From Technical Pitfall to Clinical Consequences: Leadless Pacing as a Rescue Solution
Abstract
1. Introduction and Clinical Significance
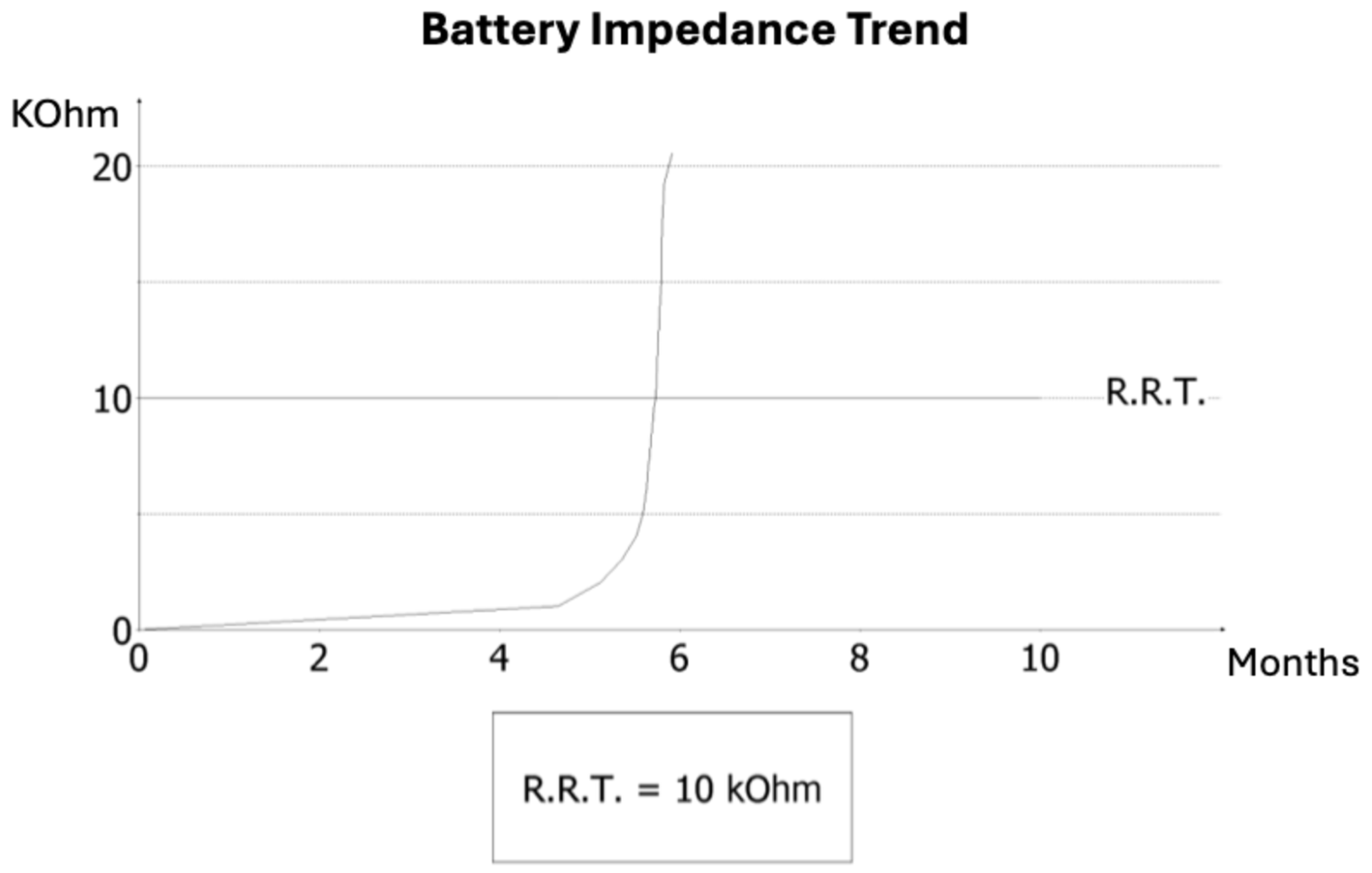
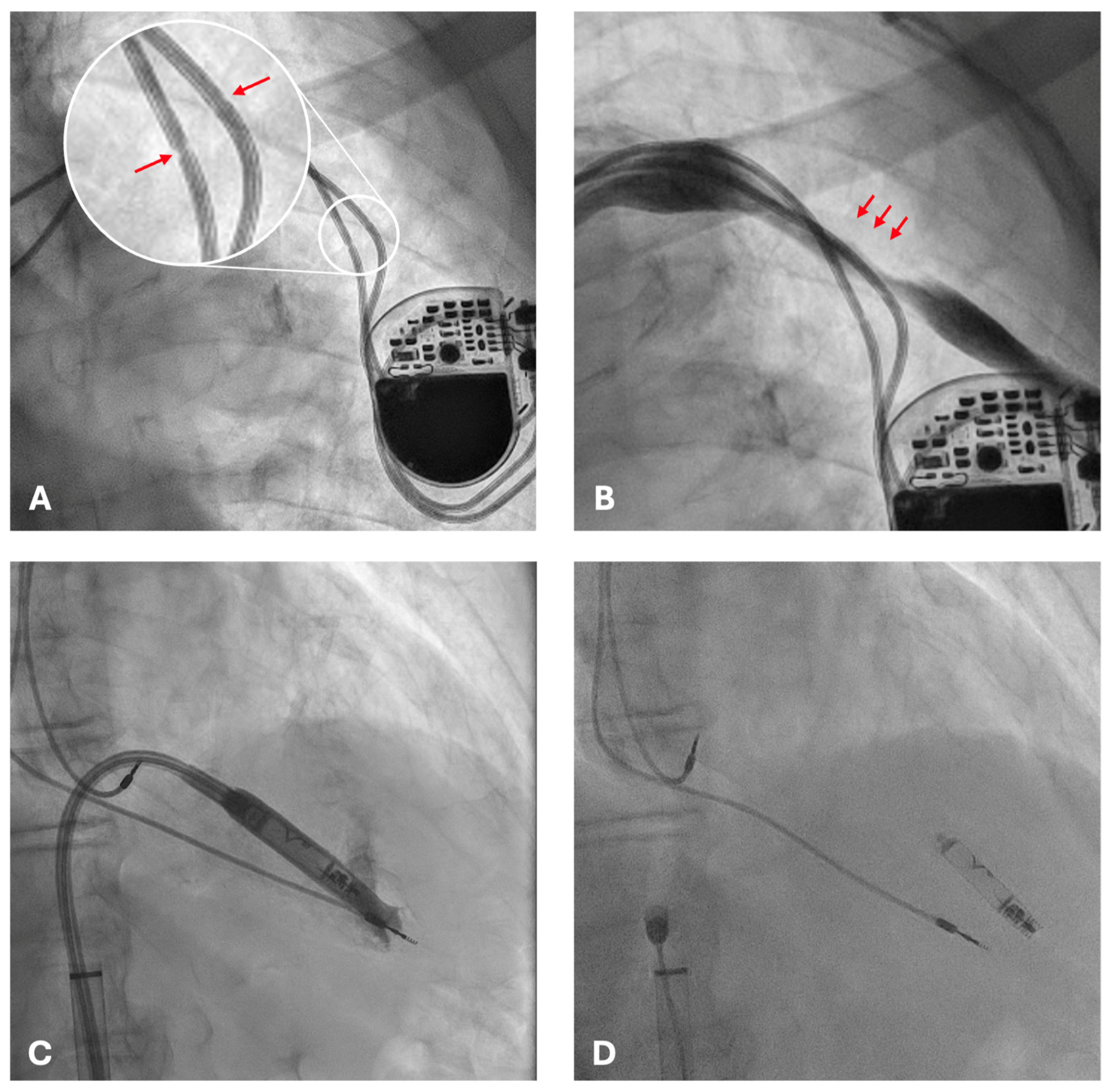
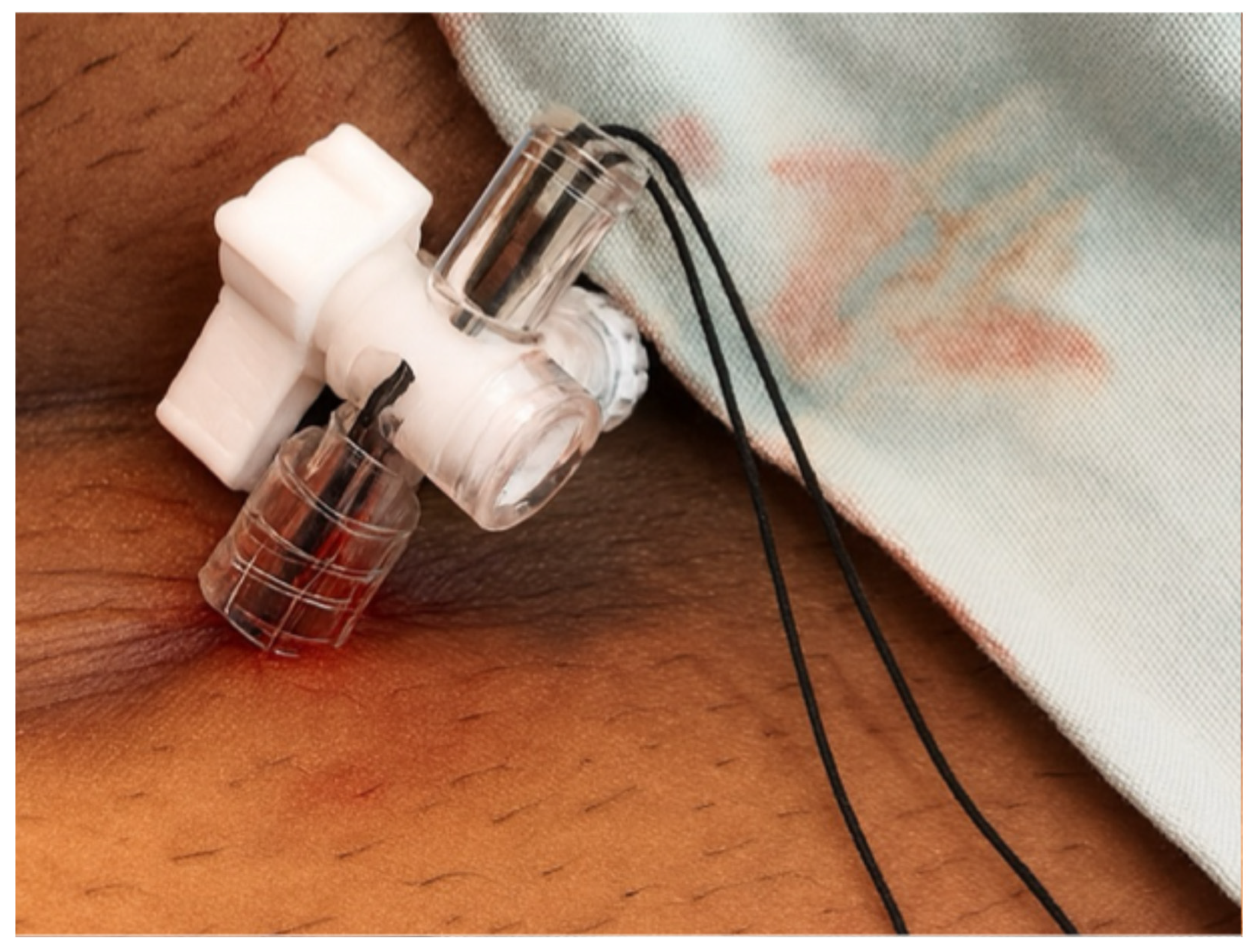
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| NIHSS | National Institutes of Health Stroke Scale |
| ERI | Elective Replacement Indicator |
| RRT | Recommended Replacement Time |
| mRS | Modified Rankin Scale |
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520, Erratum in Eur. Heart J. 2022, 43, 1651. https://doi.org/10.1093/eurheartj/ehac075. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, e51–e156, Erratum in J. Am. Coll. Cardiol. 2019, 74, 1016–1018. https://doi.org/10.1016/j.jacc.2019.06.048. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jørgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2014, 35, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schnorr, B.; Kelsch, B.; Cremers, B.; Clever, Y.P.; Speck, U.; Scheller, B. Contemporary issues in cardiac pacing. Minerva Cardioangiol. 2010, 58, 677–690. [Google Scholar] [PubMed]
- Witkowski, M.; Bissinger, A.; Grycewicz, T.; Lubinski, A. Asymptomatic atrial fibrillation in patients with atrial fibrillation and implanted pacemaker. Int. J. Cardiol. 2017, 227, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Chan, N.Y.; Kwong, N.P.; Cheong, A.P. Venous access and long-term pacemaker lead failure: Comparing contrast-guided axillary vein puncture with subclavian puncture and cephalic cutdown. Europace 2017, 19, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Meng, L.; Lin, H.; Xu, W.; Guo, H.; Peng, F. Systematic review of leadless pacemaker. Acta Cardiol. 2024, 79, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Mitacchione, G.; Schiavone, M.; Gasperetti, A.; Arabia, G.; Breitenstein, A.; Cerini, M.; Palmisano, P.; Montemerlo, E.; Ziacchi, M.; Gulletta, S.; et al. Outcomes of leadless pacemaker implantation following transvenous lead extraction in high-volume referral centers: Real-world data from a large international registry. Heart Rhythm 2023, 20, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Garweg, C.; Chinitz, J.S.; Marijon, E.; Haeberlin, A.; Winter, S.; Iacopino, S.; Curnis, A.; Breitenstein, A.; Hussin, A.; Mela, T.; et al. A leadless ventricular pacemaker providing atrioventricular synchronous pacing in the real-world setting: 12-Month results from the Micra AV post-approval registry. Heart Rhythm 2024, 21, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Garweg, C.; Clementy, N.; Al-Samadi, F.; Iacopino, S.; Martinez-Sande, J.L.; Roberts, P.R.; Tondo, C.; Johansen, J.B.; Vinolas-Prat, X.; et al. Leadless pacemakers at 5-year follow-up: The Micra transcatheter pacing system post-approval registry. Eur. Heart J. 2024, 45, 1241–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doshi, R.N.; Ip, J.E.; Defaye, P.; Reddy, V.Y.; Exner, D.V.; Canby, R.; Shoda, M.; Bongiorni, M.G.; Hindricks, G.; Neuzil, P.; et al. Dual-chamber leadless pacemaker implant procedural outcomes: Insights from the AVEIR DR i2i study. Heart Rhythm 2025, 22, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.N.; Ip, J.E.; Defaye, P.; Exner, D.V.; Reddy, V.Y.; Hindricks, G.; Canby, R.; Shoda, M.; Bongiorni, M.G.; Neužil, P.; et al. Chronic wireless communication between dual-chamber leadless pacemaker devices. Heart Rhythm 2025, 22, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Sterns, L.D. Pacemaker lead surveillance and failure: Is there a signal in the noise? Heart Rhythm 2019, 16, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Haeberlin, A.; Anwander, M.T.; Kueffer, T.; Tholl, M.; Baldinger, S.; Servatius, H.; Lam, A.; Franzeck, F.; Asatryan, B.; Zurbuchen, A.; et al. Unexpected high failure rate of a specific MicroPort/LivaNova/Sorin pacing lead. Heart Rhythm 2021, 18, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Kumar, V.; Talwar, K.K. Traumatic Fracture of Pacemaker Lead by Suture Transfixation to Pectoral Muscle. J. Invasive Cardiol. 2018, 30, E156. [Google Scholar] [PubMed]
- Rezazadeh, S.; Wang, S.; Rizkallah, J. Evaluation of common suturing techniques to secure implantable cardiac electronic device leads: Which strategy best reduces the lead dislodgement risk? Can. J. Surg. 2019, 62, E10–E13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Gibbawi, M.; Ayinde, H.O.; Bhatia, N.K.; El-Chami, M.F.; Westerman, S.B.; Leon, A.R.; Shah, A.D.; Patel, A.M.; De Lurgio, D.B.; Tompkins, C.M.; et al. Relationship between device-detected burden and duration of atrial fibrillation and risk of ischemic stroke. Heart Rhythm 2021, 18, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129, Erratum in N. Engl. J. Med. 2016, 374, 998. https://doi.org/10.1056/NEJMx160004. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Lopes, R.D.; Granger, C.B.; Alings, M.; Rivard, L.; McIntyre, W.F.; Atar, D.; Birnie, D.H.; Boriani, G.; Camm, A.J.; et al. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. N. Engl. J. Med. 2024, 390, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Toennis, T.; Goette, A.; Camm, A.J.; Diener, H.C.; Becher, N.; Bertaglia, E.; Blomstrom Lundqvist, C.; Borlich, M.; Brandes, A.; et al. Anticoagulation with Edoxaban in Patients with Atrial High-Rate Episodes. N. Engl. J. Med. 2023, 389, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ze, F.; Wang, L.; Li, D.; Duan, J.; Guo, F.; Yuan, C.; Li, Y.; Guo, J. Prevalence of venous occlusion in patients referred for lead extraction: Implications for tool selection. Europace 2014, 16, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Darlington, D.; Brown, P.; Carvalho, V.; Bourne, H.; Mayer, J.; Jones, N.; Walker, V.; Siddiqui, S.; Patwala, A.; Kwok, C.S. Efficacy and safety of leadless pacemaker: A systematic review, pooled analysis and meta-analysis. Indian Pacing Electrophysiol. J. 2022, 22, 77–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jelisejevas, J.; Breitenstein, A.; Hofer, D.; Winnik, S.; Steffel, J.; Saguner, A.M. Left femoral venous access for leadless pacemaker implantation: Patient characteristics and outcomes. Europace 2021, 23, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.F.; Carvalho, M.M.; Adão, L.; Nunes, J.P. Clinical outcomes of leadless pacemaker: A systematic review. Minerva Cardiol. Angiol. 2021, 69, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Kadado, A.J.; Chalhoub, F. Periprocedural anticoagulation therapy in patients undergoing micra leadless pacemaker implantation. Int. J. Cardiol. 2023, 371, 221–225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciapuoti, F.; Mauro, C.; Casolaro, F.; Torsi, A.; Crispo, S.; Volpicelli, M. From Technical Pitfall to Clinical Consequences: Leadless Pacing as a Rescue Solution. Reports 2025, 8, 206. https://doi.org/10.3390/reports8040206
Cacciapuoti F, Mauro C, Casolaro F, Torsi A, Crispo S, Volpicelli M. From Technical Pitfall to Clinical Consequences: Leadless Pacing as a Rescue Solution. Reports. 2025; 8(4):206. https://doi.org/10.3390/reports8040206
Chicago/Turabian StyleCacciapuoti, Fulvio, Ciro Mauro, Flavia Casolaro, Antonio Torsi, Salvatore Crispo, and Mario Volpicelli. 2025. "From Technical Pitfall to Clinical Consequences: Leadless Pacing as a Rescue Solution" Reports 8, no. 4: 206. https://doi.org/10.3390/reports8040206
APA StyleCacciapuoti, F., Mauro, C., Casolaro, F., Torsi, A., Crispo, S., & Volpicelli, M. (2025). From Technical Pitfall to Clinical Consequences: Leadless Pacing as a Rescue Solution. Reports, 8(4), 206. https://doi.org/10.3390/reports8040206




