Erosion Induced Heterogeneity of Soil Organic Matter in Catenae from the Baltic Sea Catchment
Abstract
:1. Introduction
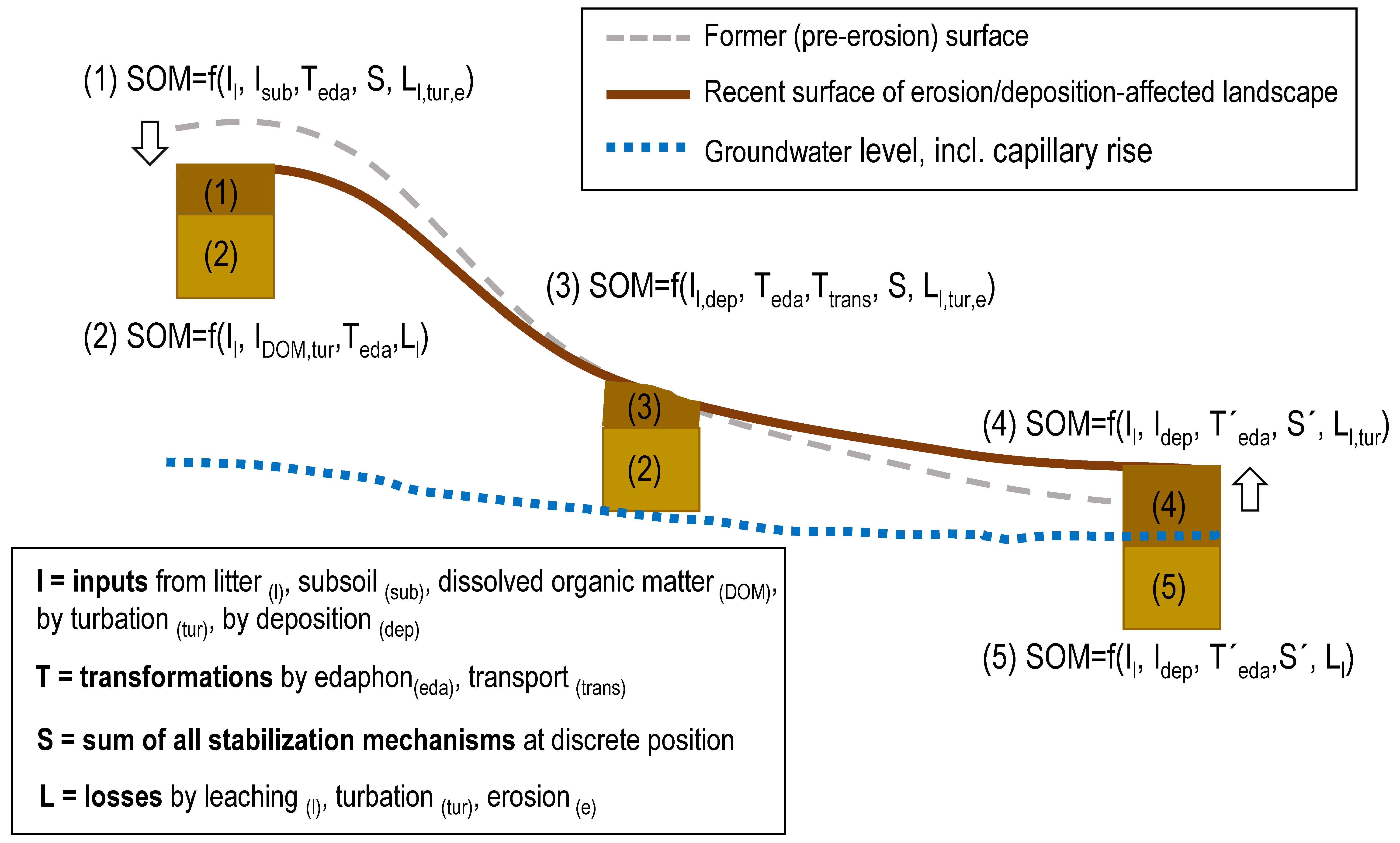
2. Materials and Methods
2.1. Description of Sampling Areas
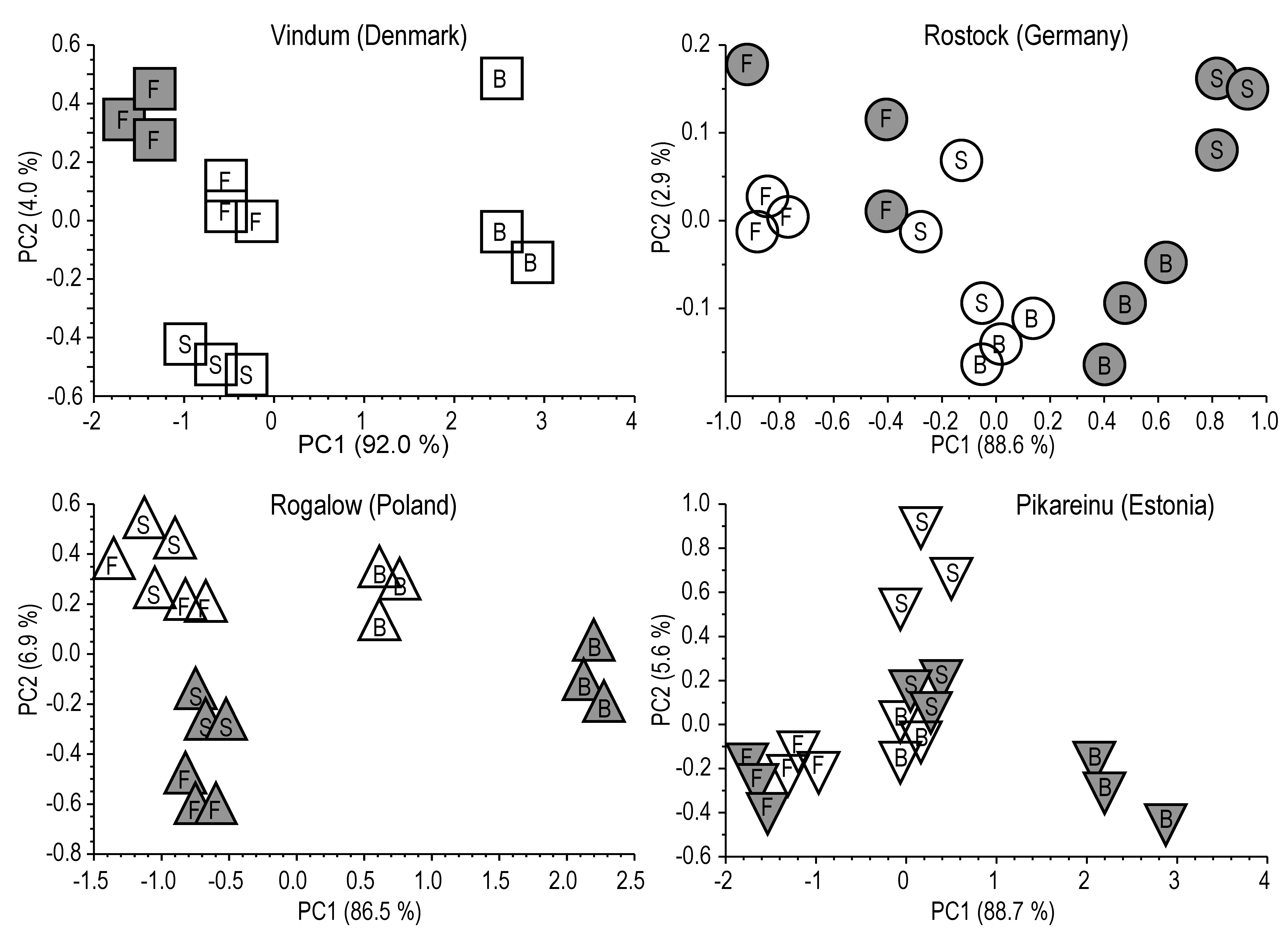
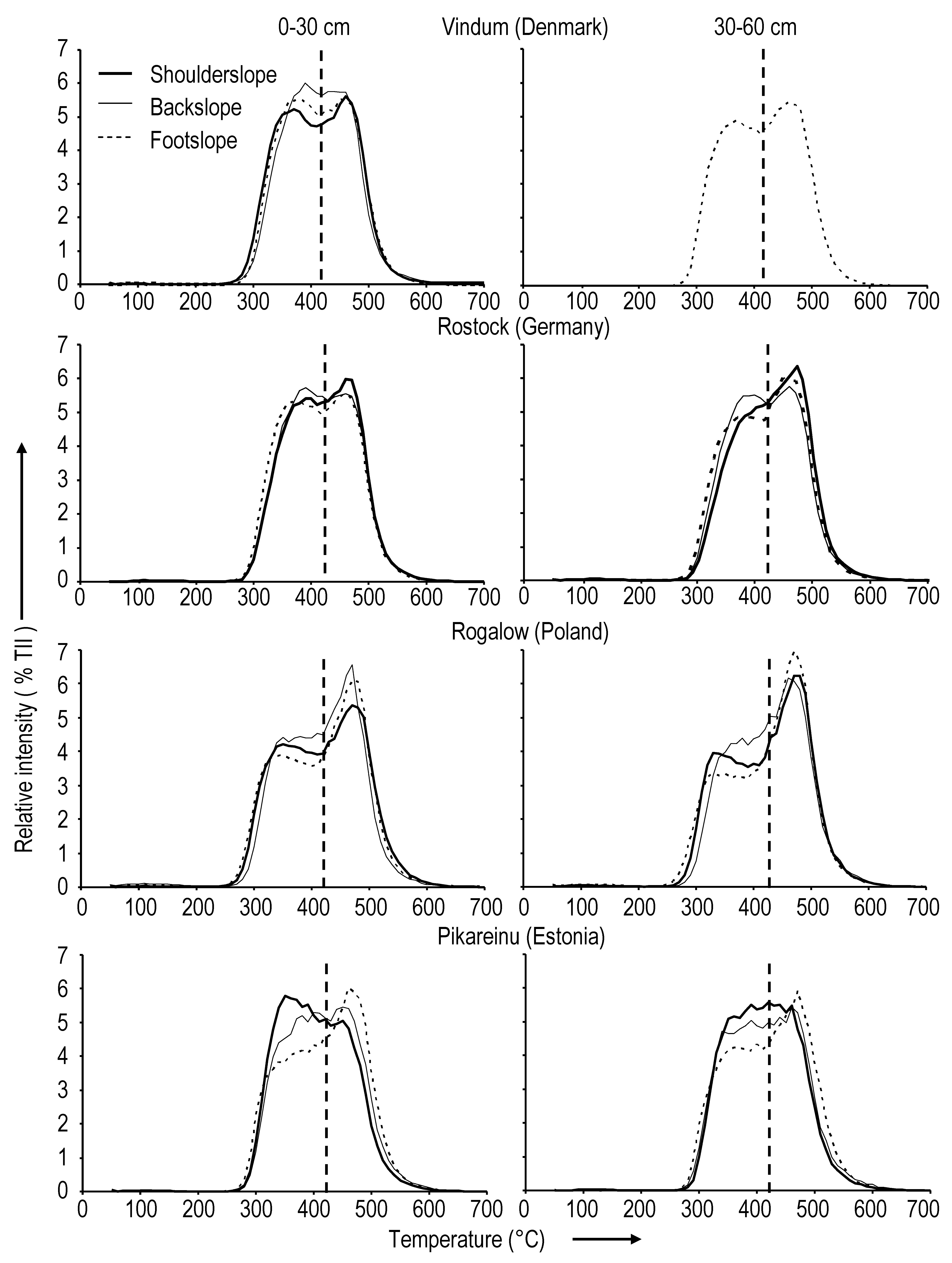
2.1.1. Vindum (Denmark)
2.1.2. Rostock (Germany)
2.1.3. Rogalow (Poland)
2.1.4. Pikareinu (Estonia)
2.2. Soil Sampling, Sample Codes and Pretreatments
2.3. Analytical Methods
2.3.1. Physical Soil Properties: Texture, Bulk Density, Porosity and Water Retention
2.3.2. Chemical Soil Properties: Elemental Concentrations, pH and Cation Exchange Capacity
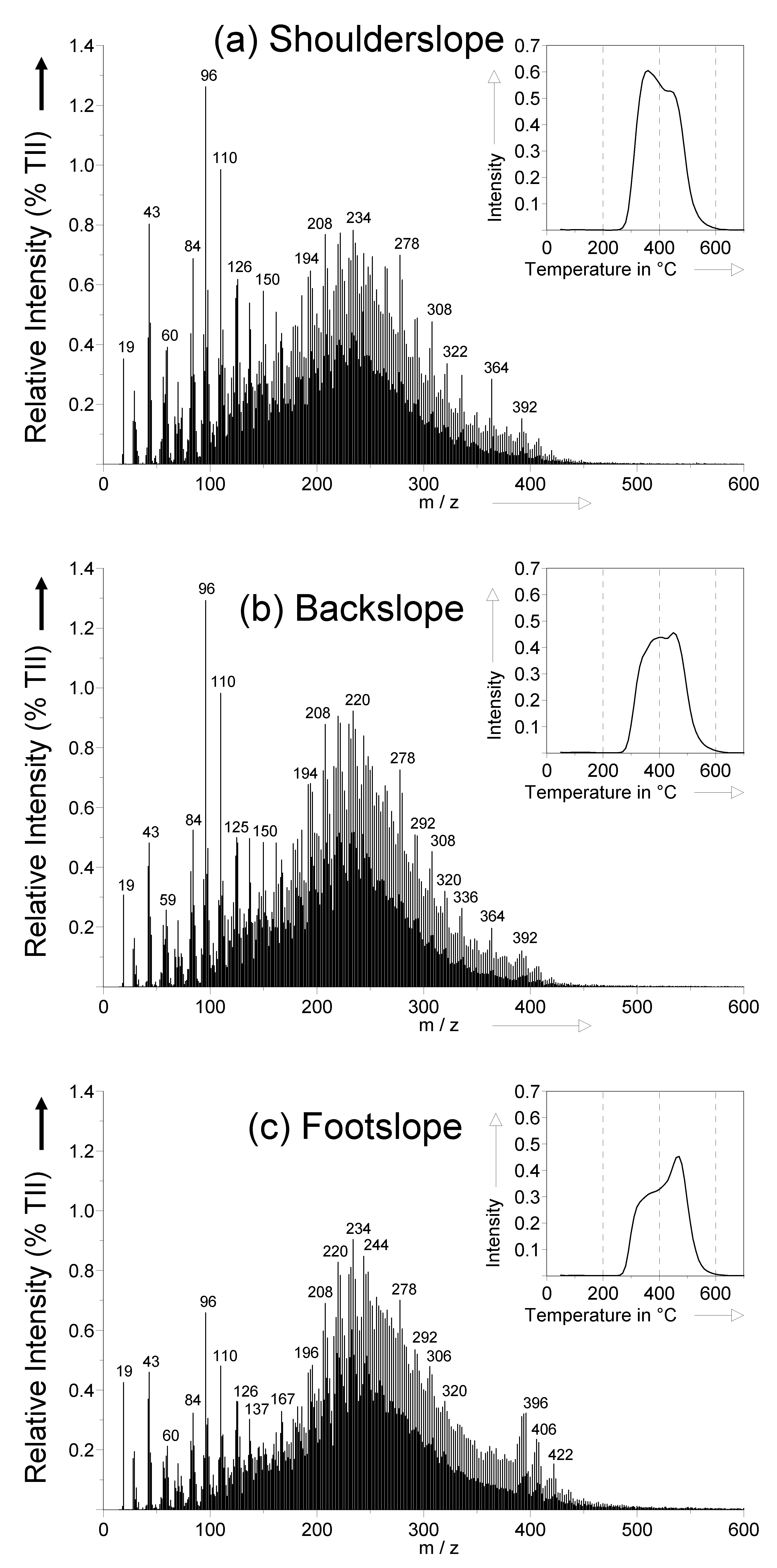
2.3.3. Soil Organic Matter Quality by Pyrolysis-Field Ionization Mass Spectrometry (Py-FIMS)
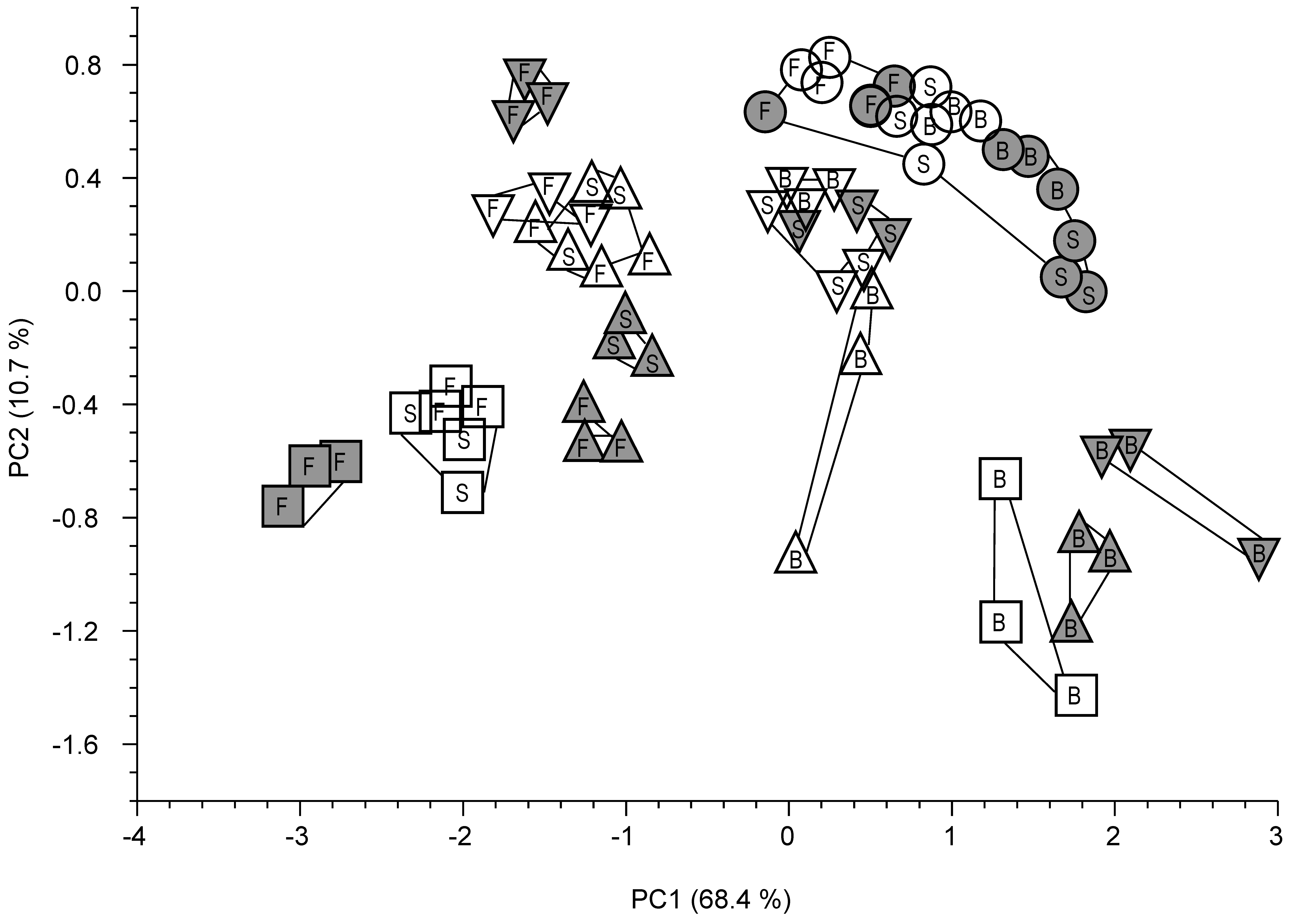
2.3.4. Statistical Analyses
3. Results and Discussion
3.1. Physical Soil Properties of the Baltic Catenae
3.2. General Chemical Soil Properties
3.3. Detailed SOM Characterization by Py-FIMS
3.4. Summarizing Discussion
4. Conclusions
- (i)
- Since we found some common properties for specific catena positions despite quite different geographic origins and management histories in the catenae under study, we can conclude that the experimental approach to combine basic soil physical and chemical soil parameters with a very detailed data set on the SOM molecular composition and thermal stability from Py-FIMS was suitable to describe the effects of soil erosion on SOM and on the overall soil fertility in agricultural fields from the Weichselian glacial and periglacial (i.e., loess) deposits in the Baltic Sea catchment.
- (ii)
- Since the special character of the backslope positions has been disclosed as a common characteristic of all these catenae for the first time, forthcoming studies should not be restricted to top and bottom positions in fields but also involve the intermediate positions that are characterized by transitions between erosive losses and depositional accumulations. Balancing these material transfers for field situations with a different curvature of the slopes seems to be an attractive scientific approache that may lead to sound data inputs into coupled erosion/SOM turnover models.
- (iii)
- The heterogeneity in the distribution of SOM-stocks, and related nutrient reserves along the catenae, representing field heterogeneity, call for an adapted soil management that (1) generally reduces soil erosion by covering the soil surface and reducing the length of erosive slopes (e.g., avoiding the “classical” backslope positions that are found here as hot spots of the SOM turnover, e.g., by establishment of barriers, hedges, etc.), and establishing soil amendments, such as manure and compost, preferentially at field sites of special demand (shoulderslope, backslope > footslope).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vente, J.; Poesen, J.; Verstraeten, G.; Govers, G.; Vanmaercke, M.; van Rompaey, A.; Arabkhedri, M.; Boix-Fayos, C. Predicting soil erosion and sediment yield at regional scales: Where do we stand? Earth-Sci. Rev. 2013, 127, 16–29. [Google Scholar] [CrossRef]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Enrique, A.; Bescansa, P. Review Soil Degradation and Soil Quality in Western Europe: Current Situation and Future Perspectives. Sustainability 2015, 7, 313–365. [Google Scholar] [CrossRef]
- Lugato, E.; Smith, P.; Borrelli, P.; Panagos, P.; Ballabio, C.; Orgiazzi, A.; Oihane, F.-U.; Montanarella, L.; Jones, A. Soil erosion is unlikely to drive a future carbon sink in Europe. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Pennock, D.J.; Anderson, D.W.; de Jong, E. Landscape-scale changes in indicators of soil quality due to cultivation in Saskatchewan, Canada. Geoderma 1994, 64, 1–19. [Google Scholar] [CrossRef]
- Landi, A.; Mermut, A.R.; Anderson, D.W. Carbon distribution in a hummocky landscape from Saskatchewan, Canada. Soil Sci. Soc. Am. J. 2004, 68, 175–184. [Google Scholar] [CrossRef]
- Mayer, S.; Schwindt, D.; Steffens, M.; Völkel, J.; Kögel-Knabner, I. Drivers of organic carbon allocation in a temperate slope-floodplain catena under agricultural use. Geoderma 2018, 327, 63–72. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Greer, K.J.; Anderson, D.W.; Liang, B.C. Carbon distribution and losses: Erosion and deposition effects. Soil Tillage Res. 1998, 47, 291–302. [Google Scholar] [CrossRef]
- Ma, W.K.; Bedard-Haughn, A.; Siciliano, S.D.; Farrell, R.E. Relationship between nitrifier and denitrifier community composition and abundance in predicting nitrous oxide emissions from ephemeral wetland soils. Soil Biol. Biochem. 2008, 40, 1114–1123. [Google Scholar] [CrossRef]
- Walley, F.L.; van Kessel, C.; Pennock, D.J. Landscape-scale variability of N mineralization in forest soils. Soil Biol. Biochem. 1996, 28, 383–391. [Google Scholar] [CrossRef]
- Pennock, D.J.; van Kessel, C.; Farrell, R.E.; Sutherland, R.A. Landscape-scale variations in denitrifi cation. Soil Sci. Soc. Am. J. 1992, 56, 770–776. [Google Scholar] [CrossRef]
- Van Kessel, C.; Pennock, D.J.; Farrell, R.E. Seasonal variations in denitrification and nitrous oxide evolution at the landscape scale. Soil Sci. Soc. Am. J. 1993, 57, 988–995. [Google Scholar] [CrossRef]
- Gillespie, A.W.; Walley, F.L.; Farrell, R.E.; Leinweber, P.; Eckhardt, K.-U.; Regier, T.Z.; Blyth, R.I.R. XANES and pyrolysis-FIMS evidence of organic matter composition in a hummocky landscape. Soil Sci. Soc. Am. J. 2011, 75, 1741–1755. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Eckhardt, K.-U.; Schlichting, A.; Hofmann, D.; Schulten, H.-R. Analytical Pyrolysis and Soft-Ionization Mass Spectrometry. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2009; pp. 539–588. [Google Scholar]
- Thiele-Bruhn, S.; Leinweber, P.; Eckhardt, K.-U.; Siemc, H.K.; Blume, H.-P. Chernozem properties of Black Soils in the Baltic region of Germany as revealed by mass-spectrometric fingerprinting of organic matter. Geoderma 2014, 213, 144–154. [Google Scholar] [CrossRef]
- Acksel, A.; Amelung, W.; Kühn, P.; Gehrt, E.; Regier, T.; Leinweber, P. Soil organic matter characteristics as indicator of Chernozem genesis in the Baltic Sea region. Geoderma Reg. 2016, 7, 187–200. [Google Scholar] [CrossRef]
- Acksel, A.; Kappenberg, A.; Kühn, P.; Leinweber, P. Human activity formed deep, dark topsoils around the Baltic Sea. Geoderma Reg. 2017, 10, 93–101. [Google Scholar] [CrossRef]
- Acksel, A.; Giani, L.; Stasch, C.; Kühn, P.; Eiter, S.; Potthoff, K.; Regier, T.; Leinweber, P. 2019. Humus-rich topsoils in SW Norway–Molecular and isotopic signatures of soil organic matter as indicators for anthropo-pedogensis. Catena 2019, 12, 831–845. [Google Scholar] [CrossRef]
- Jandl, G.; Acksel, A.; Baum, C.; Leinweber, P. Indicators for soil organic matter quality in no-till soils under perennial crops in Central Sweden. Soil Tillage Res. 2015, 148, 74–84. [Google Scholar] [CrossRef]
- Beyer, L.; Blume, H.-P.; Köbbemann, C. Colluvisols under cultivation in Schleswig-Holstein. 3. Soil organic matter transformation after translocation. J. Plant Nutr. Soil Sci. 1999, 162, 61–69. [Google Scholar] [CrossRef]
- Doetterl, S.; Six, J.; Van Wesemael, B.; Van Oost, K. Carbon cycling in eroding landscapes: Geomorphic controls on soil organic C pool composition and C stabilization. Glob. Chang. Biol. 2012, 18, 2218–2232. [Google Scholar] [CrossRef]
- Kirkels, F.M.S.A.; Cammeraat, L.H.; Kuhn, N.J. The fate of soil organic carbon upon erosion, transport and deposition in agricultural landscapes—A review of different concepts. Geomorphology 2014, 226, 94–105. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion and the global carbon budget. Environ. Int. 2003, 29, 437–450. [Google Scholar] [CrossRef]
- Aldana Jague, E.; Sommer, M.; Saby, N.P.A.; Cornelis, J.-T.; van Wesemael, B.; Van Oost, K. High resolution characterization of the soil organic carbon depth profile in a soil landscape affected by erosion. Soil Tillage Res. 2016, 156, 185–193. [Google Scholar] [CrossRef]
- Angst, G.; Kögel-Knabner, I.; Kirfel, K.; Hertel, D.; Mueller, C.W. Spatial distribution and chemical composition of soil organic matter fractions in rhizosphere and non-rhizosphere soil under European beech (Fagus sylvatica L.). Geoderma 2016, 264, 179–187. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2010, 338, 143–158. [Google Scholar] [CrossRef]
- Lal, R. Accelerated soil erosion as a source of atmospheric CO2. Soil. Tillage Res. 2019, 188, 35–40. [Google Scholar] [CrossRef]
- Djurhuus, J.; Olesen, J.E. Characterisation of Four Sites in Denmark for Long-Term Experiments on Crop Rotations for Organic Farming; DIAS Report Plant Production; Danmarks JordbrugsForskning: Tjele, Denmark, 2000; Volume 33, 74p. [Google Scholar]
- Ruhe, R.V. Elements of the soil landscape. In Transactions of 7th International Congress of Soil Science; International Society of Soil Science: Madison, WI, USA, 1996; pp. 165–170. [Google Scholar]
- Soil Survey Division Staff. Soil Survey Manual Soil Conservation Service; Handbook 18; US. Department of Agriculture: Washington, DC, USA, 1993.
- Klute, A. (Ed.) Water retention: Laboratory methods. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986; pp. 635–662. [Google Scholar]
- Resurreccion, A.C.; Moldrup, P.; Tuller, M.; Ferre, T.; Kawamoto, K.; Komatsu, T.; de Jonge, L.W. The soil-water characteristic curve at low soil-water contents: Relationships with soil specific surface area and texture. Water Resourc. Res. 2011, 47, W06522. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chem. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Kafkafi, U. Soil phosphorus. In Analytical Chemistry of Phosphorus Compounds; Halmann, M., Ed.; John Wiley: New York, NY, USA, 1972; pp. 727–741. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Evaluation of the Soils and Plant Properties; IOŚ: Warsaw, Poland, 1991. (In Polish) [Google Scholar]
- Schulten, H.-R.; Leinweber, P.; Schnitzer, M. Analytical pyrolysis and computer modelling of humic and soil particles. In IUPAC Environmental Analytical and Physical Chemistry Series; Environmental Particles: Structure and Surface Reactions of Soil Particles; Huang, P.M., Senesi, N., Buffle, J., Eds.; Wiley: Chichester, UK, 1998; Volume 4, pp. 281–324. [Google Scholar]
- Leinweber, P.; Kruse, J.; Baum, C.; Arcand, M.; Knight, J.D.; Farrell, R.; Eckhardt, K.-U.; Kiersch, K.; Jandl, G. Advances in understanding organic nitrogen chemistry in soils using state-of-the-art analytical techniques. Adv. Agron. 2012, 119, 83–151. [Google Scholar]
- Kalbitz, K.; Schwesig, D.; Schmerwitz, J.; Kaiser, K.; Haumaier, L.; Glaser, B.; Ellerbrock, R.; Leinweber, P. Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol. Biochem. 2003, 35, 1129–1142. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Baum, C.; Eckhardt, K.-U.; Kandeler, E. Stability and composition of soil organic matter control respiration and soil enzyme activities. Soil Biol. Biochem. 2008, 40, 1496–1505. [Google Scholar] [CrossRef]
- STATISTICA. STATISTICA for Windows Software, Release 5.10; StatSoft: Tulsa, OK, USA, 1996. [Google Scholar]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 9 October 2018).
- Adhikari, K.; Minasny, B.; Greve, M.B.; Greve, M.H. Constructing a soil class map of Denmark based on the FAO legend using digital techniques. Geoderma 2014, 214–215, 101–113. [Google Scholar] [CrossRef]
- Madsen, H.B.; Nørr, A.H.; Holst, K.A. Atlas over Danmark. Serie I, Bind 3; Den Danske Jordklassificering; Det Kongelige Danske Geografiske Selskab: København, Denmark, 1992. [Google Scholar]
- Adhikari, K.; Bou Kheir, R.; Greve, M.B.; Greve, M.H.; Malone, B.P.; Minasny, B.; McBratney, A.B. Mapping soil pH and bulk density at multiple soil depths in Denmark. In Proceedings of the 1st GlobalSoilMap Conference: Basis of the Global Spatial Soil Information System, Orléans, France, 7–9 October 2013; pp. 155–160. [Google Scholar]
- Leinweber, P.; Menning, P. Character of compacted layers in arable soils on loamy Moraine sites in Mecklenburg [Germany]. J. Rural Eng. Dev. 1992, 33, 100–107. (in Germany). [Google Scholar]
- Brzezińska, M.; Lipiec, J.; Frąc, M.; Oszust, K.; Szarlip, P.; Turski, M. Quantitative interactions between total and specific enzyme activities and C and N contents in earthworm-affected pear orchard soil. Land Degrad. Dev. 2018, 1–11. [Google Scholar] [CrossRef]
- Lipiec, J.; Frac, M.; Brzezinska, M.; Turski, M.; Oszust, K. Linking microbial enzymatic activities and functional diversity of soil around earthworm burrows and casts. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Adamczyk, B.; Bialousz, S.; Borkowski, J.; Borowiec, J.; Ciesla, W.; Dobrzanski, B.; Konecka-Betley, K.; Kowalinski, S.; Kowalkowski, A.; Królikowski, L.; et al. Polskie Towarzystwo Gleboznawcze; Album, G.P., Królikowski, L., Eds.; Panstwowe Wydawnictwo Naukowe: Warszawa, Poland, 1986. [Google Scholar]
- Turski, M.; Witkowska-Walczak, B. Soil physical properties of Luvisols developed from silty material of various origins. Rozprawy I Monografie 2004, 101, 1–58. (In Polish) [Google Scholar]
- Rejman, J. Redistribution of basic physical properties and water content of soils in a loess catchment. Acta Agrophys. Mono. 2013, 5, 1–106. [Google Scholar]
- Turski, R.; Paluszek, J.; Słowińska-Jurkiewicz, A. Erosion effect on physical properties of soils developed from loess. Roczniki Gleboznawcze 1987, 38, 37–49. (In Polish) [Google Scholar]
- Suuster, E.; Ritz, C.; Roostalu, H.; Reintam, E.; Kõlli, R.; Astover, A. Soil bulk density pedotransfer functions of the humus horizon in arable soils. Geoderma 2011, 163, 74–82. [Google Scholar] [CrossRef]
- Auerswald, K.; Fiener, P. Soil organic carbon storage following conversion from cropland to grassland on sites differing in soil drainage and erosion history. Sci. Total Environ. 2019, 661, 481–491. [Google Scholar] [CrossRef]
- Chirinda, N.; Roncossek, S.D.; Heckrath, G.; Elsgaard, L.; Thomsen, I.K.; Olesen, J.E. Root and soil carbon distribution at shoulderslope and footslope positions of temperate toposequences cropped to winter wheat. Catena 2014, 123, 99–105. [Google Scholar] [CrossRef]
- Reuter, G.; Böttcher, H.; Honermeier, B.; Kopp, H.; Lange, G.; Makowski, N.; Mueller, K.; Teltschik, K. Diversity of humus stocks in the arable soil of the Late Pleistoceine ground morains in Mecklenburg, West Pomerania, Germany. Arch. Agron. Soil Sci. 2007, 53, 231–240. [Google Scholar] [CrossRef]
- Kõlli, R.; Ellermäe, O.; Kauer, K.; Köster, T. Erosion-affected soils in the Estonian landscape: Humus status, patterns and classification. Arch. Agron. Soil Sci. 2010, 56, 149–164. [Google Scholar]
- Blume, H.-P.; Brümmer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M.; Thiele-Bruhn, S.; Welp, G. Scheffer/Schachtschabel: Lehrbuch der Bodenkunde; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010; p. 569. [Google Scholar]
- Chirinda, N.; Elsgaard, L.; Thomsen, I.K.; Heckrath, G.; Olesen, J.E. Carbon dynamics in topsoil and subsoil along a cultivated toposequence. Catena 2014, 120, 20–28. [Google Scholar] [CrossRef]
- Quantin, P.; Spaargaren, O. Classification of the Reference Pedons: World Reference Base for Soil Resources and Soil Taxonomy. In Soils of Volcanic Regions in Europe; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Walter, C.; Merot, P.; Layer, B.; Dutin, G. The effect of hedgerows on soil organic carbon storage in hillslopes. Soil Use Manag. 2003, 19, 201–207. [Google Scholar] [CrossRef]
- Heckrath, G.; Djurhuus, J.; Quine, T.A.; Van Oost, K.; Govers, G.; Zhang, Y. Tillage erosion and its effect on soil properties and crop yield in Denmark. J. Environ. Qual. 2005, 34, 312–324. [Google Scholar] [PubMed]
- Li, Z.; Liu, C.; Dong, Y.; Chang, X.; Nie, X.; Liu, L.; Xiao, H.; Lu, Y.; Zeng, G. response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly–gully region of China. Soil Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]
- Chen, C.; Leinweber, P.; Eckhardt, K.-U.; Sparks, D.L. The composition and stability of clay-associated organic matter along a soil profile. Soil Syst. 2018, 2, 16. [Google Scholar] [CrossRef]
- Salazar, S.; Sanchez, L.; Alvarez, J.; Valverde, A.; Galindo, P.; Igual, J.M.; Peix, A.; Santa-Regina, I. Correlation among soil enzyme activities under different forest system management practices. Ecol. Eng. 2011, 37, 1123–1131. [Google Scholar] [CrossRef]
- Koch, M.; Kruse, J.; Eichler-Löbermann, B.; Zimmer, D.; Willbold, S.; Leinweber, P.; Siebers, N. Phosphorus stocks and speciation in soil profiles of a long-term fertilizer experiment: Evidence from sequential fractionation, P K -edge XANES, and 31 P NMR spectroscopy. Geoderma 2018, 316, 115–126. [Google Scholar] [CrossRef]
| Geographical Coordinates | Soil Unit 1 | Average Annual Temperature (°C) | Average Precipitation (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoulderslope | Backslope | Footslope | Shoulderslope | Backslope | Footslope | ||||
| Vindum | 56°22′30.9″ N 9°33′32.8″ E | 56°22′31.0″ N 9°33′33.5″ E | 56°22′31.1″ N 9°33′34.2″ E | Stagnic Podzoluvisol | Stagnic Podzoluvisol | Aric Anthrosol | 7.3 | 704 | |
| Terrain slope (degrees) | 4.8 | 11.5 | 6.7 | ||||||
| Profile curvature 2 | −0.013 | −0.002 | 0.011 | ||||||
| Horizontal distance (m) | 0 | 11 | 23 | ||||||
| Elevation a.s.l. (m) 3 | 64 | 62 | 59 | ||||||
| Rostock | 54°1′58.51″ N 12°6′12.92″ E | 54°1′57.63″ N 12°6′17.74″ E | 54°1′56.83″ N 12°6′22.33″ E | Eutric Leptosol | Stagnic Anthrosol | Gleyic Anthrosol | 8.1 | 600 | |
| Terrain slope (degrees) | 4.5 | 3.0 | 1.6 | ||||||
| Profile curvature 2 | −0.087 | 0.002 | 0.547 | ||||||
| Horizontal distance (m) | 0 | 91 | 179 | ||||||
| Elevation a.s.l. (m) 3 | 43 | 37 | 34 | ||||||
| Rogalow | 51°19′06″ N 22°08′30″ E | 51°19′05.5″ N 22°08′32.1″ E | 51°19′06.8″ N 22°08′31.8″ E | Halpic Luvisol | Haplic Luvisol | Haplic Luvisol | 7.7 | 550 | |
| Horizontal distance (m) | 0 | 29 | 64 | ||||||
| Elevation a.s.l. (m) 3 | 197 | 191 | 185 | ||||||
| Pikareinu | 58°6′13.7″ N 26°39′55.4″ E | 58°6′14.6″ N 26°39′57″ E | 58°6′71″ N 26°40′2.8″ E | Aric Regosol | Aric Regosol | Thaptomollic Luvisol | 5.7 | 719 | |
| Horizontal distance (m) | 0 | 42 | 81 | ||||||
| Elevation a.s.l. (m) 3 | 162 | 160 | 153 |
| Soil Depth (cm) | Clay (%) | Silt (%) | Sand (%) | Texture Class | Bulk Density (g·cm−3) | Porosity (vol.%) | Water Retention Capacity (v/v) (vol.%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| FC (pF 1.7) | PWP (pF 4.2) | PAW | ||||||||
| Vindum | ||||||||||
| Shoulderslope | 0–30 | 3 | 23 | 74 | Loamy sand | 1.43 | 46 | 27 | 8 | 19 |
| 30–60 | 12 | 20 | 68 | Sandy loam | 1.76 | 33 | 28 | 11 | 17 | |
| Backslope | 0–30 | 9 | 23 | 68 | Sandy loam | 1.62 | 39 | 26 | 9 | 18 |
| 30–60 | 13 | 25 | 62 | Sandy loam | 1.74 | 34 | 27 | 7 | 20 | |
| Footslope | 0–30 | 5 | 25 | 70 | Sandy loam | 1.56 | 41 | 29 | 8 | 21 |
| 30–60 | 4 | 25 | 71 | Sandy loam | 1.56 | 41 | 28 | 8 | 20 | |
| Rostock | ||||||||||
| Shoulderslope | 0–30 | 6 | 29 | 65 | Sandy loam | 1.34 | 49 | 33 | 6 | 27 |
| 30–60 | 6 | 28 | 66 | Sandy loam | 1.59 | 40 | 30 | 7 | 23 | |
| Backslope | 0–30 | 4 | 32 | 64 | Sandy loam | 1.51 | 43 | 31 | 7 | 24 |
| 30–60 | 6 | 30 | 64 | Sandy loam | 1.51 | 43 | 29 | 6 | 23 | |
| Footslope | 0–30 | 2 | 29 | 69 | Sandy loam | 1.51 | 43 | 31 | 7 | 24 |
| 30–60 | 2 | 30 | 68 | Sandy loam | 1.57 | 41 | 29 | 6 | 23 | |
| Rogalow | ||||||||||
| Shoulderslope | 0–30 | 3 | 75 | 22 | Silt loam | 1.21 | 54 | 40 | 4 | 36 |
| 30–60 | 2 | 76 | 22 | Silt loam | 1.38 | 48 | 41 | 4 | 37 | |
| Backslope | 0–30 | 3 | 74 | 23 | Silt loam | 1.27 | 52 | 37 | 5 | 32 |
| 30–60 | 3 | 76 | 21 | Silt loam | 1.43 | 46 | 39 | 5 | 34 | |
| Footslope | 0–30 | 4 | 71 | 25 | Silt loam | 1.46 | 45 | 40 | 6 | 35 |
| 30–60 | 5 | 72 | 23 | Silt loam | 1.45 | 45 | 40 | 5 | 35 | |
| Pikareinu | ||||||||||
| Shoulderslope | 0–30 | 7 | 35 | 58 | Sandy loam | 1.28 | 52 | 27 | 5 | 22 |
| 30–60 | 8 | 36 | 56 | Sandy loam | 1.57 | 41 | 33 | 5 | 28 | |
| Backslope | 0–30 | 7 | 35 | 58 | Sandy loam | 1.32 | 50 | 28 | 5 | 24 |
| 30–60 | 6 | 35 | 59 | Sandy loam | 1.64 | 38 | 24 | 6 | 30 | |
| Footslope | 0–30 | 4 | 34 | 62 | Sandy loam | 1.37 | 48 | 35 | 5 | 31 |
| 30–60 | 3 | 36 | 61 | Sandy loam | 1.48 | 44 | 36 | 4 | 32 | |
| Soil Depth (cm) | pH (in KCl) | C (g·kg−1) | N (g·kg−1) | C:N Ratio | C Stock (kg·m−2) | Σ C Stock 0–60 cm (kg·m−2) | S (g·kg−1) | Pt (mg·kg−1) | CEC (cmol(+)·kg−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vindum | ||||||||||
| Shoulderslope | 0–30 | 6.0 | 10.4 | 1.1 | 9.5 | 4.5 | 5.4 | 0.19 | 533 | 7.37 |
| 30–60 | 5.2 | 1.7 | 0.3 | 5.7 | 0.9 | 0.1 | 601 | 7.61 | ||
| Backslope | 0–30 | 5.5 | 6.1 | 0.7 | 8.7 | 3.0 | 3.8 | 0.1 | 513 | 8.45 |
| 30–60 | 4.7 | 1.6 | 0.3 | 5.3 | 0.8 | 0.07 | 410 | 8.92 | ||
| Footslope | 0–30 | 6.1 | 12.7 | 1.3 | 9.8 | 5.9 | 12 | 0.18 | 613 | 8.59 |
| 30–60 | 6.7 | 12.8 | 1.2 | 10.7 | 6.0 | 0.16 | 643 | 9.61 | ||
| Rostock | ||||||||||
| Shoulderslope | 0–30 | 5.4 | 14.1 | 1.8 | 7.8 | 5.7 | 8.5 | 0.22 | 598 | 7.46 |
| 30–60 | 5.5 | 5.9 | 0.8 | 7.4 | 2.8 | 0.09 | 520 | 7.21 | ||
| Backslope | 0–30 | 5.8 | 6.6 | 0.8 | 8.3 | 3.0 | 5.4 | 0.14 | 498 | 7.65 |
| 30–60 | 5.8 | 5.2 | 0.6 | 8.7 | 2.4 | 0.11 | 498 | 7.35 | ||
| Footslope | 0–30 | 5.4 | 9.3 | 1.1 | 8.5 | 4.2 | 9.1 | 0.16 | 641 | 8.56 |
| 30–60 | 5.8 | 10.4 | 1.2 | 8.7 | 4.9 | 0.15 | 571 | 8.04 | ||
| Rogalow | ||||||||||
| Shoulderslope | 0–30 | 6.6 | 9.2 | 1.0 | 9.2 | 3.4 | 6.1 | 0.18 | 533 | 6.76 |
| 30–60 | 6.2 | 6.6 | 0.8 | 8.3 | 2.7 | 0.14 | 507 | 5.74 | ||
| Backslope | 0–30 | 6.1 | 8.4 | 0.9 | 9.3 | 3.2 | 5.7 | 0.13 | 461 | 6.24 |
| 30–60 | 5.7 | 5.7 | 0.7 | 8.1 | 2.5 | 0.09 | 438 | 6.19 | ||
| Footslope | 0–30 | 5.3 | 10.6 | 1.1 | 9.6 | 4.7 | 8.9 | 0.22 | 560 | 6.63 |
| 30–60 | 4.1 | 9.6 | 1.0 | 9.6 | 4.2 | 0.12 | 487 | 6.55 | ||
| Pikareinu | ||||||||||
| Shoulderslope | 0–30 | 6.4 | 13.2 | 1.5 | 8.8 | 5.1 | 12 | 0.18 | 560 | 10.71 |
| 30–60 | 6.6 | 13.5 | 1.5 | 9.0 | 6.4 | 0.15 | 490 | 11.03 | ||
| Backslope | 0–30 | 6.1 | 11.8 | 1.3 | 9.1 | 4.7 | 8.5 | 0.15 | 457 | 9.93 |
| 30–60 | 5.8 | 7.7 | 0.9 | 8.6 | 3.8 | 0.1 | 447 | 7.53 | ||
| Footslope | 0–30 | 6.7 | 16.5 | 1.7 | 9.7 | 6.8 | 15 | 0.46 | 525 | 11.3 |
| 30–60 | 6.6 | 17.7 | 1.8 | 9.8 | 7.9 | 0.2 | 498 | 11.03 |
| Soil Depth (cm) | Carbohydrates | Phenols + Lignin Monomers | Lignin Dimers | Lipids 1 | Alkylaromatics | N-Compounds 2 | Sterols | Amides 3 | Suberin | Free Fatty Acids 4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vindum | |||||||||||
| Shoulderslope | 0–30 cm | 3.3 | 6.4 | 3.2 | 5.8 | 9.5 | 1.6 | 4.3 | 4.0 | 0.2 | 1.6 |
| 30–60 cm | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Backslope | 0–30 cm | 7.1 | 12.0 | 1.9 | 5.1 | 13.7 | 3.5 | 0.6 | 5.8 | 0.0 | 0.4 |
| 30–60 cm | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Footslope | 0–30 cm | 3.4 | 7.2 | 3.1 | 6.1 | 9.9 | 1.7 | 4.4 | 3.8 | 0.2 | 1.3 |
| 30–60 cm | 2.3 | 5.5 | 3.6 | 6.4 | 8.7 | 1.2 | 6.2 | 3.1 | 0.3 | 2.0 | |
| Rostock | |||||||||||
| Shoulderslope | 0–30 cm | 6.0 | 11.8 | 2.2 | 4.4 | 12.1 | 3.3 | 0.3 | 5.7 | 0.0 | 0.4 |
| 30–60 cm | 7.1 | 13.1 | 2.0 | 4.3 | 12.9 | 4.2 | 0.1 | 6.3 | 0.0 | 0.2 | |
| Backslope | 0–30 cm | 6.1 | 12.5 | 1.9 | 4.3 | 12.3 | 3.5 | 0.3 | 6.0 | 0.0 | 0.4 |
| 30–60 cm | 6.7 | 13.2 | 1.8 | 4.2 | 12.6 | 3.9 | 0.2 | 6.2 | 0.0 | 0.3 | |
| Footslope | 0–30 cm | 5.5 | 10.7 | 2.3 | 4.3 | 11.1 | 3.1 | 0.7 | 5.8 | 0.0 | 0.7 |
| 30–60 cm | 5.7 | 10.6 | 2.3 | 4.2 | 11.2 | 3.3 | 0.7 | 6.0 | 0.1 | 0.5 | |
| Rogalow | |||||||||||
| Shoulderslope | 0–30 cm | 4.0 | 7.5 | 3.3 | 5.3 | 10.1 | 2.3 | 2.8 | 4.7 | 0.1 | 1.4 |
| 30–60 cm | 4.5 | 8.1 | 3.4 | 5.9 | 11.1 | 2.5 | 3.2 | 4.7 | 0.1 | 1.2 | |
| Backslope | 0–30 cm | 6.3 | 10.5 | 2.4 | 5.1 | 11.8 | 3.3 | 1.4 | 5.7 | 0.2 | 0.6 |
| 30–60 cm | 8.1 | 12.7 | 2.2 | 5.2 | 13.7 | 4.3 | 0.5 | 6.4 | 0.0 | 0.2 | |
| Footslope | 0–30 cm | 4.5 | 7.7 | 3.5 | 5.4 | 10.6 | 2.1 | 2.6 | 4.7 | 0.1 | 1.3 |
| 30–60 cm | 4.3 | 7.6 | 3.6 | 6.5 | 11.4 | 2.2 | 3.5 | 4.3 | 0.1 | 1.1 | |
| Pikareinu | |||||||||||
| Shoulderslope | 0–30 cm | 6.6 | 10.3 | 1.9 | 4.4 | 10.8 | 3.5 | 1.2 | 6.4 | 0.0 | 0.8 |
| 30–60 cm | 6.0 | 10.2 | 2.1 | 4.8 | 11.4 | 3.4 | 0.9 | 5.9 | 0.0 | 0.6 | |
| Backslope | 0–30 cm | 5.8 | 9.9 | 2.3 | 5.1 | 11.6 | 3.1 | 1.1 | 5.6 | 0.0 | 0.6 |
| 30–60 cm | 9.4 | 12.8 | 1.7 | 4.8 | 12.4 | 5.1 | 0.2 | 7.5 | 0.0 | 0.3 | |
| Footslope | 0–30 cm | 3.5 | 6.6 | 3.3 | 5.6 | 10.1 | 2.2 | 2.9 | 4.4 | 0.2 | 1.1 |
| 30–60 cm | 3.1 | 6.7 | 3.4 | 5.5 | 9.9 | 1.9 | 2.3 | 4.1 | 0.2 | 1.2 |
| Top: Sub | Vindum | Rostock | Rogalow | Pikareinu | ||||||||
| SS | BS | FS | SS | BS | FS | SS | BS | FS | SS | BS | FS | |
| Carbohydrates | - | - | > ** | < ** | < * | < ** | < * | |||||
| Phenols + lignin monomers | - | - | > ** | < ** | < * | < * | < * | < ** | ||||
| Lignin dimers | - | - | > * | |||||||||
| Lipids 1 | - | - | < ** | > * | < ** | < *** | ||||||
| Alkylaromatics | - | - | > ** | < * | < ** | < ** | < * | < ** | ||||
| N-compounds 2 | - | - | > *** | < * | < * | < ** | < ** | |||||
| Sterols | - | - | < ** | > * | < * | > *** | > * | |||||
| Amides 3 | - | - | > ** | < ** | < ** | |||||||
| Suberin | - | - | < * | > ** | > *** | |||||||
| Free fatty acids 4 | - | - | < ** | > *** | > ** | > * | > ** | |||||
| Low masses | - | - | > * | > * | < * | > * | < ** | |||||
| 0–30 cm | Vindum | Rostock | Rogalow | Pikareinu | ||||||||
| SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | |
| Carbohydrates | < * | > * | > * | > ** | < ** | > ** | > *** | > ** | ||||
| Phenols + lignin monomers | < *** | > *** | < * | > * | > ** | < * | > ** | > *** | > ** | |||
| Lignin dimers | > *** | < ** | > * | < ** | > ** | < ** | < ** | < *** | ||||
| Lipids 1 | > * | < ** | < * | < ** | ||||||||
| Alkylaromatics | < * | > * | < * | > *** | > ** | < * | < * | > *** | > * | |||
| N-compounds 2 | > * | < ** | > ** | > ** | > *** | |||||||
| Sterols | > *** | < *** | < ** | < ** | > ** | < ** | < ** | < *** | ||||
| Amides 3 | < * | > * | < * | > * | > ** | > ** | ||||||
| Suberin | > ** | < ** | < * | > ** | > *** | |||||||
| Free fatty acids 4 | > * | < *** | < ** | < *** | > ** | < * | > ** | > ** | ||||
| 30–60 cm | Vindum | Rostock | Rogalow | Pikareinu | ||||||||
| SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | |
| Carbohydrates | - | - | - | > * | > * | < *** | > *** | < * | > ** | > ** | ||
| Phenols + lignin monomers | - | - | - | > * | > * | < *** | > *** | < ** | > *** | > ** | ||
| Lignin dimers | - | - | - | < * | > ** | < ** | < *** | < ** | ||||
| Lipids 1 | - | - | - | > ** | < ** | < * | < * | < * | ||||
| Alkylaromatics | - | - | - | > * | > * | < *** | > *** | < ** | > *** | > ** | ||
| N-compounds 2 | - | - | - | > * | > * | < *** | > *** | < ** | > *** | > ** | ||
| Sterols | - | - | - | < * | > *** | < *** | < * | > ** | < *** | < *** | ||
| Amides 3 | - | - | - | < ** | > *** | < ** | > *** | > *** | ||||
| Suberin | - | - | - | > ** | < * | < * | < * | |||||
| Free fatty acids 4 | - | - | - | < * | < ** | < *** | > ** | < *** | > * | < ** | < ** |
| Top: Sub | Vindum | Rostock | Rogalow | Pikareinu | ||||||||
| SS | BS | FS | SS | BS | FS | SS | BS | FS | SS | BS | FS | |
| TII | - | - | ||||||||||
| Carbohydrates | - | - | < * | |||||||||
| Phenols + lignin monomers | - | - | < * | < * | < * | < * | ||||||
| Lignin dimers | - | - | < * | < ** | < * | < ** | ||||||
| Lipids 1 | - | - | < * | < * | < * | < * | < * | < * | ||||
| Alkylaromatics | - | - | < * | < * | ||||||||
| N-compounds 2 | - | - | ||||||||||
| Sterols | - | - | > * | > ** | ||||||||
| Amides 3 | - | - | < * | < * | ||||||||
| Suberin | - | - | ||||||||||
| Free fatty acids 4 | - | - | > * | < * | < * | < * | ||||||
| 0–30 cm | Vindum | Rostock | Rogalow | Pikareinu | ||||||||
| SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | |
| TII | > * | > ** | < ** | > * | > ** | |||||||
| Carbohydrates | > ** | < * | < ** | |||||||||
| Phenols + lignin monomers | < * | > ** | > * | > ** | > ** | > * | < * | < ** | < ** | |||
| Lignin dimers | > * | > ** | < * | < ** | < ** | |||||||
| Lipids 1 | < ** | > ** | > ** | > ** | < * | < ** | < * | |||||
| Alkylaromatics | > *** | < * | < ** | < ** | ||||||||
| N-compounds 2 | > * | > * | ||||||||||
| Sterols | ||||||||||||
| Amides 3 | > * | > ** | < ** | < * | < *** | |||||||
| Suberin | < ** | < * | < * | |||||||||
| Free fatty acids 4 | < ** | > * | > ** | |||||||||
| 30–60 cm | Vindum | Rostock | Rogalow | Pikareinu | ||||||||
| SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | SS vs. BS | BS vs. FS | SS vs. FS | |
| TII | - | - | - | |||||||||
| Carbohydrates | - | - | - | > * | > ** | |||||||
| Phenols + lignin monomers | - | - | - | > * | > * | |||||||
| Lignin dimers | - | - | - | > * | > * | < * | < * | > * | ||||
| Lipids 1 | - | - | - | > * | < ** | > *** | < * | > ** | ||||
| Alkylaromatics | - | - | - | > * | ||||||||
| N-compounds 2 | - | - | - | > * | ||||||||
| Sterols | - | - | - | > * | ||||||||
| Amides 3 | - | - | - | < * | ||||||||
| Suberin | - | - | - | |||||||||
| Free fatty acids 4 | - | - | - | > * | > * | > ** | < *** | > *** | < * | < * | > ** | > * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jandl, G.; Baum, C.; Heckrath, G.; Greve, M.H.; Kanal, A.; Mander, Ü.; Maliszewska-Kordybach, B.; Niedzwiecki, J.; Eckhardt, K.-U.; Leinweber, P. Erosion Induced Heterogeneity of Soil Organic Matter in Catenae from the Baltic Sea Catchment. Soil Syst. 2019, 3, 42. https://doi.org/10.3390/soilsystems3020042
Jandl G, Baum C, Heckrath G, Greve MH, Kanal A, Mander Ü, Maliszewska-Kordybach B, Niedzwiecki J, Eckhardt K-U, Leinweber P. Erosion Induced Heterogeneity of Soil Organic Matter in Catenae from the Baltic Sea Catchment. Soil Systems. 2019; 3(2):42. https://doi.org/10.3390/soilsystems3020042
Chicago/Turabian StyleJandl, Gerald, Christel Baum, Goswin Heckrath, Mogens H. Greve, Arno Kanal, Ülo Mander, Barbara Maliszewska-Kordybach, Jacek Niedzwiecki, Kai-Uwe Eckhardt, and Peter Leinweber. 2019. "Erosion Induced Heterogeneity of Soil Organic Matter in Catenae from the Baltic Sea Catchment" Soil Systems 3, no. 2: 42. https://doi.org/10.3390/soilsystems3020042
APA StyleJandl, G., Baum, C., Heckrath, G., Greve, M. H., Kanal, A., Mander, Ü., Maliszewska-Kordybach, B., Niedzwiecki, J., Eckhardt, K.-U., & Leinweber, P. (2019). Erosion Induced Heterogeneity of Soil Organic Matter in Catenae from the Baltic Sea Catchment. Soil Systems, 3(2), 42. https://doi.org/10.3390/soilsystems3020042







