Geochemical Fingerprint and Soil Carbon of Sandy Alfisols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Soil Sampling and Soil Properties
2.3. Elemental Analysis
2.4. XRD Analysis
2.5. Weathering Indices
2.6. Statistics
3. Results
3.1. Particle-Size Fractions
3.2. Carbon and Iron
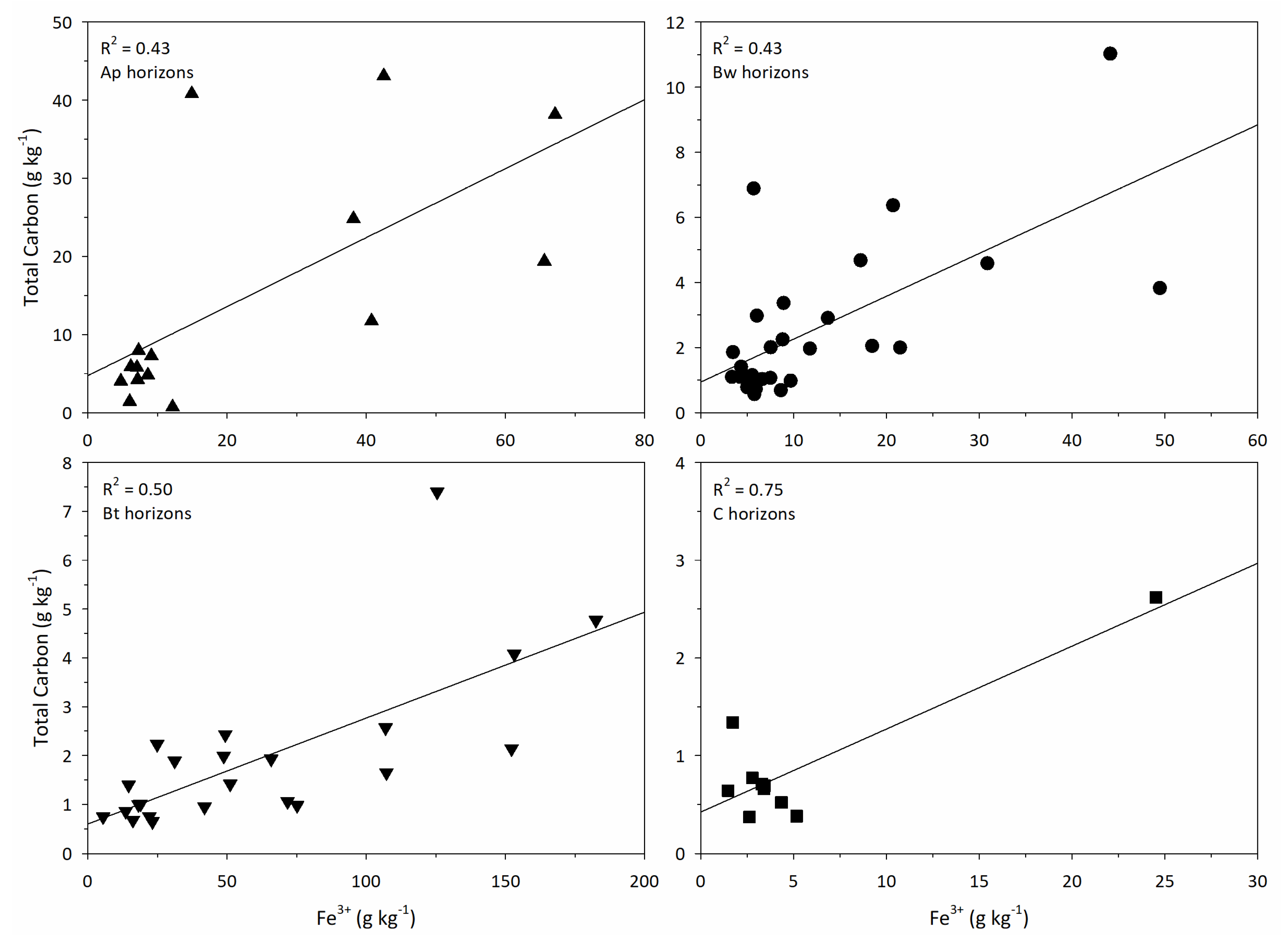
3.2.1. Bulk Soil
3.2.2. Particle-Size Fractions Carbon
3.2.3. Particle-Size Fractions Iron
3.3. Elemental Analysis by pXRF and EDS
3.3.1. Bulk Soil (pXRF)
3.3.2. Particle-Size Fractions (EDS)
3.4. Correlations
3.4.1. Bulk Soil (pXRF)
3.4.2. Particle-Size Fractions (EDS)
3.5. Mineralogy
3.6. Weathering Indices
4. Discussion
4.1. Total Carbon
4.2. Carbon Storage
4.3. Elements, Mineralogy, and Weathering
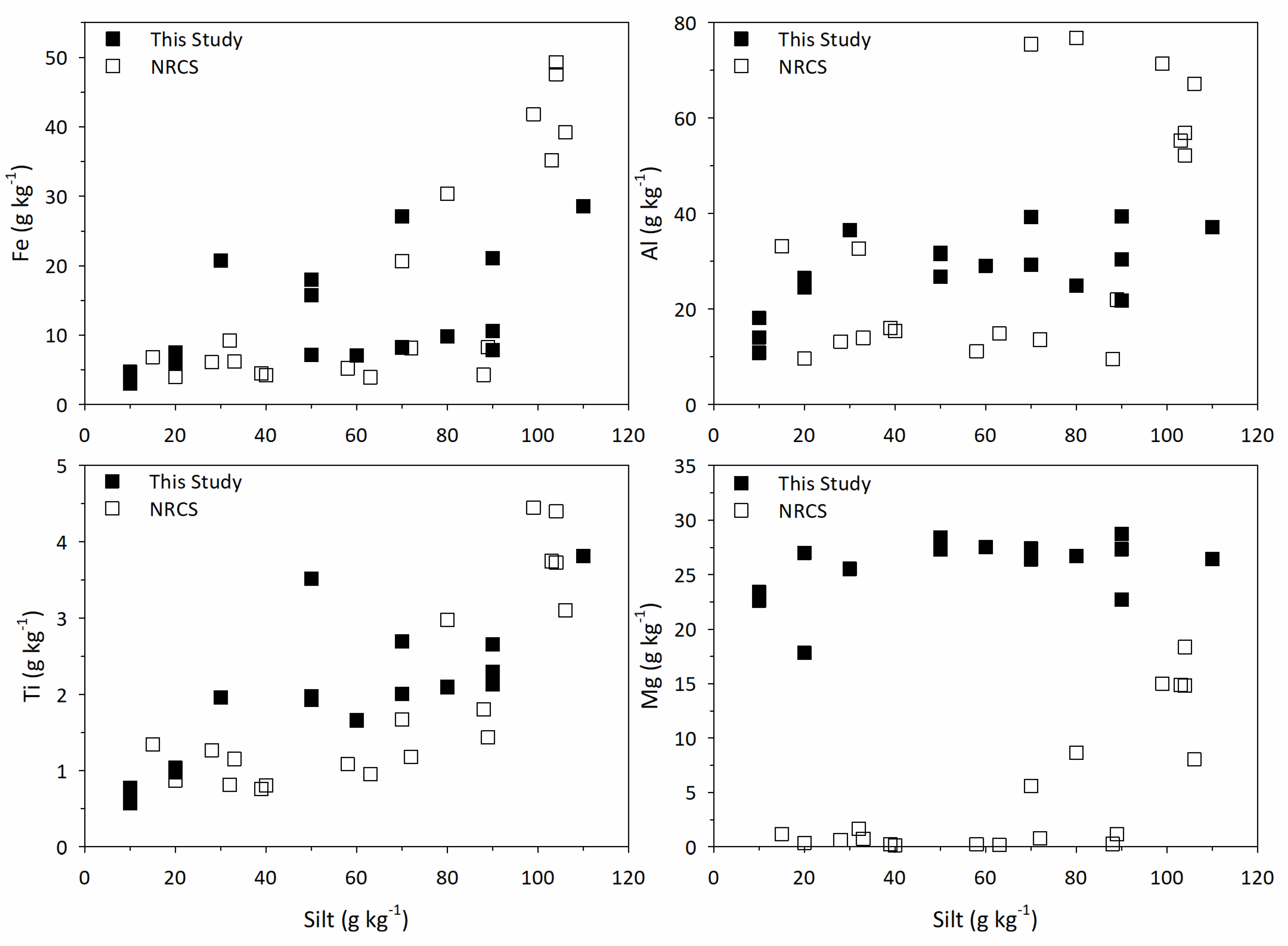
4.4. Global Soils Database
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| B | B Index |
| CTR | calcium/titanium ratio |
| EDS | energy dispersive spectrometry |
| PI | product index |
| pXRF | portable x-ray fluorescence |
| R | Ruxton ratio |
| SEM | scanning electron microscope |
| STI | silica-titania index |
| V | Vogt’s residual index |
| WIP | weather index of Parker |
| WPI | weathering potential index |
| XRD | x-ray diffractometer |
References
- Lal, R.; Kimble, J.M.; Follett, R.F. Pedospheric processes and the carbon cycle. In Soil Processes and the Carbon Cycle; Lal, R., Kimble, J.M., Follett, R.F., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; De Courcelles, V.D.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Liang, A.; Yang, X.; Zhang, X.; McLaughlin, N.; Shen, Y.; Li, W. Soil organic carbon changes in particle-size fractions following cultivation of Black soils in China. Soil Tillage Res. 2009, 105, 21–26. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Organic carbon and nitrogen associated with soil aggregates and particle sizes under different land uses in Tigray, Northern Ethiopia. L. Degrad. Dev. 2015, 26, 690–700. [Google Scholar] [CrossRef]
- Staaf, H. Release of plant nutrients from decomposing leaf litter in a south Swedish beech forest. Holarct. Ecol. 1980, 3, 129–136. [Google Scholar] [CrossRef]
- Dick, W.A. Organic carbon, nitrogen, and phosphorus concentrations and pH in soil profiles as affected by tillage intensity. Soil Sci. Soc. Am. J. 1983, 47, 102–107. [Google Scholar] [CrossRef]
- Rhoton, F.E. Influence of time on soil response to no-till practices. Soil Sci. Soc. Am. J. 2000, 64, 700–709. [Google Scholar] [CrossRef]
- Shuman, L.M. Effect of organic matter on the distribution of manganese, copper, iron, and zinc in soil fractions. Soil Sci. 1988, 146, 192–198. [Google Scholar] [CrossRef]
- Schwertmann, U.; Kodama, H.; Fischer, W.R. Mutual interactions between organics and iron oxides. In Interactions of Soil Minerals with Natural Organics and Microbes; SSSA: Madison, WI, USA, 1986; pp. 223–250. [Google Scholar]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Heckman, K.; Lawrence, C.R.; Harden, J.W. A sequential selective dissolution method to quantify storage and stability of organic carbon associated with Al and Fe hydroxide phases. Geoderma 2018, 312, 24–35. [Google Scholar] [CrossRef]
- Buck, B.J.; Brock, A.L.; Johnson, W.H.; Ulery, A.L. Corrosion of depleted uranium in an arid environment: Soil-geomorphology, SEM/EDS, XRD, and electron microprobe analysis. Soil Sediment Contam. 2004, 13, 545–561. [Google Scholar] [CrossRef]
- Price, J.R.; Velbel, M.A. Chemical weathering indices applied to weathering profiles developed on heterogeneous felsic metamorphic parent rocks. Chem. Geol. 2003, 202, 397–416. [Google Scholar] [CrossRef]
- Van Wambeke, A. Soils of the Tropics: Properties and Appraisal; McGraw Hill: New York, USA, 1992. [Google Scholar]
- Yost, J.L.; Hartemink, A.E. Soil carbon in sandy soils – a review. Adv. Agron. 2019, 158. [Google Scholar]
- Hartemink, A.E.; Huting, J. Land cover, extent, and properties of Arenosols in Southern Africa. Arid L. Res. Manag. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Yost, J.L.; Hartemink, A.E. Effects of carbon on moisture storage in soils of the Wisconsin Central Sands, USA. Eur. J. Soil Sci. 2019, 70, 565–577. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- WI-DNR. Central Sand Plains Ecological Landscape. In The ecological landscape of Wisconsin: An Assessment of Ecological Resources and a Guide to Planning Sustainable Management; Wisconsin Department of Natural Resources: Madison, WI, USA, 2014; p. 108. [Google Scholar]
- Kraft, G.J.; Clancy, K.; Mechenich, D.J.; Haucke, J. Irrigation effects in the Northern Lake States: Wisconsin Central Sands revisited. Ground Water 2012, 50, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.D.; Hartemink, A.E. Rapid changes in sandy soils under intensive agriculture in Wisconsin. Soil Horizons 2015, 56, 1–6. [Google Scholar] [CrossRef]
- Adhikari, K.; Hartemink, A.E. Soil organic carbon increases under intensive agriculture in the Central Sands, Wisconsin, USA. Geoderma Reg. 2017, 10, 115–125. [Google Scholar] [CrossRef]
- Madison, F.W.; Lee, G.B. Some mineralogic characteristics of sandy soils in Wisconsin. Trans. Wisconsin Acad. Sci. Arts Lett. 1965, 54, 223–230. [Google Scholar]
- Otter, A.J.; Simeth, F.J.; Simonson, D.T. Soil Survey of Waushara County, Wisconsin; United States Department of Agriculture: Washington, DC, USA, 1989.
- Colgan, P.M.; Mickelson, D.M. Genesis of streamlined landforms and flow history of the Green Bay Lobe, Wisconsin, USA. Sediment. Geol. 1997, 111, 7–25. [Google Scholar] [CrossRef]
- Arguez, A.; Durre, I.; Applequist, S.; Vose, R.S.; Squires, M.F.; Yin, X.; Heim, R.R., Jr.; Owen, T.W. NOAA’s 1981–2010 U.S. climate normals: An overview. Bull. Am. Meteorol. Soc. 2012, 93, 1687–1697. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meterologische Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Ruxton, B.P. Measures of the degree of chemical weathering in rocks. J. Geol. 1968, 76, 518–527. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Parker, A. An index of weathering for silicate rocks. Geol. Mag. 1970, 107, 501–504. [Google Scholar] [CrossRef]
- Vogt, T. Sultijelmafeltets geologi og petrografi. Norges Geol. Unders. 1927, 121, 1–560. [Google Scholar]
- Jayawardena, U.; Izawa, E. A new chemical index of weathering for metamorphic silicate rocks in tropical regions: A study from Sri Lanka. Eng. Geol. 1994, 36, 303–310. [Google Scholar] [CrossRef]
- Kronberg, B.I.; Nesbitt, H.W. Quantification of weathering, soil geochemistry and soil fertility. J. Soil Sci. 1981, 32, 453–459. [Google Scholar] [CrossRef]
- Kristiansen, S.M. Present-day soil distribution explained by prehistoric land-use: Podzol-Arenosol variation in an ancient woodland in Denmark. Geoderma 2001, 103, 273–289. [Google Scholar] [CrossRef]
- Majgier, L.; Rahmonov, O.; Bednarek, R. Features of abandoned cemetery soils on sandy substrates in Northern Poland. Eurasian Soil Sci. 2014, 47, 621–629. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Amelung, W.; Zech, W.; Zhang, X.; Follett, R.F.; Tiessen, H.; Knox, E.; Flach, K.-W. Carbon, nitrogen, and sulfur pools in particle-size fractions as influenced by climate. Soil Sci. Soc. Am. J. 1998, 62, 172–181. [Google Scholar] [CrossRef]
- Chivenge, P.P.; Murwira, H.K.; Giller, K.E.; Mapfumo, P.; Six, J. Long-term impact of reduced tillage and residue management on soil carbon stabilization: Implications for conservation agriculture on contrasting soils. Soil Tillage Res. 2007, 94, 328–337. [Google Scholar] [CrossRef]
- Dalal, R.C.C.; Mayer, R.J.J. Long-term trends in fertility of soils under continuous cultivation and cereal cropping in southern Queensland. III Distribution and kinetics of soil organic carbon in particle-size fractions. Aust. J. Soil Resour. 1986, 24, 293–300. [Google Scholar] [CrossRef]
- Desjardins, T.; Andreux, F.; Volkoff, B.; Cerri, C.C. Organic carbon and 13C contents in soils and soil size-fractions, and their changes due to deforestation and pasture installation in eastern Amazonia. Geoderma 1994, 61, 103–118. [Google Scholar] [CrossRef]
- Nelson, P.N.; Dictor, M.-C.; Soulas, G. Availability of organic carbon in soluble and particle-size fractions from a soil profile. Soil Biol. Biochem. 1994, 26, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Gulde, S.; Chung, H.; Amelung, W.; Chang, C.; Six, J. Soil carbon saturation controls labile and stable carbon pool dynamics. Soil Sci. Soc. Am. J. 2008, 72, 605–612. [Google Scholar] [CrossRef]
- Singh, B.P.; Setia, R.; Wiesmeier, M.; Kunhikrishnan, A. Agricultural management practices and soil organic carbon storage. In Soil Carbon Storage; Singh, B.K., Ed.; Academic Press: London, UK, 2018; pp. 207–244. ISBN 9780128127667. [Google Scholar]
- Souza, I.F.; Almeida, L.F.J.; Jesus, G.L.; Kleber, M.; Silva, I.R. The mechanisms of organic carbon protection and dynamics of C-saturation in Oxisols vary with particle-size distribution. Eur. J. Soil Sci. 2017, 68, 726–739. [Google Scholar] [CrossRef]
- Kiem, R.; Knicker, H.; Kögel-Knabner, I. Refractory organic carbon in particle-size fractions of arable soils I: Distribution of refractory carbon between the size fractions. Org. Geochem. 2002, 33, 1683–1697. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay addition to sandy soil—Influence of clay type and size on nutrient availability in sandy soils amended with residues differing in C/N ratio. Pedosphere 2017, 27, 293–305. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.C.; Calvert, D.V.; Alva, A.K. Fraction of iron, manganese, aluminum, and phosphorus in selected sandy soils under citrus production. Soil Sci. Soc. Am. J. 1997, 61, 794–801. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochimica 2007, 71, 25–35. [Google Scholar] [CrossRef]
- Zhao, Q.; Poulson, S.R.; Obrist, D.; Sumaila, S.; Dynes, J.J.; Mcbeth, J.M.; Yang, Y. Iron-bound organic carbon in forest soils: Quantification and characterization. Biogeosciences 2016, 13, 4777–4788. [Google Scholar] [CrossRef]
- Eusterhues, K.; Rumpel, C.; Kögel-Knabner, I. Organo-mineral associations in sandy acid forest soils: Importance of specific surface area, iron oxides and micropores. Eur. J. Soil Sci. 2005, 56, 753–763. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Iron promotes the preservation of organic matter in sediments. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Sudom, M.D.; Arnaud, R.J. Use of quartz, zirconium and titanium as indices in pedological studies. Can. J. Soil Sci. 1971, 51, 385–396. [Google Scholar] [CrossRef]
- National Cooperative Soil Survey. National Cooperative Soil Survey Characterization Database; United States Department of Agriculture: Washington, DC, USA, 2016.
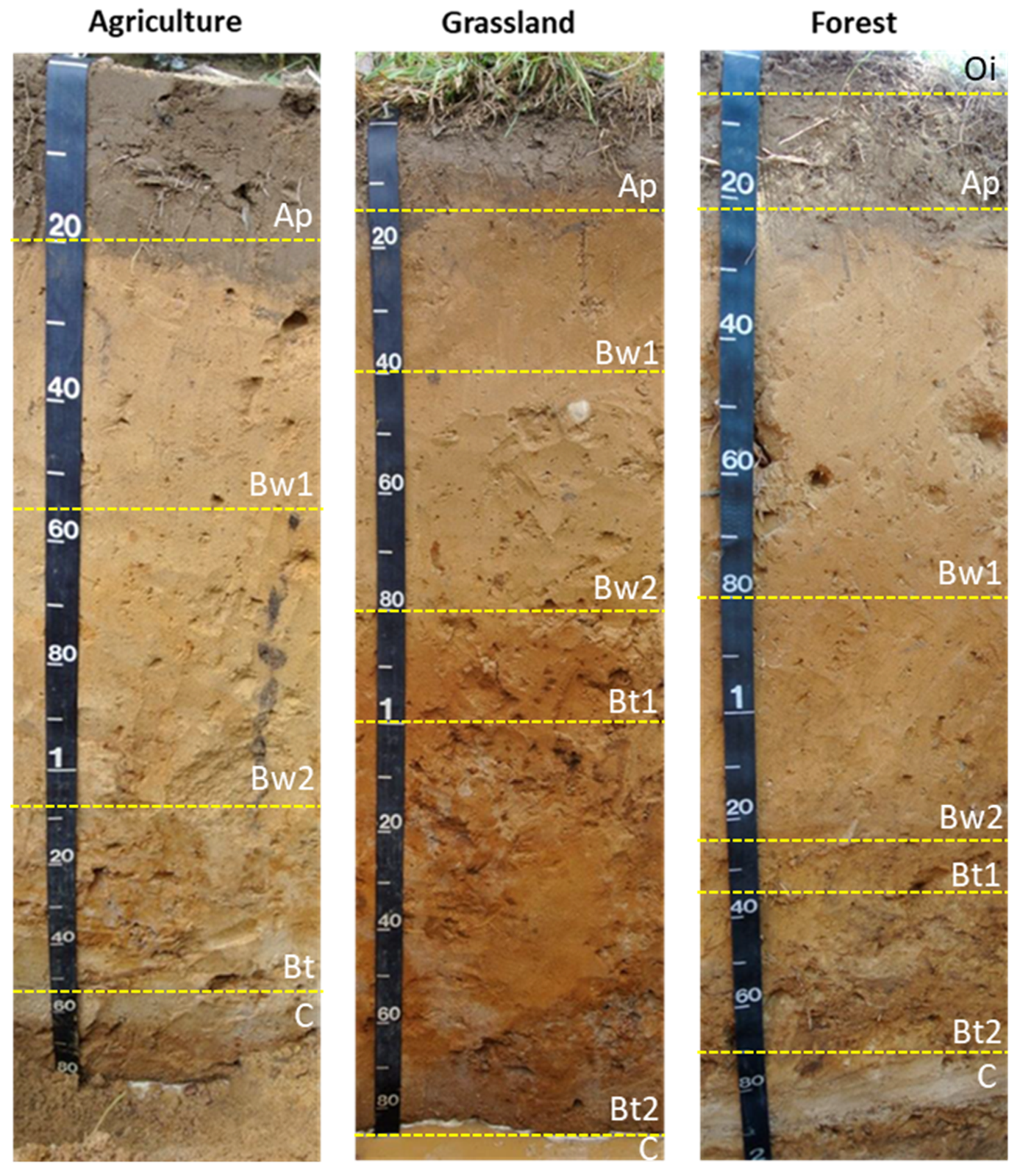

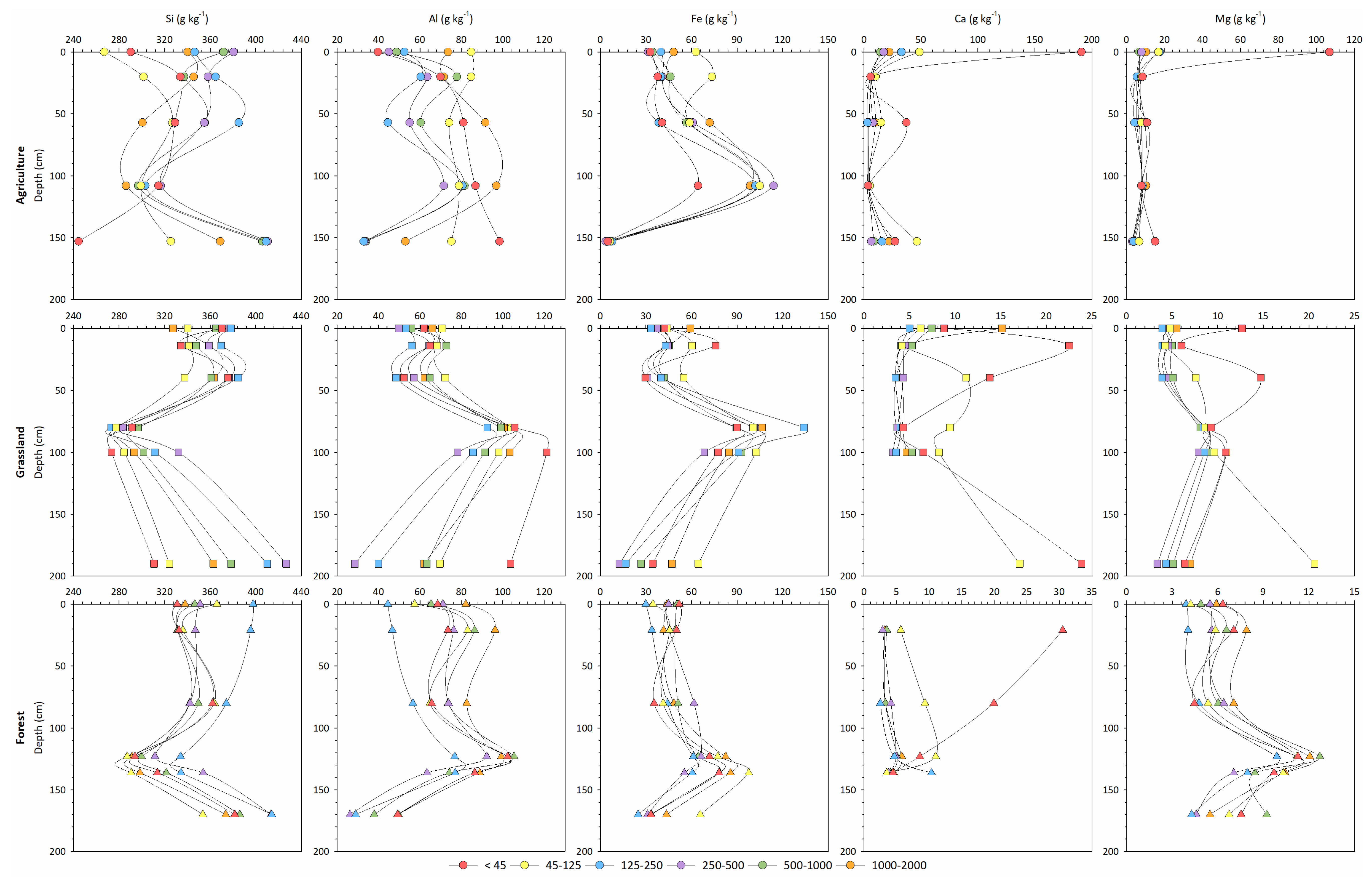
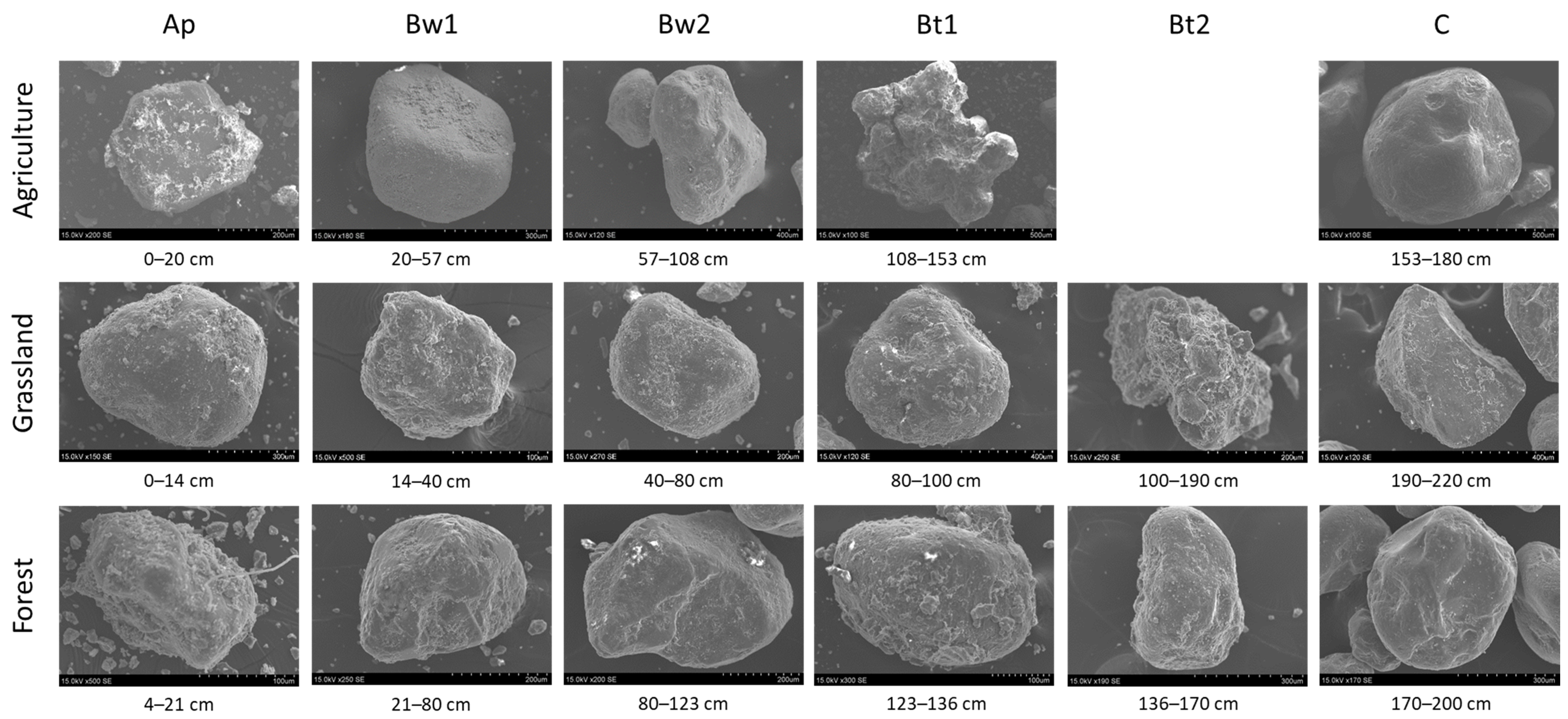


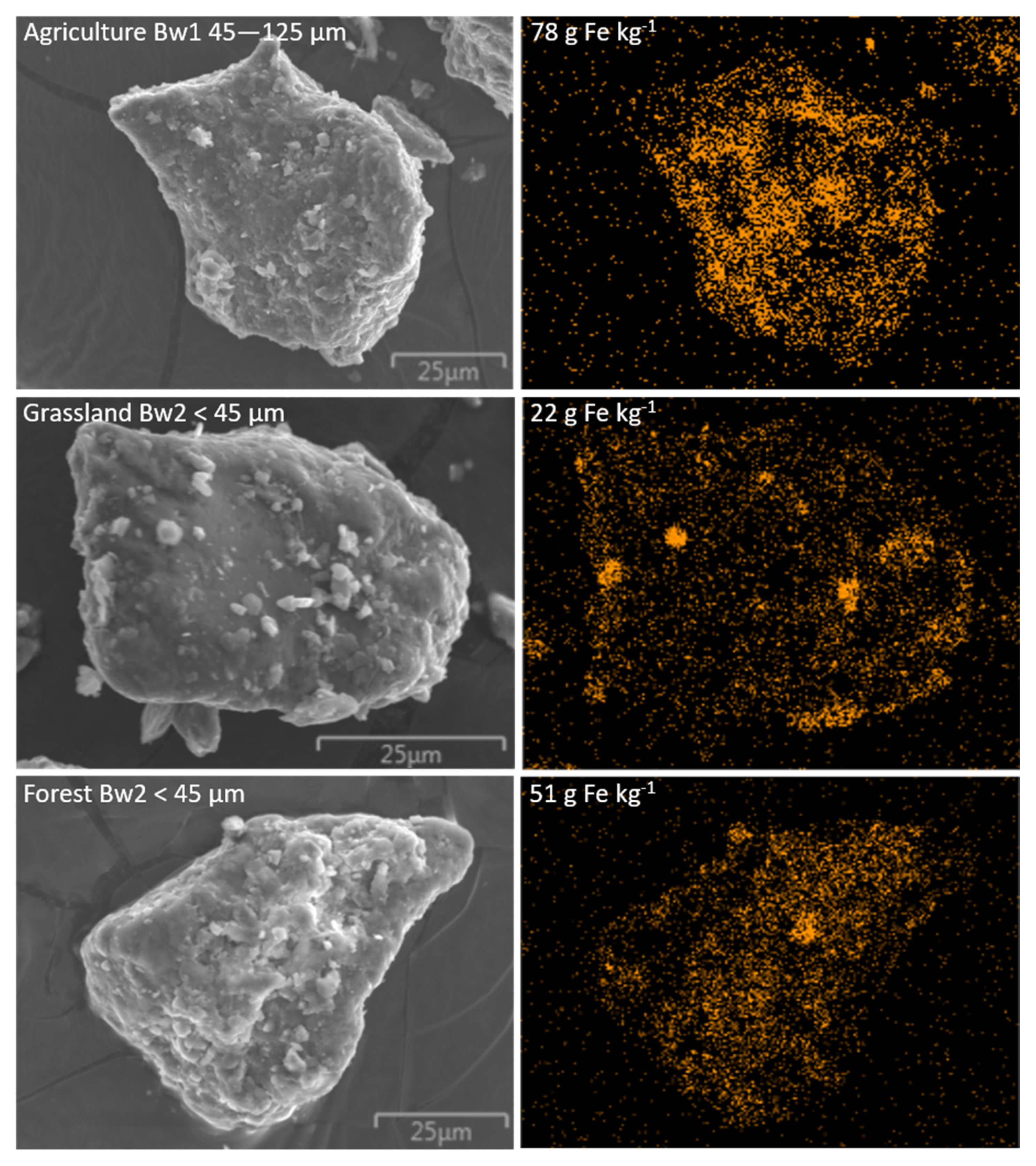
| Land Use | Horizon | Depth | Munsell | Total Carbon | Carbon Stock | Total Fe | pH | Clay | Silt | Sand | CF | Bulk Density |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (dry) | (g kg−1) | (Mg ha−1) | (mg kg−1) | (1:1 H2O) | (g kg−1) | (%) | (Mg m−3) | ||||
| Agriculture | Ap | 0–20 | 10YR 5/3 | 12.7 | 40 | 3077 | 7.4 | 20 | 90 | 890 | 1 | 1.57 |
| Bw1 | 20–57 | 10YR 6/6 | 1.1 | 7 | 2809 | 7.3 | 0 | 60 | 940 | 2 | 1.70 | |
| Bw2 | 57–108 | 10YR 6/6 | 0.9 | 7 | 5111 | 6.4 | 10 | 20 | 970 | 6 | 1.63 | |
| Bt | 108–153 | 10YR 5/6 | 1.9 | 13 | 23,135 | 5.1 | 80 | 110 | 810 | 9 | 1.68 | |
| C | >153 | 10YR 7/4 | 0.3 | 2913 | 5.2 | 10 | 10 | 980 | 7 | 1.68 | ||
| Grassland | Ap | 0–14 | 10YR 5/3 | 12.9 | 27 | 3798 | 5.1 | 40 | 80 | 880 | 0 | 1.50 |
| Bw1 | 14–40 | 10YR 6/6 | 3.7 | 16 | 8776 | 5.3 | 30 | 70 | 900 | 0 | 1.65 | |
| Bw2 | 40–80 | 10YR 5/6 | 1.7 | 11 | 6800 | 5.5 | 20 | 50 | 930 | 1 | 1.62 | |
| Bt1 | 80–100 | 10YR 5/6 | 3.5 | 10 | 14,468 | 5.5 | 100 | 70 | 830 | 3 | 1.51 | |
| Bt2 | 100–190 | 10YR 6/6 | 0.8 | 11 | 9289 | 5.4 | 70 | 30 | 900 | 6 | 1.59 | |
| C | >190 | 10YR 7/4 | 0.3 | 4273 | 5.3 | 10 | 10 | 980 | 7 | 1.59 | ||
| Forest | Oi | 0–4 | ||||||||||
| Ap | 4–21 | 10YR 5/3 | 4.7 | 12 | 6057 | 4.4 | 50 | 90 | 860 | 0 | 1.48 | |
| Bw1 | 21–80 | 10YR 5/6 | 1.0 | 9 | 5335 | 5.1 | 20 | 50 | 930 | 1 | 1.49 | |
| Bw2 | 80–123 | 10YR 5/6 | 0.4 | 3 | 3360 | 5.7 | 10 | 20 | 970 | 3 | 1.58 | |
| Bt1 | 123–136 | 10YR 5/8 | 0.6 | 1 | 13,154 | 5.7 | 80 | 90 | 830 | 8 | 1.55 | |
| Bt2 | 136–170 | 10YR 5/8 | 0.9 | 4 | 6130 | 5.4 | 50 | 50 | 900 | 9 | 1.57 | |
| C | >170 | 10YR 7/4 | 0.2 | 2532 | 5.9 | 0 | 10 | 990 | 5 | 1.57 | ||
| Index | Formula | Optimum Fresh Value | Optimum Weathered Value | Increase in Weathering Trend | Reference |
|---|---|---|---|---|---|
| Ruxton Ratio (R) | >10 | 0 | Negative | [14,30] | |
| Weather Potential Index (WPI) | [30] | ||||
| Product Index (PI) | [30] | ||||
| Calcium/Titanium Ratio (CTR) | [31] | ||||
| Weather Index of Parker (WIP) | >100 | 0 | Negative | [14,32] | |
| Vogt’s Residual Index (V) | <1 | Indefinite | Positive | [14,33] | |
| Silica-Titania Index (STI) | >90 | 0 | Negative | [14,34] | |
| B Index (B) | [35] |
| Land Use | Horizon | Depth | Si | Al | Fe | Ti | Ca | Mg |
|---|---|---|---|---|---|---|---|---|
| (cm) | (g kg−1) | |||||||
| Agriculture | Ap | 0–20 | 228 | 22 | 8 | 2 | 15 | 23 |
| Bw1 | 20–57 | 268 | 29 | 7 | 2 | 0.5 | 28 | |
| Bw2 | 57–108 | 273 | 27 | 6 | 1 | 27 | ||
| Bt | 108–153 | 210 | 37 | 29 | 4 | 1 | 26 | |
| C | >153 | 294 | 14 | 5 | 0.8 | 23 | ||
| Grassland | Ap | 0–14 | 252 | 25 | 10 | 2 | 0.3 | 27 |
| Bw1 | 14–40 | 264 | 29 | 8 | 2 | 26 | ||
| Bw2 | 40–80 | 281 | 27 | 7 | 2 | 27 | ||
| Bt1 | 80–100 | 191 | 39 | 27 | 3 | 0.4 | 27 | |
| Bt2 | 100–190 | 194 | 37 | 21 | 2 | 0.5 | 26 | |
| C | >190 | 297 | 18 | 3 | 0.6 | 23 | ||
| Forest | Ap | 4–21 | 243 | 30 | 11 | 2 | 27 | |
| Bw1 | 21–80 | 264 | 32 | 16 | 4 | 1 | 28 | |
| Bw2 | 80–123 | 247 | 25 | 8 | 1 | 18 | ||
| Bt1 | 123–136 | 218 | 39 | 21 | 3 | 1 | 29 | |
| Bt2 | 136–170 | 224 | 32 | 18 | 2 | 0.7 | 27 | |
| C | >170 | 285 | 11 | 3 | 0.6 | 23 | ||
| Land Use | ANOVA | Si | Al | Fe | Ti | Ca | Mg |
|---|---|---|---|---|---|---|---|
| Agriculture | Depth | *** | *** | *** | n.s. | *** | *** |
| Fraction | *** | *** | * | n.s. | *** | *** | |
| Depth × Fraction | *** | *** | ** | * | *** | *** | |
| Grassland | Depth | *** | *** | *** | n.s. | ** | ** |
| Fraction | *** | *** | * | *** | *** | ** | |
| Depth × Fraction | * | *** | n.s. | ** | * | ** | |
| Forest | Depth | *** | *** | *** | n.s. | n.s. | *** |
| Fraction | *** | *** | ** | ** | n.s. | *** | |
| Depth × Fraction | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Land Use | Horizon | Depth (cm) | R | WPI | PI | CTR | WIP | V | STI | B |
|---|---|---|---|---|---|---|---|---|---|---|
| Agriculture | Ap | 0–20 | 20 | 14 | 94 | 8.40 | 16 | 0.31 | 86 | 0.48 |
| Bw1 | 20–57 | 18 | 10 | 94 | 0.39 | 13 | 0.47 | 89 | 0.02 | |
| Bw2 | 57–108 | 20 | 10 | 94 | n.d. | 12 | 0.44 | 91 | 0.00 | |
| Bt | 108–153 | 11 | 12 | 88 | 0.44 | 13 | 0.61 | 83 | 0.05 | |
| C | >153 | 40 | 8 | 97 | n.d. | 11 | 0.27 | 92 | 0.00 | |
| Grassland | Ap | 0–14 | 19 | 10 | 94 | 0.20 | 12 | 0.42 | 87 | 0.02 |
| Bw1 | 14–40 | 17 | 10 | 93 | n.d. | 12 | 0.50 | 88 | 0.00 | |
| Bw2 | 40–80 | 20 | 10 | 94 | n.d. | 12 | 0.44 | 88 | 0.00 | |
| Bt1 | 80–100 | 9 | 13 | 87 | 0.15 | 13 | 0.64 | 84 | 0.01 | |
| Bt2 | 100–190 | 10 | 12 | 88 | 0.32 | 12 | 0.64 | 86 | 0.02 | |
| C | >190 | 32 | 8 | 97 | n.d. | 11 | 0.35 | 93 | 0.00 | |
| Forest | Ap | 4–21 | 15 | 11 | 92 | n.d. | 12 | 0.50 | 87 | 0.00 |
| Bw1 | 21–80 | 16 | 11 | 92 | 0.35 | 13 | 0.49 | 84 | 0.04 | |
| Bw2 | 80–123 | 19 | 7 | 94 | n.d. | 8 | 0.62 | 91 | 0.00 | |
| Bt1 | 123–136 | 11 | 12 | 89 | 0.51 | 14 | 0.60 | 85 | 0.04 | |
| Bt2 | 136–170 | 14 | 12 | 91 | 0.42 | 13 | 0.51 | 87 | 0.03 | |
| C | >170 | 50 | 8 | 98 | n.d. | 10 | 0.22 | 93 | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yost, J.L.; Roden, E.E.; Hartemink, A.E. Geochemical Fingerprint and Soil Carbon of Sandy Alfisols. Soil Syst. 2019, 3, 59. https://doi.org/10.3390/soilsystems3030059
Yost JL, Roden EE, Hartemink AE. Geochemical Fingerprint and Soil Carbon of Sandy Alfisols. Soil Systems. 2019; 3(3):59. https://doi.org/10.3390/soilsystems3030059
Chicago/Turabian StyleYost, Jenifer L., Eric E. Roden, and Alfred E. Hartemink. 2019. "Geochemical Fingerprint and Soil Carbon of Sandy Alfisols" Soil Systems 3, no. 3: 59. https://doi.org/10.3390/soilsystems3030059





