Hemostasis Using Esophageal Balloon of Sengstaken–Blakemore Tube for Ulcer Bleeding at Esophagogastric Anastomosis: A Case Report
Abstract
1. Introduction and Clinical Significance
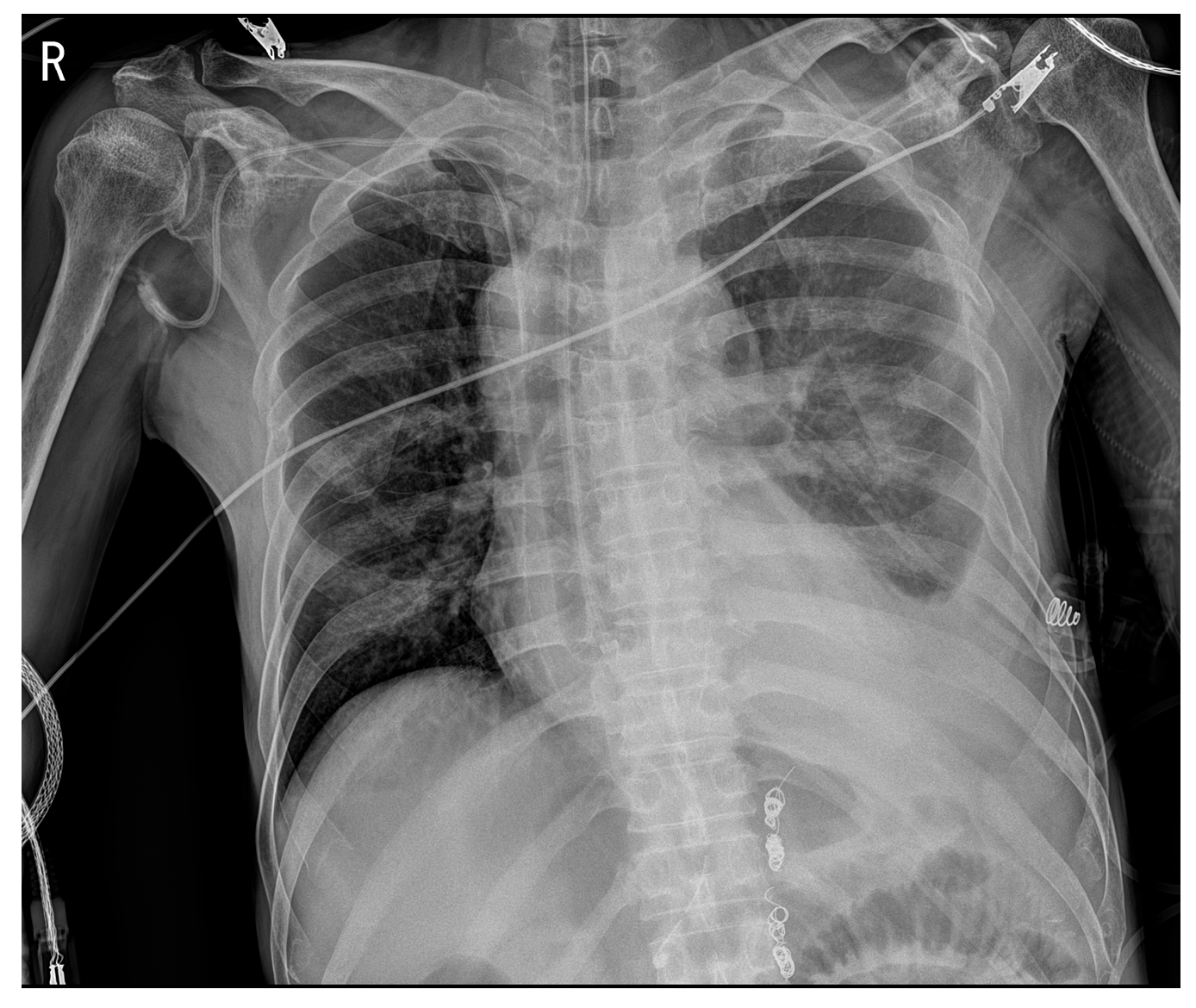
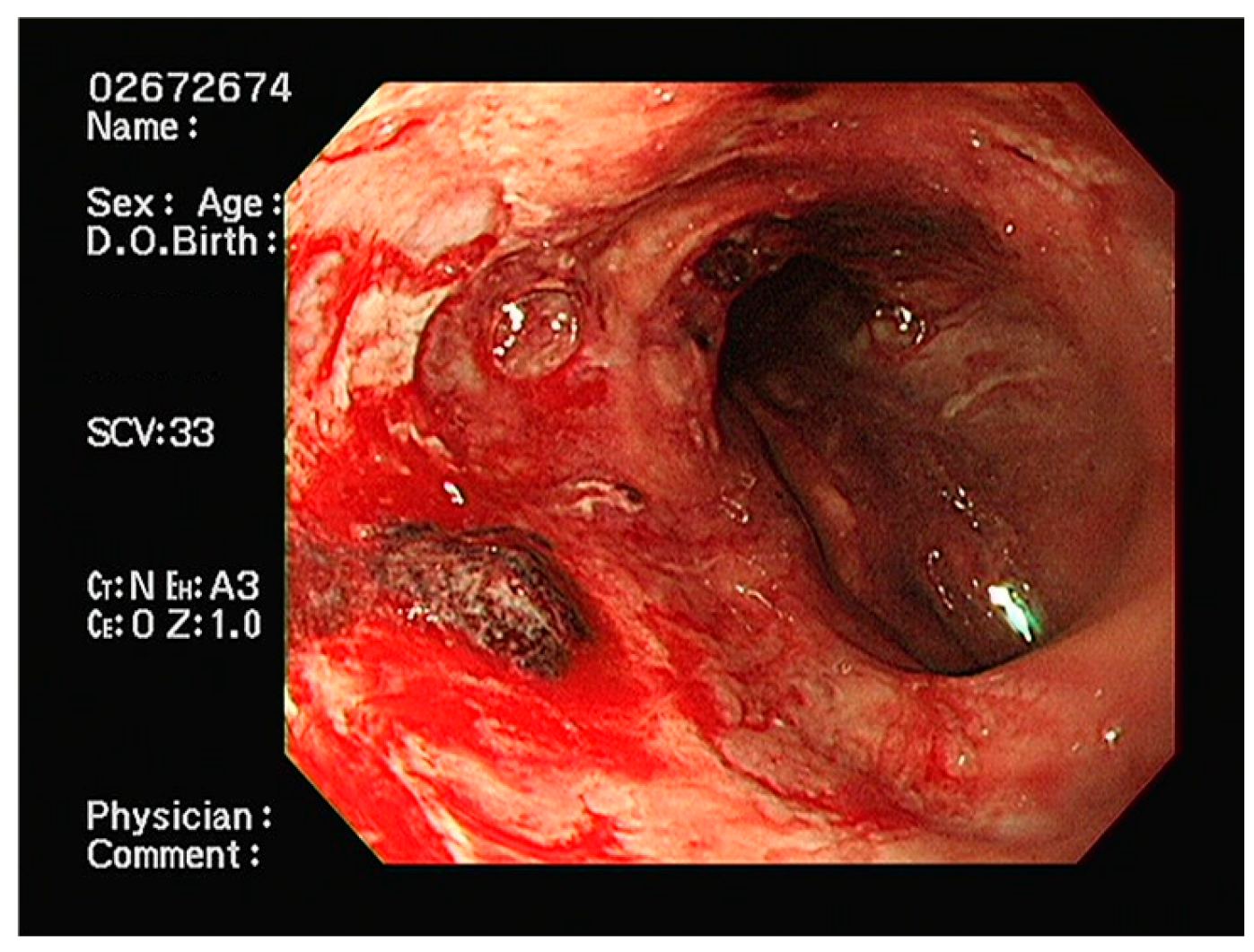
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laine, L.; Yang, H.; Chang, S.C.; Datto, C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am. J. Gastroenterol. 2012, 107, 1190–1195. [Google Scholar] [CrossRef]
- Hearnshaw, S.A.; Logan, R.F.; Lowe, D.; Travis, S.P.; Murphy, M.F.; Palmer, K.R. Acute upper gastrointestinal bleeding in the UK: Patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011, 60, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.J.; Laine, L. Management of acute upper gastrointestinal bleeding. Bmj 2019, 364, l536. [Google Scholar] [CrossRef] [PubMed]
- Sass, D.A.; Chopra, K.B. Portal hypertension and variceal hemorrhage. Med. Clin. North Am. 2009, 93, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Barkun, A.N.; Bardou, M.; Kuipers, E.J.; Sung, J.; Hunt, R.H.; Martel, M.; Sinclair, P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann. Intern. Med. 2010, 152, 101–113. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Dumonceau, J.M.; Kuipers, E.J.; Lanas, A.; Sanders, D.S.; Kurien, M.; Rotondano, G.; Hucl, T.; Dinis-Ribeiro, M.; Marmo, R.; et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, a1–a46. [Google Scholar] [CrossRef]
- D’Amico, G.; De Franchis, R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003, 38, 599–612. [Google Scholar] [CrossRef]
- Laine, L.; McQuaid, K.R. Endoscopic therapy for bleeding ulcers: An evidence-based approach based on meta-analyses of randomized controlled trials. Clin. Gastroenterol. Hepatol. 2009, 7, 33–47. [Google Scholar] [CrossRef]
- Sung, J.J.; Tsoi, K.K.; Lai, L.H.; Wu, J.C.; Lau, J.Y. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: A meta-analysis. Gut 2007, 56, 1364–1373. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Young, S.D.; Inadomi, J.M.; Schoenfeld, P.; Laine, L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am. J. Gastroenterol. 2008, 103, 2625–2632. [Google Scholar] [CrossRef]
- Sengstaken, R.W.; Blakemore, A.H. Balloon tamponage for the control of hemorrhage from esophageal varices. Ann. Surg. 1950, 131, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.; Journey, J.D. Sengstaken-Blakemore Tube. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Seet, E.; Beevee, S.; Cheng, A.; Lim, E. The Sengstaken-Blakemore tube: Uses and abuses. Singap. Med. J. 2008, 49, e195–e197. [Google Scholar]
- Pasquale, M.D.; Cerra, F.B. Sengstaken-Blakemore tube placement. Use of balloon tamponade to control bleeding varices. Crit. Care Clin. 1992, 8, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.F. Esophageal rupture due to Sengstaken-Blakemore tube misplacement. World J. Gastroenterol. 2005, 11, 6563–6565. [Google Scholar] [CrossRef]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef]
- Markar, S.R.; Arya, S.; Karthikesalingam, A.; Hanna, G.B. Technical factors that affect anastomotic integrity following esophagectomy: Systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 4274–4281. [Google Scholar] [CrossRef]
- Kassis, E.S.; Kosinski, A.S.; Ross, P., Jr.; Koppes, K.E.; Donahue, J.M.; Daniel, V.C. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 2013, 96, 1919–1926. [Google Scholar] [CrossRef]
- Schizas, D.; Giannopoulos, S.; Vailas, M.; Mylonas, K.S.; Giannopoulos, S.; Moris, D.; Rouvelas, I.; Felekouras, E.; Liakakos, T. The impact of cirrhosis on esophageal cancer surgery: An up-to-date meta-analysis. Am. J. Surg. 2020, 220, 865–872. [Google Scholar] [CrossRef]
- Schmidt, A.; Gölder, S.; Goetz, M.; Meining, A.; Lau, J.; von Delius, S.; Escher, M.; Hoffmann, A.; Wiest, R.; Messmann, H.; et al. Over-the-Scope Clips Are More Effective Than Standard Endoscopic Therapy for Patients With Recurrent Bleeding of Peptic Ulcers. Gastroenterology 2018, 155, 674–686.e6. [Google Scholar] [CrossRef]
- Chen, Y.I.; Barkun, A.N. Hemostatic Powders in Gastrointestinal Bleeding: A Systematic Review. Gastrointest. Endosc. Clin. North Am. 2015, 25, 535–552. [Google Scholar] [CrossRef]
- Sung, J.J.; Luo, D.; Wu, J.C.; Ching, J.Y.; Chan, F.K.; Lau, J.Y.; Mack, S.; Ducharme, R.; Okolo, P.; Canto, M.; et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy 2011, 43, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Favelier, S.; Pottecher, P.; Estivalet, L.; Genson, P.Y.; Gehin, S.; Cercueil, J.P.; Krausé, D. Transcatheter arterial embolization for acute nonvariceal upper gastrointestinal bleeding: Indications, techniques and outcomes. Diagn. Interv. Imaging 2015, 96, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Wong, K.T.; Chiu, P.W.; Teoh, A.Y.; Yu, S.C.; Au, K.W.; Lau, J.Y. A comparison of angiographic embolization with surgery after failed endoscopic hemostasis to bleeding peptic ulcers. Gastrointest. Endosc. 2011, 73, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.P.; Seder, C.W.; Wright, C.D.; Magee, M.J.; Kosinski, A.S.; Cassivi, S.D.; Grogan, E.L.; Blackmon, S.H.; Allen, M.S.; Park, B.J.; et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann. Thorac. Surg. 2016, 102, 207–214. [Google Scholar] [CrossRef]
- Müller, M.; Seufferlein, T.; Perkhofer, L.; Wagner, M.; Kleger, A. Self-Expandable Metal Stents for Persisting Esophageal Variceal Bleeding after Band Ligation or Injection-Therapy: A Retrospective Study. PLoS ONE 2015, 10, e0126525. [Google Scholar] [CrossRef]
- Bridwell, R.E.; Long, B.; Ramzy, M.; Gottlieb, M. Balloon Tamponade for the Management of Gastrointestinal Bleeding. J. Emerg. Med. 2022, 62, 545–558. [Google Scholar] [CrossRef]
- Escorsell, À.; Pavel, O.; Cárdenas, A.; Morillas, R.; Llop, E.; Villanueva, C.; Garcia-Pagán, J.C.; Bosch, J. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: A multicenter randomized, controlled trial. Hepatology 2016, 63, 1957–1967. [Google Scholar] [CrossRef]
- Panés, J.; Terés, J.; Bosch, J.; Rodés, J. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig. Dis. Sci. 1988, 33, 454–459. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Charles, A.V. Lethal esophageal rupture following treatment with Sengstaken-Blakemore tube in management of variceal bleeding: A 10-year autopsy study. Forensic Sci. Int. 2012, 222, e19–e22. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef]
- de Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- García-Pagán, J.C.; Reverter, E.; Abraldes, J.G.; Bosch, J. Acute variceal bleeding. Semin. Respir. Crit. Care Med. 2012, 33, 46–54. [Google Scholar] [CrossRef]
- Hsieh, W.Y.; Chen, P.H.; Lin, I.Y.; Su, C.W.; Chao, Y.; Huo, T.I.; Huang, Y.H.; Hou, M.C.; Lin, H.C.; Wu, J.C. The impact of esophagogastric varices on the prognosis of patients with hepatocellular carcinoma. Sci. Rep. 2017, 7, 42577. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Kim, T. Hemostasis Using Esophageal Balloon of Sengstaken–Blakemore Tube for Ulcer Bleeding at Esophagogastric Anastomosis: A Case Report. Reports 2025, 8, 241. https://doi.org/10.3390/reports8040241
Yoo J, Kim T. Hemostasis Using Esophageal Balloon of Sengstaken–Blakemore Tube for Ulcer Bleeding at Esophagogastric Anastomosis: A Case Report. Reports. 2025; 8(4):241. https://doi.org/10.3390/reports8040241
Chicago/Turabian StyleYoo, Jonghoon, and Taekwon Kim. 2025. "Hemostasis Using Esophageal Balloon of Sengstaken–Blakemore Tube for Ulcer Bleeding at Esophagogastric Anastomosis: A Case Report" Reports 8, no. 4: 241. https://doi.org/10.3390/reports8040241
APA StyleYoo, J., & Kim, T. (2025). Hemostasis Using Esophageal Balloon of Sengstaken–Blakemore Tube for Ulcer Bleeding at Esophagogastric Anastomosis: A Case Report. Reports, 8(4), 241. https://doi.org/10.3390/reports8040241





