on 13 April 2017).
- Molt, K.; Telgheder, U. Berechnung der Verfahrensstandardabweichung und Nachweis-, Erfassungs- und Bestimmungsgrenze Einer Kalibrierung Gemäß DIN 32645. Available online: https://www.uni-due.de/imperia/md/content/iac/git_erw_1.pdf (accessed on 13 April 2017).
- JCGM = Joint Committee for Guides in Metrology. Evaluation of Measurement Data. Guide to the Expression of Uncertainty in Measurement. Available online: http://www.bipm.org/en/publication/guides/gum.html (accessed on 12 January 2020).
- Durner, W.; Iden, S.C.; von Unold, G. The integral suspension pressure method (ISP) for precise particle-size analysis by gravitational sedimentation. Water Resour. Res. 2017, 53, 33–48. [Google Scholar] [CrossRef]
- Weihrauch, C.; Schupp, A.; Söder, U.; Opp, C. Could oxalate-extractable phosphorus replace phosphorus fractionation schemes in soil phosphorus prospections? A case study in the prehistoric Milseburg hillfort (Germany). Geoarchaeology 2020, 35, 98–111. [Google Scholar] [CrossRef]
- Thomas, R. A beginner’s guide to ICP-MS—Part VII: Mass separation devices—Double-focusing magnetic-sector technology. Spectroscopy 2001, 16, 22. [Google Scholar]
- Thomas, R. A beginner’s guide to ICP-MS—Part VIII—Mass analyzers: Time-of-flight technology. Spectroscopy 2002, 17, 36. [Google Scholar]
- Weihrauch, C. Phosphor-Dynamiken in Böden: Grundlagen, Konzepte und Untersuchungen zur Räumlichen Verteilung des Nährstoffs; Springer: Wiesbaden, Germany, 2018; ISBN 9783658223489. [Google Scholar]
- Deutscher Wetterdienst. Precipitation and temperature data 2018–2019 climate station Alsfeld-Eifa (DWD91). In Wetterextreme Hessen; Hessian Agency for Nature Conservation, Environment and Geology: Wiesbaden, Germany, 2019. [Google Scholar]
- Deutscher Wetterdienst. Precipitation and temperature data 2018–2019 climate station Neustadt (DWD 3561). In Wetterextreme Hessen; Hessian Agency for Nature Conservation, Environment and Geology: Wiesbaden, Germany, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2018. [Google Scholar]
- Wei, T.; Simko, V. R Package “corrplot”: Visualization of a Correlation. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 21 December 2019).
- Zimmermann-Janschitz, S. Statistik in der Geographie. Eine Exkursion durch die Deskriptive Statistik; Springer: Berlin, Germany, 2014; ISBN 9783827426116. [Google Scholar]
- Sharpley, A.N.; Chapra, S.C.; Wedepohl, R.; Sims, J.T.; Daniel, T.C.; Reddy, K.R. Managing Agricultural Phosphorus for Protection of Surface Waters: Issues and Options. J. Environ. Qual. 1994, 23, 437–451. [Google Scholar] [CrossRef]
- Bol, R.; Gruau, G.; Mellander, P.-E.; Dupas, R.; Bechmann, M.; Skarbøvik, E.; Bieroza, M.; Djodjic, F.; Glendell, M.; Jordan, P.; et al. Challenges of Reducing Phosphorus Based Water Eutrophication in the Agricultural Landscapes of Northwest Europe. Front. Mar. Sci. 2018, 5, 296. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jarvie, H.P. Delivery and cycling of phosphorus in rivers: A review. Sci. Total Environ. 2008, 400, 379–395. [Google Scholar] [CrossRef]
- Dunkerley, D.L. Rainfall intensity bursts and the erosion of soils: An analysis highlighting the need for high temporal resolution rainfall data for research under current and future climates. Earth Surf. Dynam. 2019, 7, 345–360. [Google Scholar] [CrossRef]
- Lane, L.J.; Shirley, E.D.; Singh, V.P. Modeling erosion on hillslopes. In Modeling Geomorphological Systems; Abderson, M.G., Ed.; Wiley: New York, NY, USA, 1988; pp. 287–308. [Google Scholar]
- Piacentini, T.; Galli, A.; Marsala, V.; Miccadei, E. Analysis of Soil Erosion Induced by Heavy Rainfall: A Case Study from the NE Abruzzo Hills Area in Central Italy. Water 2018, 10, 1314. [Google Scholar] [CrossRef]
- Reid, K.; Schneider, K.; McConkey, B. Components of Phosphorus Loss from Agricultural Landscapes, and How to Incorporate Them into Risk Assessment Tools. Front. Earth Sci. 2018, 6, 179. [Google Scholar] [CrossRef]
- Wetterextreme Hessen; Hessian Agency for Nature Conservation, Environment and Geology, Ed.; Wiesbaden. 2019. Available online: https://www.hlnug.de/?id=11522 (accessed on 17 December 2019).
- Hessian Agency for Nature Conservation, Environment and Geology. Hochwasservorwarnung 20.05.2019. (Flood Advance Warning). Available online: http://www2.hochwasser-hessen.de/hochwasserportal-hessen/aktuelle-hochwasserlage/meldung.html?tx_pghochwasser_pi1%5Baction%5D=show&tx_pghochwasser_pi1%5Bmeldung%5D=1157&tx_pghochwasser_pi1%5Bcontroller%5D=Meldung&cHash=1cd845bb5cc04584adace543515f5498 (accessed on 6 July 2019).
- Umweltbundesamt. Erosion. Available online: https://www.umweltbundesamt.de/en/topics/soil-agriculture/land-a-precious-resource/erosion#textpart-1 (accessed on 17 December 2019).
- Schwertmann, U.; Vogel, W.; Kainz, M. Bodenerosion durch Wasser—Vorhersage des Abtrags und Bewertung von Gegenmaßnahmen (Soil Erosion by Water—Prediction of the Removal and Evaluation of Countermeasures); Ulmer: Stuttgart, Germany, 1987. [Google Scholar]
- Hessisches Ministerium für Umwelt, Klimaschutz, Landwirtschaft und Verbraucherschutz. Integrierter Klimaschutzplan Hessen 2025; Hessisches Ministerium für Umwelt, Energie, Landwirtschaft und Verbraucherschutz: Wiesbaden, Germany, 2019.
- Foley, B.; Jones, I.D.A.N.; Maberly, S.C.; Rippey, B. Long-term changes in oxygen depletion in a small temperate lake: Effects of climate change and eutrophication. Freshwater Biol. 2012, 57, 278–289. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Richardson, J.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Change Biol. 2019, 25, 3365–3380. [Google Scholar] [CrossRef]
- Neufeld, J.D. Can Shade Structures Help Riparian Areas? A look at using constructed shades to pull cattle off riparian areas in northeastern Nevada. Rangelands 2005, 27, 24–30. [Google Scholar] [CrossRef]
- Rutherford, J.C. Some approaches for measuring and modelling riparian shade. Ecol. Eng. 2005, 24, 525–530. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Liu, W.-C.; Chen, W.-B.; Kimura, N. Impact of phosphorus load reduction on water quality in a stratified reservoir-eutrophication modeling study. Environ. Monit. Assess. 2009, 159, 393–406. [Google Scholar] [CrossRef]
- Ansari, A.A.; Gill, S.S. (Eds.) Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hudnell, H.K. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: New York, NY, USA, 2008. [Google Scholar]
- Kernan, M.R.; Battarbee, R.W.; Moss, B. (Eds.) Climate Change Impacts on Freshwater Ecosystems; Wiley: Chichester, UK, 2010; ISBN 9781405179133. [Google Scholar]
- Williams, R.J.; White, C.; Harrow, M.L.; Neal, C. Temporal and small-scale spatial variations of dissolved oxygen in the Rivers Thames, Pang and Kennet, UK. Sci. Total Environ. 2000, 497–510. [Google Scholar] [CrossRef]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Jones, J.I. How green is my river? A new paradigm of eutrophication in rivers. Sci. Total Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef]
- Dorgham, M.M. Effects of Eutrophication. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 29–44. [Google Scholar]
- Young, K.; Morse, G.K.; Scrimshaw, M.D.; Kinniburgh, J.H.; MacLoad, C.L.; Lester, J.N. The relation between phosphorus and eutrophication in the Thames catchment, UK. Sci. Total Environ. 1999, 228, 157–183. [Google Scholar] [CrossRef]
- Djodjic, F.; Markensten, H. From single fields to river basins: Identification of critical source areas for erosion and phosphorus losses at high resolution. Ambio 2019, 48, 1129–1142. [Google Scholar] [CrossRef]
- Cassidy, R.; Thomas, I.A.; Higgins, A.; Bailey, J.S.; Jordan, P. A carrying capacity framework for soil phosphorus and hydrological sensitivity from farm to catchment scales. Sci. Total Environ. 2019, 687, 277–286. [Google Scholar] [CrossRef]
- Kleinman, P.J.A.; Church, C.; Saporito, L.S.; McGrath, J.M.; Reiter, M.S.; Allen, A.L.; Tingle, S.; Binford, G.D.; Han, K.; Joern, B.C. Phosphorus leaching from agricultural soils of the delmarva peninsula, USA. J. Environ. Qual. 2015, 44, 524–534. [Google Scholar] [CrossRef]
- Weihrauch, C. Dynamics need space—A geospatial approach to soil phosphorus’ reactions and migration. Geoderma 2019. [Google Scholar] [CrossRef]
- Christianson, L.E.; Harmel, R.D.; Smith, D.; Williams, M.R.; King, K. Assessment and Synthesis of 50 Years of Published Drainage Phosphorus Losses. J. Environ. Qual. 2016, 45, 1467–1477. [Google Scholar] [CrossRef]
- Blume, H.-P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Scheffer/Schachtschabel Soil Science, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783642309410. [Google Scholar]
- Miall, A. Fluvial Depositional Systems; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Robert, A. River Processes: An Introduction to Fluvial Dynamics; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Fryirs, K.A.; Brierley, G.J. Geomoprhic Analysis of River Systems: An Approach to Reading the Landscape; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Holliday, V.T.; Gartner, W.G. Methods of soil P analysis in archaeology. J. Archaeol. Sci. 2007, 34, 301–333. [Google Scholar] [CrossRef]
- Amberger, A. Pflanzenernährung. Ökologische und Physiologische Grundlagen, Dynamik und Stoffwechsel der Nährelemente, 3rd ed.; 74 Tabellen; Ulmer: Stuttgart, Germany, 1988; ISBN 3800125536. [Google Scholar]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kuehn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rueger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Prietzel, J.; Klysubun, W.; Werner, F. Speciation of phosphorus in temperate zone forest soils as assessed by combined wet-chemical fractionation and XANES spectroscopy. J. Plant Nutr. Soil Sci. 2016, 179, 168–185. [Google Scholar] [CrossRef]
- Binner, I.; Dultz, S.; Schenk, M.K. Phosphorus buffering capacity of substrate clays and its significance for plant cultivation. J. Plant Nutr. Soil Sci. 2015, 178, 155–164. [Google Scholar] [CrossRef]
- Sharpley, A.N. Soil phosphorus dynamics: Agronomic and environmental impacts. Ecol. Eng. 1995, 5, 261–279. [Google Scholar] [CrossRef]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Reddy, K.R.; Wetzel, R.G.; Kadlec, R.H. Biogeochemistry of phosphorus in wetlands. In Phosphorus: Agriculture and the Environment; Sims, J.T., Sharpley, A.N., Eds.; American Society of Agronomy: Madison, WI, USA, 2005; pp. 263–316. ISBN 9780891181576. [Google Scholar]
- Samadi, A. Phosphorus sorption characteristics in relation to soil properties in some calcareous soils of Western Azarbaijan Province. J. Agric. Sci. Technol. 2006, 8, 251–264. [Google Scholar]
- Holford, O.C.R.; Mattingly, G.E.G. phosphate sorption by jurassic oolitic limestones. Geoderma 1975, 13, 257–264. [Google Scholar] [CrossRef]
- Alvarez, R.; Evans, L.A.; Milham, P.J.; Wilson, M.A. Effects of humic material on the precipitation of calcium phosphate. Geoderma 2004, 118, 245–260. [Google Scholar] [CrossRef]
- Holzmann, S.; Missong, A.; Puhlmann, H.; Siemens, J.; Bol, R.; Klumpp, E.; von Wilpert, K. Impact of anthropogenic induced nitrogen input and liming on phosphorus leaching in forest soils. J. Plant Nutr. Soil Sci. 2016, 179, 443–453. [Google Scholar] [CrossRef]
- Cosette Galvan-Tejada, N.; Pena-Ramirez, V.; Mora-Palomino, L.; Siebe, C. Soil P fractions in a volcanic soil chronosequence of Central Mexico and their relationship to foliar P in pine trees. J. Plant Nutr. Soil Sci. 2014, 177, 792–802. [Google Scholar] [CrossRef]
- Smeck, N.E. Phosphorus dynamics in soils and landscapes. Geoderma 1985, 36, 185–199. [Google Scholar] [CrossRef]
- Wang, M.K.; Tzou, Y.M. Phosphate sorption by calcite, and iron-rich calcareous soils. Geoderma 1995, 65, 249–261. [Google Scholar] [CrossRef]
- Yang, X.-E.; Wu, X.; Hao, H.-L.; He, Z.-L. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef]
- Trimble, S.W. Streams, valleys and floodplains in the sediment cascade. In Sediment Cascades: An Integrated Approach; Burt, T.P., Allison, R.J., Eds.; Wiley-Blackwell: Chichester, UK, 2010; ISBN 9780470849620. [Google Scholar]
- Meals, D.W.; Dressing, S.A.; Davenport, T.E. Lag Time in Water Quality Response to Best Management Practices: A Review. J. Environ. Qual. 2010, 39, 85–96. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]






| Water Body Features | Riparian Features | Features of Surrounding Landscape | |||
|---|---|---|---|---|---|
| Water Body Type | Sampling Stations | Bedrock/Sub-strata and Soils | Land Use | Bedrocks/Substrata and Soils | Land Use |
| Tributaries | GOE OCH | Alluvial sediments, Gleysols and Anthrosols | Grassland (meadow), strip of trees (alder) next to water courses | Basalt ridge with some loess accumulation along slopes, Leptosols, Cambisols, Luvisols | Forest (low percentage of cropland) |
| Main river | AT-1 AT-2 AT-3 | Alluvial sediments, Gleyic Fluvisols and Anthrosols | Grassland (meadow), strip of trees (alder) next to water courses | Dominantly loess, Luvisols, Stagnic Luvisols | Cropland (low percentage of grassland) |
| Flood protection reservoir | AB-1 AB-2 AB-3 | Alluvial sediments, Gleyic Fluvisols and Anthrosols | Strip of trees (alder, willow), dam structures, nature reserve | Oligocene clays (S, SE), Mesozoic sandstones (direct reservoir surroundings), Pleistocene loess (surrounding slopes), Luvisols, Stagnic Luvisols | Cropland (low percentage of forest) |
| Station | Date | pH (CaCl2) | OM a (Mass-%) | Grain Size (Mass-%) | P Fractions (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | PdHCl | Pox | PAR | ||||
| GOE | 05.11.2018 | 6.03 | 18.79 | 44.34 | 47.81 | 7.84 | 379.61 | 720.53 | 1214.73 |
| 15.03.2019 | 6.16 | 7.53 | 22.98 | 74.00 | 3.02 | 228.44 | 630.00 | 919.80 | |
| OCH | 05.11.2018 | 6.66 | 15.30 | 28.96 | 65.08 | 5.96 | 326.37 | 634.67 | 1093.40 |
| 15.03.2019 | 6.01 | 9.13 | 6.53 | 88.51 | 4.96 | 226.64 | 672.00 | 1094.80 | |
| AT1 | 05.11.2018 | n.d.b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 15.03.2019 | 6.26 | 10.72 | 10.05 | 75.83 | 14.12 | 429.26 | 796.13 | 1255.57 | |
| AT2 | 05.11.2018 | 7.17 | 8.85 | 22.12 | 54.26 | 23.61 | 559.00 | 952.23 | 1727.60 |
| 15.03.2019 | 6.26 | 11.76 | 15.59 | 73.27 | 11.14 | 601.05 | 966.47 | 1658.77 | |
| AT3 | 05.11.2018 | 7.16 | 6.92 | 18.03 | 67.61 | 14.36 | 465.44 | 796.13 | 1311.10 |
| 15.03.2019 | 6.70 | 3.92 | 5.54 | 72.28 | 22.18 | 482.89 | 767.67 | 1277.73 | |
| AB1 | 05.11.2018 | 7.03 | 7.42 | 23.72 | 66.22 | 10.06 | 331.99 | 836.73 | 1376.90 |
| 15.03.2019 | 6.71 | 17.27 | 7.66 | 65.52 | 26.82 | 666.55 | 1188.37 | 1630.53 | |
| AB2 | 05.11.2018 | 7.61 | 3.17 | 21.11 | 57.07 | 21.83 | 84.33 | 221.20 | 507.27 |
| 15.03.2019 | 7.14 | 2.89 | 12.59 | 72.86 | 14.55 | 304.29 | 546.70 | 1197.70 | |
| AB3 | 05.11.2018 | 7.48 | 3.41 | 25.77 | 66.19 | 8.04 | 3.94 | 107.33 | 429.80 |
| 15.03.2019 | 7.69 | 2.09 | 5.38 | 12.29 | 82.33 | 230.10 | 154.93 | 402.73 | |
| PdHCl a | Pml a (mg/kg) | Prc a | DPM2 a | DPM3 a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | |||||||||
| all data | 355.8 | 341.1 | 484.2 | 0.47 | 0.57 | |||||
| sampling | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| catchment | 307.2 a * | 400.2 b | 302.6 a | 381.6 a | 484.6 a | 483.8 a | 0.43 a | 0.51 a | 0.52 a | 0.62 b |
| headwaters | 353.0 a,A | 227.5 b,A | 324.6 a,A | 423.5 b,A | 476.5 a,A | 356.3 b,A | 0.52 a,A | 0.35 b,A | 0.59 a,A | 0.65 a,A |
| main river | 512.2 a,A | 504.4 a,B | 362.0 a,A | 339.0 a,A | 645.2 a,B | 553.9 a,B | 0.59 a,A | 0.60 a,B | 0.58 a,A | 0.61 a,A |
| reservoir | 140.1 a,B | 412.3 b,AB | 248.3 a,A | 407.8 a,A | 382.9 a,A | 498.6 a,AB | 0.27 a,B | 0.56 b,B | 0.44 a,A | 0.59 a,A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, C.J.; Weihrauch, C. Autogenous Eutrophication, Anthropogenic Eutrophication, and Climate Change: Insights from the Antrift Reservoir (Hesse, Germany). Soil Syst. 2020, 4, 29. https://doi.org/10.3390/soilsystems4020029
Weber CJ, Weihrauch C. Autogenous Eutrophication, Anthropogenic Eutrophication, and Climate Change: Insights from the Antrift Reservoir (Hesse, Germany). Soil Systems. 2020; 4(2):29. https://doi.org/10.3390/soilsystems4020029
Chicago/Turabian StyleWeber, Collin J., and Christoph Weihrauch. 2020. "Autogenous Eutrophication, Anthropogenic Eutrophication, and Climate Change: Insights from the Antrift Reservoir (Hesse, Germany)" Soil Systems 4, no. 2: 29. https://doi.org/10.3390/soilsystems4020029
APA StyleWeber, C. J., & Weihrauch, C. (2020). Autogenous Eutrophication, Anthropogenic Eutrophication, and Climate Change: Insights from the Antrift Reservoir (Hesse, Germany). Soil Systems, 4(2), 29. https://doi.org/10.3390/soilsystems4020029