Clinically Advanced Warty Invasive Squamous Cell Carcinoma of the Cervix with p16 Overexpression—Case Study and Literature Review
Abstract
1. Introduction and Clinical Significance
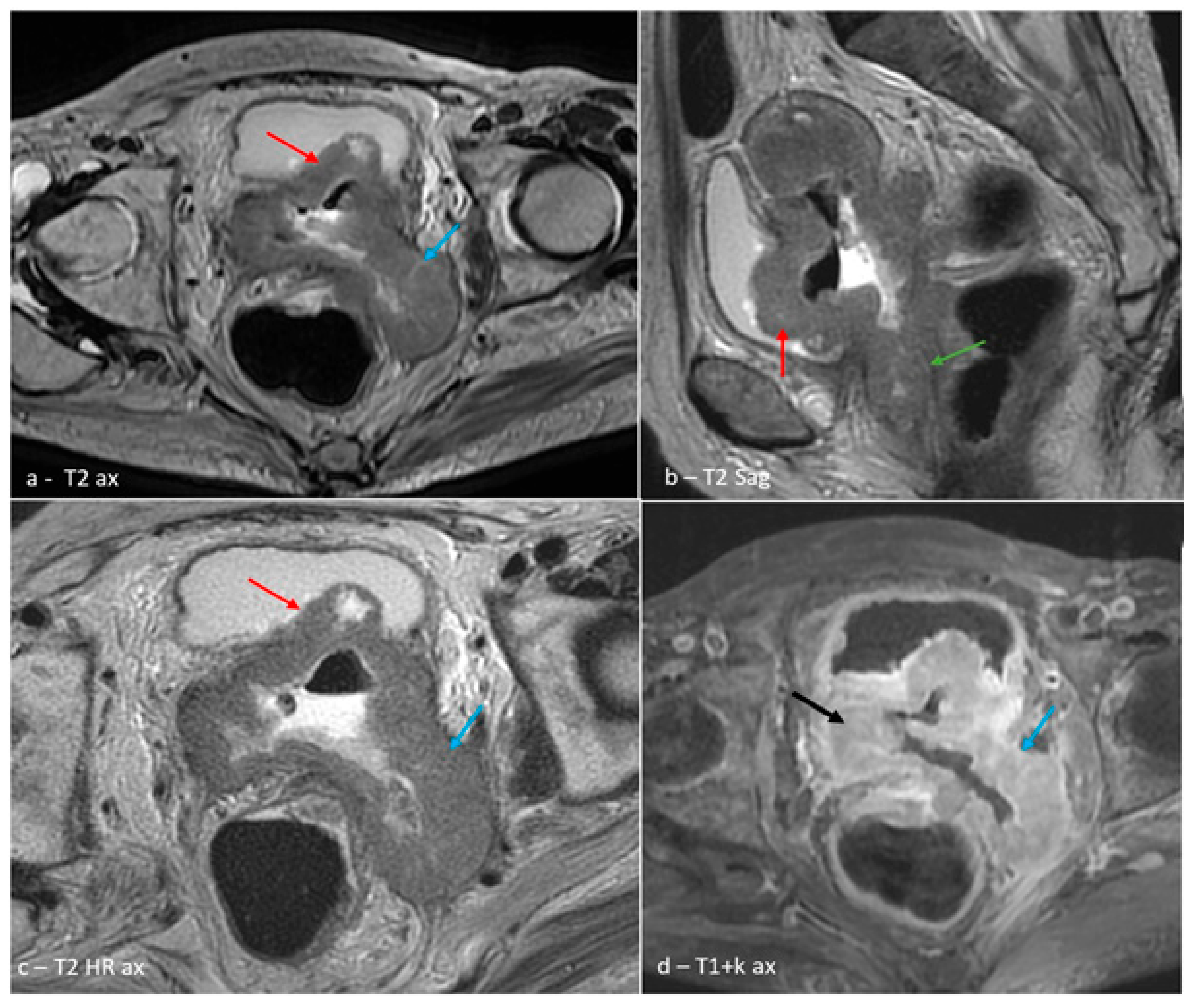
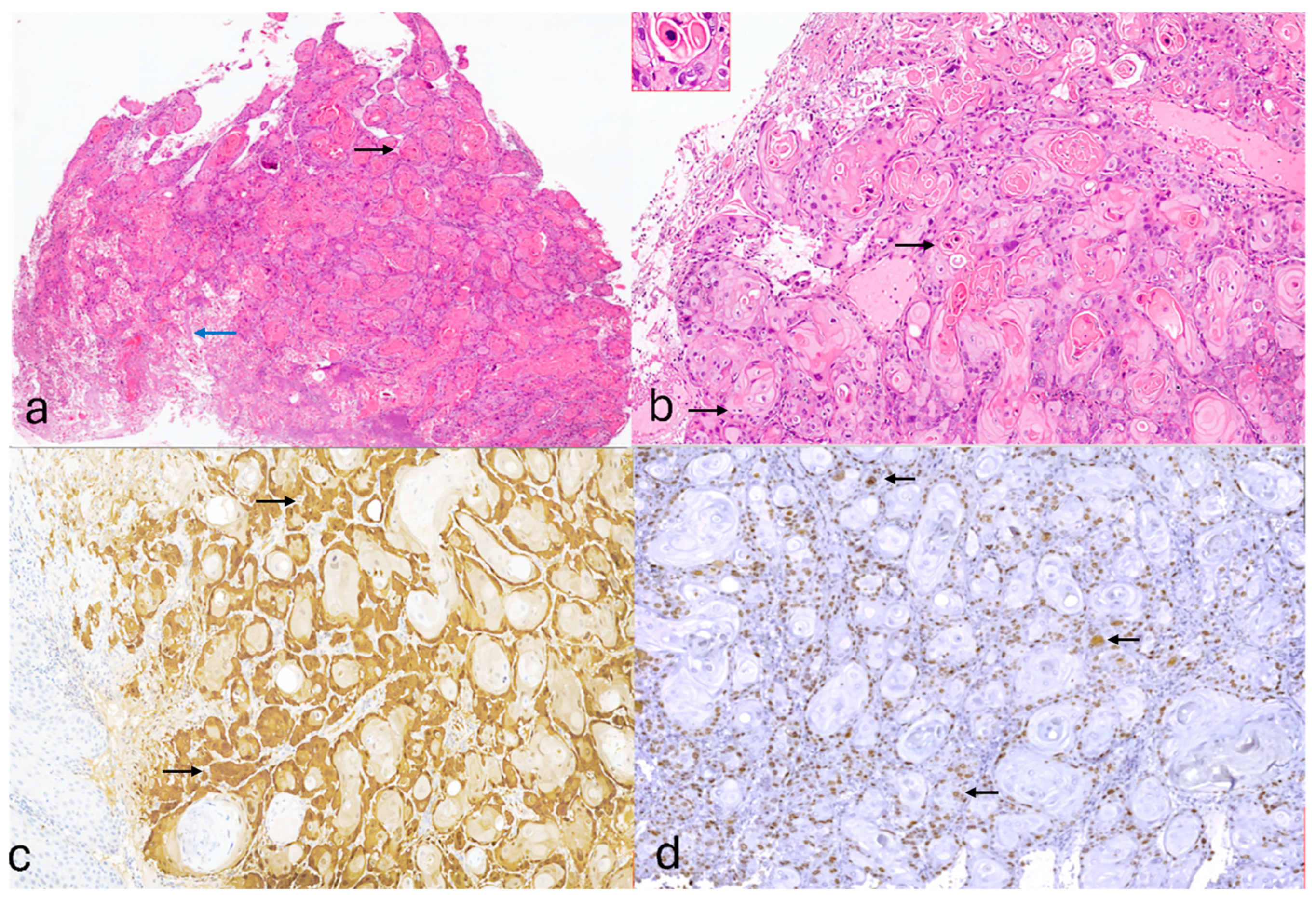
2. Case Presentation
3. Discussion
Literature Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; IARC: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 10 March 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Q.; Zhang, Y.; Ji, Y.; Li, J.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; Zhang, Y.; et al. Global burden of cervical cancer: Current estimates, temporal trend and future projections based on the GLOBOCAN 2022. J. Natl. Cancer Cent. 2024; ahead of print. [Google Scholar]
- Saco, A.; Carrilho, C.; Focchi, G.R.A.; Kong, C.S.; Mills, A.M.; Park, K.J.; Regauer, S. WHO Classification of Tumours of Female Genital Tract, 5th ed.; WHO: Geneva, Switzerland, 2020; Volume 4, pp. 347–350. [Google Scholar]
- Stolnicu, S.; Allison, D.; Patrichi, A.; Flynn, J.; Iasonos, A.; Soslow, R.A. Invasive squamous cell carcinoma of the cervix: Morphologic appearances in HPV-associated and HPV-independent tumours and precursors. Adv. Anat. Pathol. 2024, 31, 1–14. [Google Scholar] [CrossRef]
- Stoler, M.; Bergeron, C.; Colgan, T.J.; Ferenczy, A.S.; Herrington, C.S.; Kim, K.R.; Loening, T.; Schneider, A.; Sherman, M.E.; Wilbur, D.C.; et al. WHO Classification of Tumours of Female Genital Tract, 4th ed.; IARC: Lyon, France, 2014; Volume 4, pp. 172–182. [Google Scholar]
- Park, K.J.; Selinger, C.I.; Alvarado-Cabrero, I.; Duggan, M.A.; Kiyokawa, T.; Mills, A.M.; Ordi, J.; Otis, C.N.; Plante, M.; Stolnicu, S.; et al. Dataset for the reporting of carcinoma of the cervix: ICCR recommendations. Int. J. Gynecol. Pathol. 2022, 37 (Suppl. S1), S64–S89. [Google Scholar] [CrossRef]
- Padberg, B.C.; Bode, B.; Zimmermann, D.R. Metastatic warty (condylomatous) carcinoma of the uterine cervix associated with low-risk HPV type 6. Acta Cytol. 2006, 50, 235–238. [Google Scholar]
- Jang, Y.H.; Kim, Y.C.; Lee, E.S. Warty squamous cell carcinoma of the vulva in older women: Association with human papillomavirus. Yonsei Med. J. 2005, 46, 155–158. [Google Scholar] [CrossRef]
- Kurman, R.J.; Toki, T.; Schiffman, M.H. Basaloid and warty carcinomas of the vulva. Am. J. Surg. Pathol. 1993, 17, 133–145. [Google Scholar] [CrossRef]
- Yordanov, A.; Strashilov, S.; Karcheva, M.; Karamanliev, M.; Slavchev, S.; Vasileva, P. Contemporary challenges of warty carcinoma of cervix: Our experience and review. AMJ 2018, 11, 474–477. [Google Scholar] [CrossRef]
- Yordanov, A.; Ivanov, I.; Dineva, T.; Slavchev, S.; Kostov, S.; Strashilov, S.; Konsoulova, A. Warty carcinoma of the uterine cervix: A virus-induced disease? Arch. Med. Sci. 2020, 18, 1248–1252. [Google Scholar] [CrossRef]
- Chan, M.P. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch. Pathol. Lab. Med. 2019, 143, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- PRISMA 2020 Flow Diagram. Available online: https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on 10 March 2025).
- Cho, N.H.; Joo, H.J.; Ahn, H.J.; Jung, W.H.; Lee, K.G. Detection of HPV in warty carcinoma of the uterine cervix: Comparison of IHC, ISH and in situ PCR. Pathol. Res. Pract. 1998, 194, 713–720. [Google Scholar] [CrossRef]
- Ng, W.K.; Cheung, L.K.; Li, A.S. Warty (condylomatous) carcinoma of the cervix: Three cases with thin-layer cytology and HPV analysis. Acta Cytol. 2003, 47, 159–166. [Google Scholar] [CrossRef]
- Masuda, M.; Abiko, K.; Minamiguchi, S.; Murakami, R.; Baba, T.; Konishi, I. Rapidly progressing condylomatous (warty) SCC of the cervix associated with HPV-6. J. Obstet. Gynaecol. Res. 2018, 44, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Rokutan-Kurata, M.; Minamiguchi, S.; Kataoka, T.; Abiko, K.; Mandai, M.; Haga, H. Uterine cervical SCC without p16 (CDKN2A) expression: Heterogeneous causes of an unusual immunophenotype. Pathol. Int. 2020, 70, 413–421. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, C.M. p16-negative warty carcinoma of the uterine cervix with superficial endometrial invasion. Case Rep. Clin. Pathol. 2017, 4, 12–15. [Google Scholar]
- Landeyro, J.; Gutiérrez-Fornés, C.; Sirvent, J.J. Warty (condylomatous) carcinoma of the cervix with extension to the endometrial cavity. Int. J. Gynaecol. Obstet. 2011, 112, 66–67. [Google Scholar] [CrossRef]
- Olaru, O.G.; Pena, C.M.; Balalau, O.D.; Stanescu, A.D. Warty carcinoma of uterine cervix: Review and case report. J. Mind Med. Sci. 2020, 7, 100–104. [Google Scholar] [CrossRef]
- Ates, D.; Sahin, E.N.; Katipoglu, K.; Usubutun, A. Low-risk HPV–associated well-differentiated SCC of the cervix with LSIL morphology: Diagnostic difficulties and literature review. Turk. Patoloji Derg. 2024, 40, 196–201. [Google Scholar] [PubMed]
- World Health Organization. Cervical Cancer Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 10 March 2025).
- Yim, E.K.; Park, J.S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Qian, Y.S.; Lv, W.; Sui, L.H.; Wang, J. Relationship between cervical cancer genesis and infections with HPV16/18, HHV-II and CMV. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 622–625. [Google Scholar]
- Vranic, S.; Cyprian, F.S.; Akhtar, S.; Al Moustafa, A.E. The role of Epstein-Barr virus in cervical cancer: A brief update. Front. Oncol. 2018, 8, 113. [Google Scholar] [CrossRef]
- Silva, L.L.d.; Teles, A.M.; Santos, J.M.O.; Souza de Andrade, M.; Medeiros, R.; Faustino-Rocha, A.I.; Oliveira, P.A.; dos Santos, A.P.A.; Ferreira Lopes, F.; Braz, G.; et al. Malignancy Associated with Low-Risk HPV6 and HPV11: A Systematic Review and Implications for Cancer Prevention. Cancers 2023, 15, 4068. [Google Scholar] [CrossRef] [PubMed]
- Pavelescu, L.A.; Mititelu-Zafiu, N.L.; Mindru, D.E.; Vladareanu, R.; Curici, A. Molecular insights into HPV-driven cervical cancer: Oncoproteins, immune evasion, and epigenetic modifications. Microorganisms 2025, 13, 1000. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Velazquez, E.F.; Reuter, V.E.; Oliva, E.; Mihm, M.C., Jr.; Young, R.H. Warty (condylomatous) SCC of the penis: Report of 11 cases and classification of “verruciform” penile tumors. Am. J. Surg. Pathol. 2000, 24, 505–512. [Google Scholar] [CrossRef]
- Erman-Vlahovic, M.; Vlahovic, J.; Mrcela, M.; Hrgovic, Z. Coexistence of condylomata acuminata with warty squamous cell carcinoma and squamous cell carcinoma. Med. Arch. 2017, 71, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Petry, K.U.; Köchel, H.; Bode, U.; Schedel, I.; Niesert, S.; Glaubitz, M.; Maschek, H.; Kühnle, H. HPV associated with frequent detection of warty/basaloid VIN and cervical neoplasia in immunocompromised women. Gynecol. Oncol. 1996, 60, 30–34. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Law, K.S. Cervical verrucous cancer misdiagnosed as verrucous hyperplasia: Case report. Heliyon 2022, 8, e10268. [Google Scholar] [CrossRef]
- Huang, K.; Li, L.A.; Meng, Y.G.; Fu, X.Y. P16 expression in cervical cancer and prognostic significance: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 64–69. [Google Scholar] [CrossRef]
- da Mata, S.; Ferreira, J.; Nicolás, I.; Esteves, S.; Esteves, G.; Lérias, S.; Silva, F.; Saco, A.; Cochicho, D.; Cunha, M.; et al. P16 and HPV genotype significance in HPV-associated cervical cancer. Int. J. Mol. Sci. 2021, 22, 2294. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, R.D.; Arunachalam, S.; Amitkumar, K.; John, J.J.; Sudalaimuthu, M. Ki-67 expression in cervical SCC: An IHC study with clinicopathological correlation. Cureus 2023, 15, e34155. [Google Scholar]
- Pan, D.; Wei, K.; Ling, Y.; Su, S.; Zhu, M.; Chen, G. Prognostic role of Ki-67/MIB-1 in cervical cancer: Systematic review and meta-analysis. Med. Sci. Monit. 2015, 21, 882–889. [Google Scholar]
- Tu, Y.; Jiang, P.; Zhang, J.; Jiang, S.; Yi, Q.; Yuan, R. Positive threshold of Ki-67 for predicting cervical cancer recurrence. Int. J. Gynaecol. Obstet. 2022, 158, 330–337. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, M.; Yang, R.; Liu, J.; Zeng, P.; Zhao, T. Decreasing HIF-1α, VEGF-A, and Ki-67 with neoadjuvant therapy efficacy in locally advanced cervical cancer. Medicine 2023, 102, e33820. [Google Scholar] [CrossRef]
- Deacu, M.; Tuţă, L.A.; Boşoteanu, M.; Aşchie, M.; Mitroi, A.F.; Nicolau, A.A.; Enciu, M.; Cojocaru, O.; Petcu, L.C.; Bălţătescu, G.I. PD-L1 expression and its association with TILs and p53 status in triple-negative breast cancer. Rom. J. Morphol. Embryol. 2021, 62, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, A.; Mathe, E.; Kato, S.; Ishioka, C.; Tavtigian, S.V.; Hainaut, P.; Olivier, M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumour phenotype. Hum. Mutat. 2007, 28, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Obata, T.; Daikoku, T.; Fujiwara, H. The association and significance of p53 in gynecologic cancers: Potential for targeted therapy. Int. J. Mol. Sci. 2019, 20, 5482. [Google Scholar] [CrossRef]
- Dhamija, E.; Gulati, M.; Manchanda, S.; Singhal, S.; Sharma, D.; Kumar, S.; Bhatla, N. Imaging in carcinoma cervix and revised 2018 FIGO staging: Implications in radiology reporting. Indian J. Radiol. Imaging 2021, 31, 623–634. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cervical Cancer Treatment by Stage. Available online: https://www.cancer.gov/types/cervical/treatment/by-stage (accessed on 10 March 2025).
- Franco, I.; Viswanathan, A.N. Radiation oncology management of stage III and IVA cervical carcinoma. Int. J. Gynecol. Cancer 2022, 32, 231–238. [Google Scholar] [CrossRef]
| Study | Country | Nr | Age (y.o) | Clinical Stage | P16 IHC | HPV ISH | EBV ISH | PCR | Outcome | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Yordanov A (2018, 2022) [11,12] | Bulgaria | 14 | Mean 48 range 29–72 | FIGO IB1 (n = 6) FIGO IB2 (n = 8) | - | 1 case + * (HR- HPV) | 1 case + | HR-HPV poz, (n= 2) EBV poz (n = 2) HR-HPV+ EB1 poz (n = 2) * | Favorable | minimum 4 maximum 115 months |
| Cho NH (1988) [16] | Korea | 9 | Mean 54,1 range 44–77 | IA1-IIA | - | 6 + 11 (n = 1) 33 (n = 1) 16 (n = 7) | - | - | Favorable | - |
| Ng WK (2002) [17] | China | 3 | 50, 69, 81 | IB (n = 2) IIA (n = 1) | - | - | - | - | Favorable | - |
| Masuda M (2018) [18] | Japan | 1 | 43 | FIGO IB1 | neg | - | - | HPV6 | Unfavorable | 12 months DFS !—1 month |
| Rokutan-Kurata M (2020) [19] | Japan | 1 ☼ | 43 | T1bN1M0 | - | 6 | neg | - | Unfavorable | DFS ❄—10 months OS—22 months |
| Kim HJ (2017) [20] | Korea | 1 | 75 | FIGO IB1 | neg | 6 + 42 | - | HPV6 HPV42 | Favorable | follow-up: 3 months |
| Landeyro J (2011) [21] | Spain | 1 | 73 | FIGO IIA | poz | no | - | HPV16 | Unfavorable | patient died after surgery from postoperative bleeding complications |
| Olaru OG (2020) [22] | Romania | 1 | 58 | FIGO IIB | neg | - | - | HPV45 | Favorable | 5 years |
| Ates D (2024) [23] | Turky | 1 | 30 | FIGO IVA | neg | 6 + 11 | - | - | Favorable | 36 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrică, L.-A.; Deacu, M.; Cozaru, G.C.; Bălţătescu, G.I.; Aşchie, M. Clinically Advanced Warty Invasive Squamous Cell Carcinoma of the Cervix with p16 Overexpression—Case Study and Literature Review. Reports 2025, 8, 243. https://doi.org/10.3390/reports8040243
Petrică L-A, Deacu M, Cozaru GC, Bălţătescu GI, Aşchie M. Clinically Advanced Warty Invasive Squamous Cell Carcinoma of the Cervix with p16 Overexpression—Case Study and Literature Review. Reports. 2025; 8(4):243. https://doi.org/10.3390/reports8040243
Chicago/Turabian StylePetrică, Laura-Andra, Mariana Deacu, Georgeta Camelia Cozaru, Gabriela Izabela Bălţătescu, and Mariana Aşchie. 2025. "Clinically Advanced Warty Invasive Squamous Cell Carcinoma of the Cervix with p16 Overexpression—Case Study and Literature Review" Reports 8, no. 4: 243. https://doi.org/10.3390/reports8040243
APA StylePetrică, L.-A., Deacu, M., Cozaru, G. C., Bălţătescu, G. I., & Aşchie, M. (2025). Clinically Advanced Warty Invasive Squamous Cell Carcinoma of the Cervix with p16 Overexpression—Case Study and Literature Review. Reports, 8(4), 243. https://doi.org/10.3390/reports8040243






