Adjuvant A-PRF Application in Patients with Various Stages of Medication-Related Osteonecrosis of the Jaw (MRONJ): Case Series
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
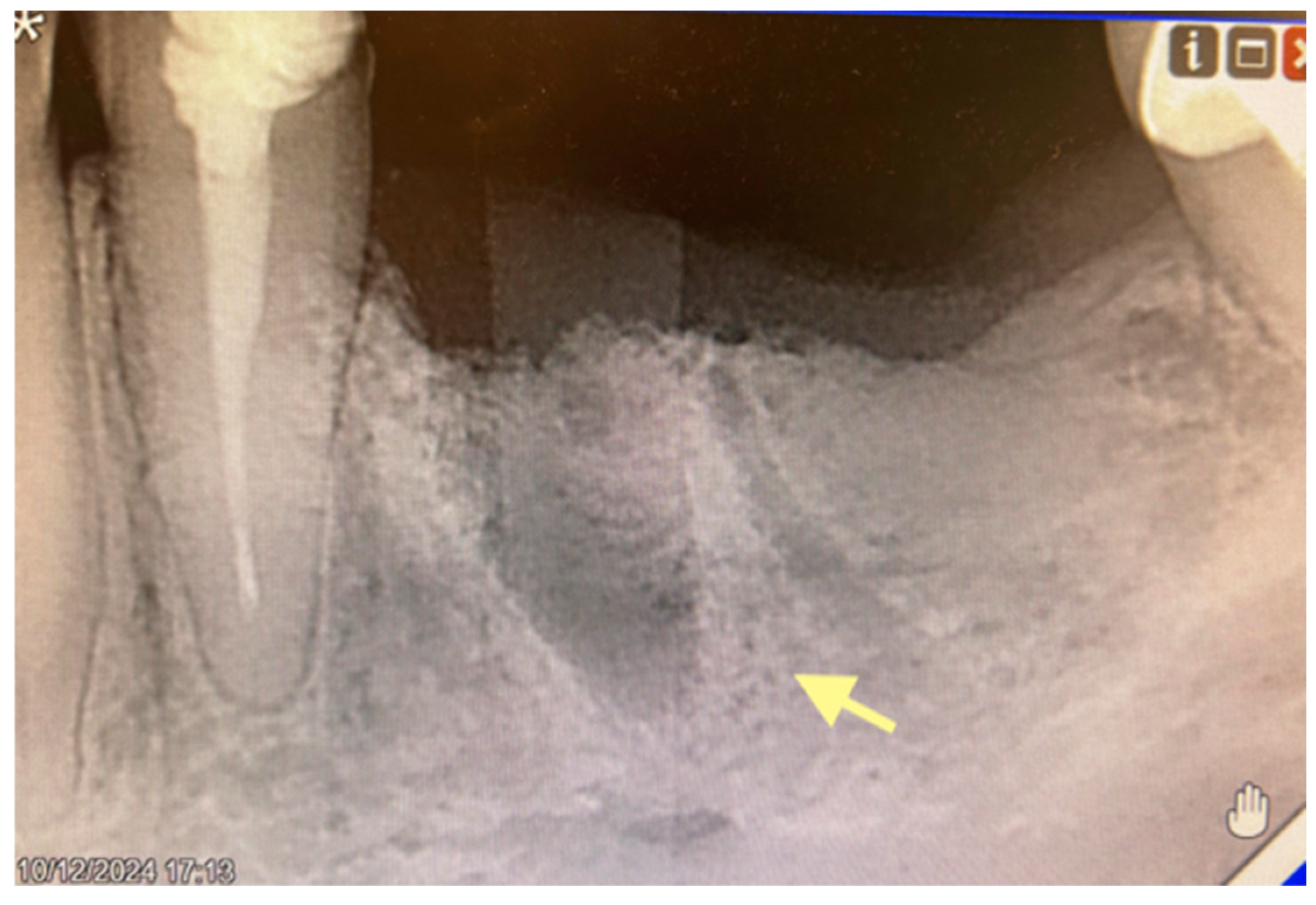
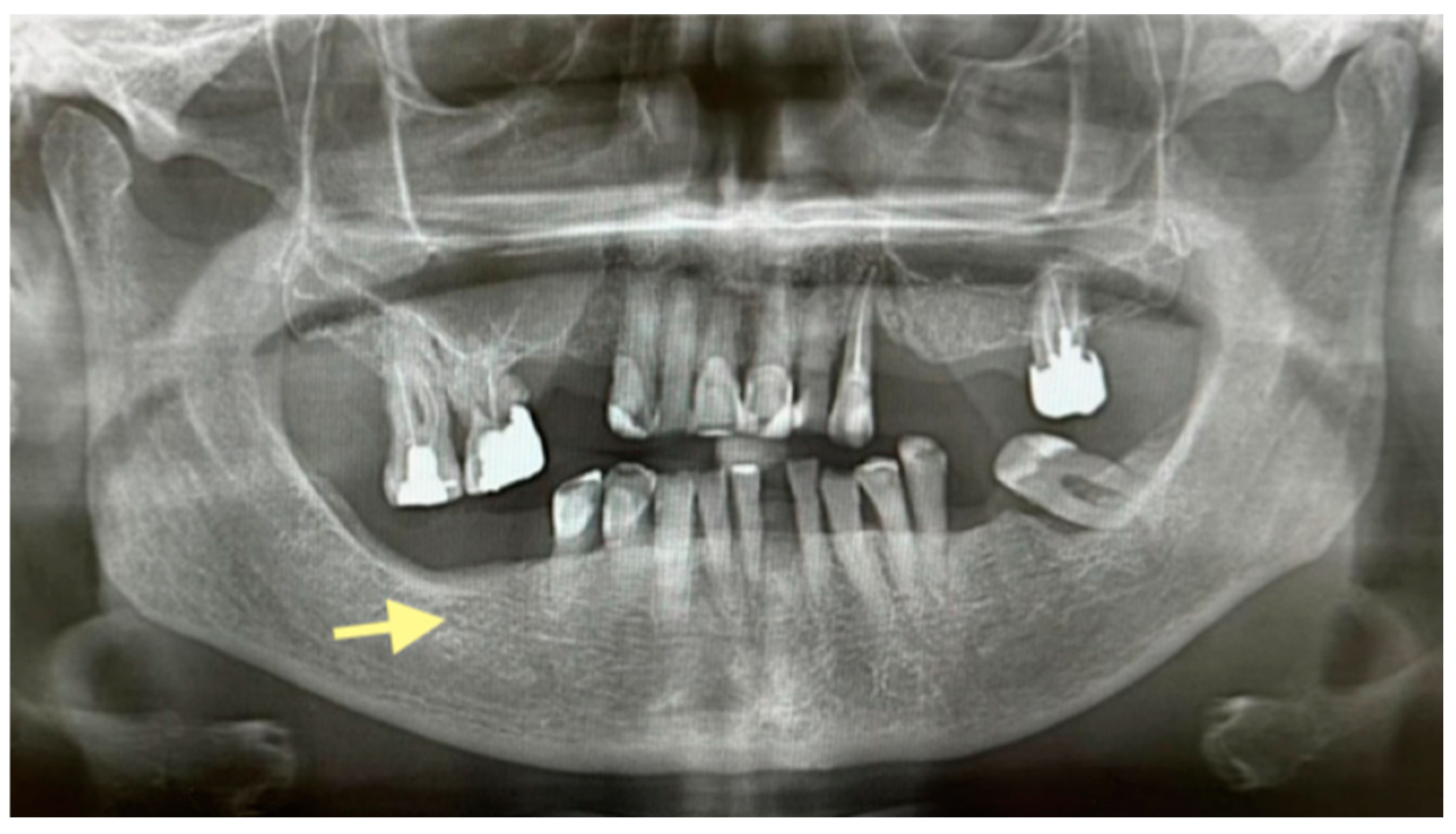
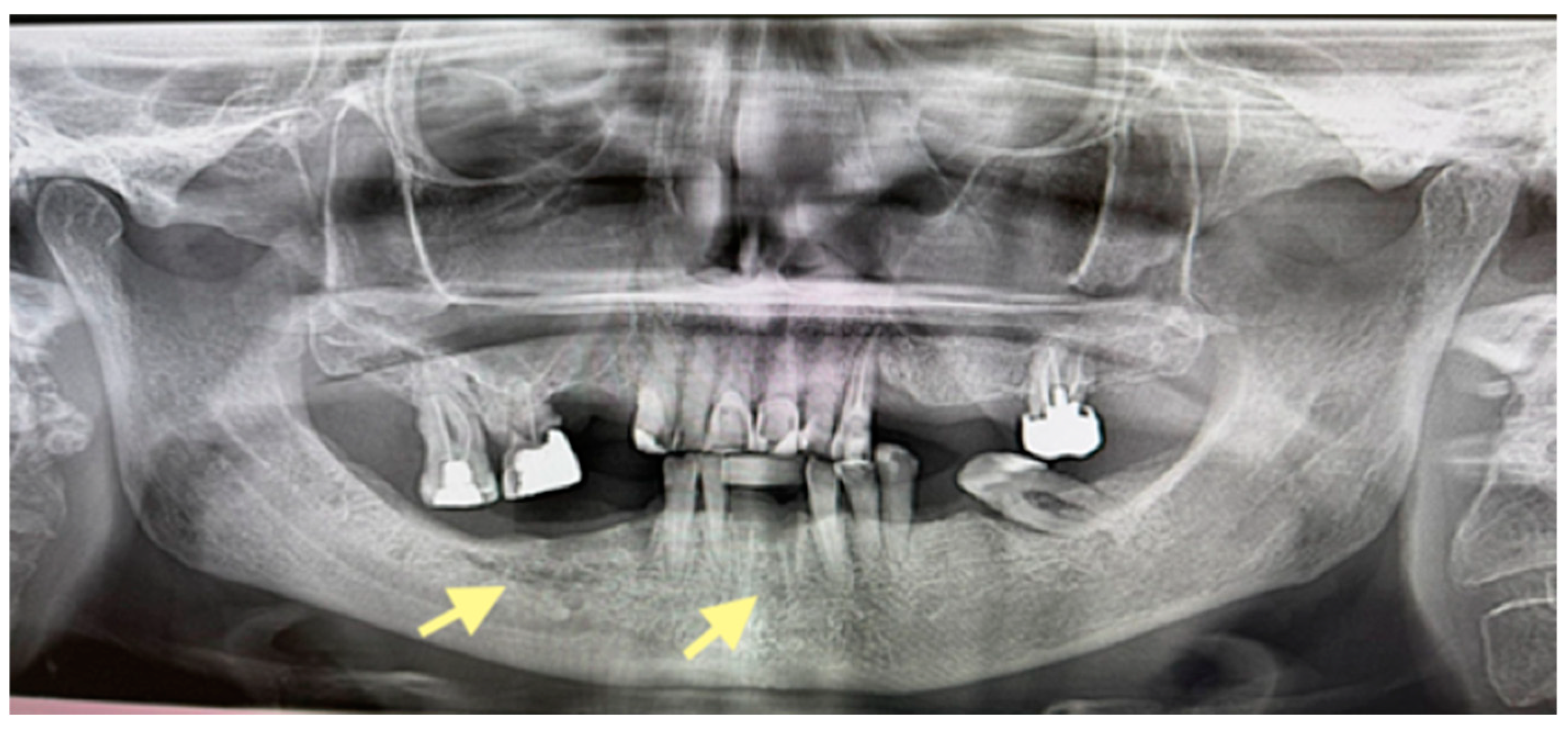
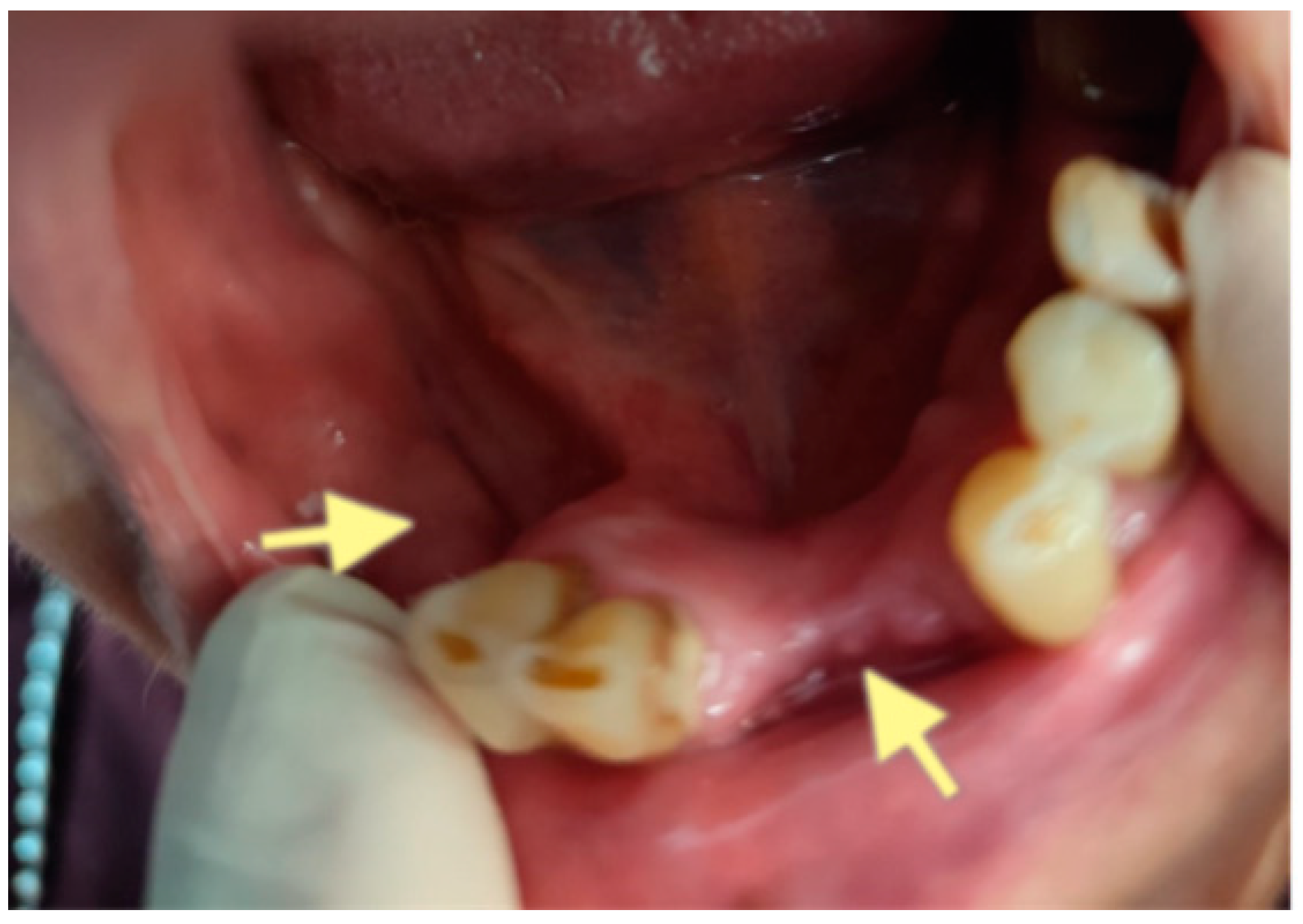
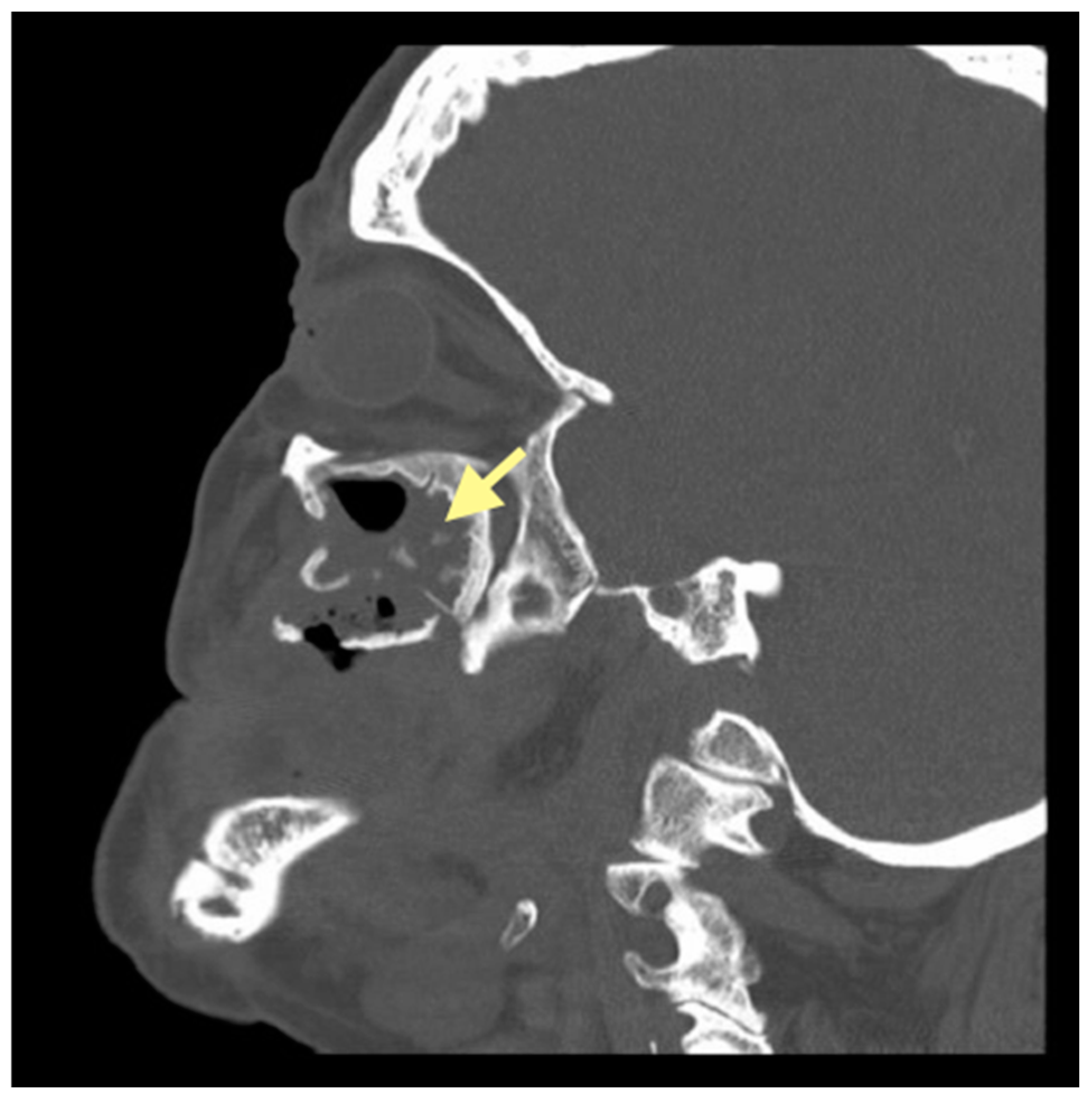
2.1. The First Case (See Table 1)
| The First Clinical Case | The Second Clinical Case | The Third Clinical Case | |
|---|---|---|---|
| Demographics | 64-years old female | 82-years old female | 75-years old female |
| Primary disease | Malignant breast tumor (IV st.); bones metastases | Malignant breast tumor (IV st.); bones metastases | Malignant breast tumor (IV st.); bones and lung metastases |
| Comorbidities | - | - | - |
| Bad habits | Non-smoker Non-alcoholic person | Non-smoker Non-alcoholic person | Non-smoker Non-alcoholic person |
| Risk factors (periodontal disease) | - | dd13,14,16,17, 22,23,38,41.42.31.32 Chronic periodontitis with deep bone pockets | Adentia of the upper jaw; hypodentia of the lower jaw; dd 32,33; Chronic periodontitis |
| Oral hygiene | Good | Satisfactory | Satisfactory |
| Drug exposure history | Zolendronic acid 5 mg (i/v) once a month, 2021.g. (March)–024.g. (November) | Zolendronic acid 5 mg (i/v) once a month, 2021.g. (January)–2024.g. (June) | Zolendronic acid 5 mg(i/v) once a month—2021.g. (August) |
| Clinical finding | MRONJ in reg.dd34-35 STAGE I | MRONJ in reg.dd45-46 and reg.dd41-31 STAGE II | MRONJ in reg.dd21-28, dd34-36, dd31-46 STAGE III |
| Surgery | 24 February 2025 Sequestrectomy of the lower jaw (reg.dd34-35) and A-PRF application | 5 May 2024 Sequestrectomy of the lower jaw (reg.dd45-46) and A-PRF application. 4 April 2025 Sequestrectomy of the lower jaw (reg.dd45-46, reg.dd41-31) and A-PRF application | 25 September 2023 Sequestrectomy of the lower jaw (III quadrant) and of the upper jaw (II quadrant) 19 February 2024 e/o incision; revision of submandibular abscess 17 December 2024 Sequestrectomy of the lower jaw (IV quadrant) 20 March 2025 Sequestrectomy of the lower jaw (IV quadrant) and of the upper jaw (II quadrant); A-PRF application |
| Outcome | Complete healing | Surgical wound on the lower jaw in reg.dd 45-47 healed completely, but closed partially in reg.dd 41-31 | Surgical wounds reg.dd32-42 and reg.dd27-28 have not healed, but surgical wounds reg.dd34-46 healed completely |
| Microbiology | Normal microflora of the upper respiratory tract grows in the culture | Streptococcus constellatus (sensitive to Penicillin, Cefotaxime, Ceftriaxone, Clindamycin, Vankomycin)—5 May 2024. Porphyromonas gingivalis, Parvimonas micra, Prevotella buccae—7 April 2025. | Streptococcus constellatus (sensitive to Penicillin, Cefotaxime, Ceftriaxone, Clindamycin, Vankomycin)—27 January 2025 Streptococcus constellatus Actinomyces odontolyticus Bacteroides thetalomicron Prevotella nigrescens—21 March 2025. |
| Morphological picture | The morphological picture corresponds to small bone fragments; soft tissue with focal intense lymphomacrophage inflammatory infiltration | The morphological picture corresponds to granulation tissue of varying degrees of maturity, with chronic purulent inflammation | The morphological picture corresponds to bone fragments with necrosis; neutrophilic leukocyte infiltration |
| Antibacterial therapy | 4 days in hospital—Amoxiclav 1.2 g 3x(i/v); after that, continued T.Amoxyclav 1 g 2x(p/o) for 4 days | 4 days in hospital—Amoxiclav 1.2 g 3x(i/v); after that, continued T.Amoxyclav 1 g 2x(p/o) for 7 days (each case) | 8 days in hospital—Amoxiclav 1.2 g 3x(i/v); after that, continued T. Amoxyclav 1 g 2x(p/o) for 10 days (February 2024) 5 days in hospital—Amoxiclav 1.2 g 3x(i/v); after that, continued T.Amoxyclav 1 g 2x(p/o) for 7 days (December 2024) 6 days in hospital—Dalacin 0.6 g-2x (i/v); after that, continued caps. Dalacini 0.3 g-3x (p/o) |
| Follow-up | The first visit is a week after the operation; then, once a month; the last visit is 30 June 2025, when complete healing is confirmed | The first visit is a week after the operation; then, once a month; last visit is 29 September 2025 (complete healing, patient uses new partial plate for lower jaw) | The first visit is a week after the operation, then as needed, but no less than once a month; the last visit was 6 October 2025 |
2.2. The Second Case (See Table 1)
2.3. The Third Case (See Table 1)
2.4. The Duration of Postoperative Follow-Up Varied Among Cases
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusco, V.; Campisi, G.; Carcieri, P.; Fagioli, F.; Bertetto, O.; Mignogna, M.D.; Bedogni, A. ONJ (MRONJ) Update 2021 Osteonecrosis of Jaw Related to Bisphosphonates and Other Drugs -Prevention, Diagnosis, Pharmacovigilance, Treatment: A 2021 Web Event. Oral 2022, 2, 137–147. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Mücke, T.; Koschinski, J.; Deppe, H.; Wagenpfeil, S.; Pautke, C.; Mitchell, D.A.; Wolff, K.D.; Hölzle, F. Outcome of treatment and parameters influencing recurrence in patients with bisphosphonate-related osteonecrosis of the jaws. J. Cancer Res. Clin. Oncol. 2011, 137, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; du Bois, A.; Buch, L.; Harter, P.; Grötz, K.A. Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 2009, 115, 1631–1637. [Google Scholar] [CrossRef]
- Van Poznak, C.H.; Unger, J.M.; Darke, A.K.; Moinpour, C.; Bagramian, R.A.; Schubert, M.M.; Hansen, L.K.; Floyd, J.D.; Dakhil, S.R.; Lew, D.; et al. Association of Osteonecrosis of the Jaw With Zoledronic Acid Treatment for Bone Metastases in Patients With Cancer. JAMA Oncol. 2021, 7, 246–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brunner, C.; Arvandi, M.; Marth, C.; Egle, D.; Baumgart, F.; Emmelheinz, M.; Walch, B.; Lercher, J.; Iannetti, C.; Wöll, E.; et al. Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Breast Cancer During a 20-Year Follow-Up: A Population-Based Multicenter Retrospective Study. J. Clin. Oncol. 2025, 43, 180–188. [Google Scholar] [CrossRef]
- Kujanpää, M.; Vuollo, V.; Tiisanoja, A.; Laitala, M.L.; Sándor, G.K.; Karki, S. Incidence of medication-related osteonecrosis of the jaw and associated antiresorptive drugs in adult Finnish population. Sci. Rep. 2025, 15, 17377. [Google Scholar] [CrossRef]
- Calvo de Mora, J.; Herencia, H.; del Amo, A.; Acero, J. Maxillary necrosis associated with biphosphonates. Diagnostic, clinical aspects and treatment. J. Craniomaxillofac. Surg. 2006, 34, 195. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Biphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Ruggiero, S.I.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Shibahara, T. Antiresorptive Agent-Related Osteonecrosis of the Jaw (ARONJ): A Twist of Fate in the Bone. Tohoku J. Exp. Med. 2019, 247, 75–86. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Barros Mourão, C.F.; Calasans-Maia, M.D.; Del Fabbro, M.; Le Drapper Vieira, F.; Coutinho de Mello Machado, R.; Capella, R.; Miron, R.J.; Gomes Alves, G. The use of Platelet-rich Fibrin in the management of medication-related osteonecrosis of the jaw: A case series. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zelinka, J.; Blahak, J.; Perina, V.; Pacasova, R.; Treglerova, J.; Bulik, O. The use of platelet-rich fibrin in the surgical treatment of medication-related osteonecrosis of the jaw: 40 patient’s prospective study. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 2021, 165, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Özalp, Ö.; Yıldırımyan, N.; Öztürk, C.; Kocabalkan, B.; ¸Simsek Kaya, G.; Sindel, A.; Altay, M.A. Promising results of surgical management of advanced medication related osteonecrosis of the jaws using adjunctive leukocyte and platelet rich fibrin. BMC Oral Health 2021, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.D.; Pitros, P.; Liew, J.; Besi, E. The adjunctive use of Leukocyte-Platelet Rich Fibrin (L-PRF) in the management of Medication Related Osteonecrosis of the Jaw (MRONJ): A retrospective observational study. Oral Maxillofac. Surg. 2024, 28, 1605–1615. [Google Scholar] [CrossRef]
- Yalcin-Ülker, G.M.; Duygu, G.; Tanan, G.; Cakir, M.; Meral, D.G. Use of Leukocyte-rich and Platelet-rich Fibrin (L-PRF) Adjunct to Surgical Debridement in the Treatment of Stage 2 and 3 Medication-Related Osteonecrosis of the Jaw. J. Craniofac. Surg. 2023, 34, 1039–1044. [Google Scholar] [CrossRef]
- Neal, T.W.; Schlieve, T. Medication-Related Osteonecrosis of the jaws in the Pediatric Population. J. Oral Maxillofac. Surg. 2022, 80, 1686–1690. [Google Scholar] [CrossRef]
- Chmielewski, M.; Pilloni, A.; Adamska, P. Application of Advanced Platelet-Rich Fibrin in Oral and Maxillofacial Surgery: A Systematic Rewiew. J. Funct. Biomater. 2024, 15, 377. [Google Scholar] [CrossRef]
- Frutuoso, F.; Frietas, F.; Vilares, M.; Francisco, H.; Marques, D.; Carames, J.; Moreira, A. A Medication-Related Osteonecrosis of the jaw: A Systematic Review of Case Reports and Case Series. Diseases 2024, 12, 205. [Google Scholar] [CrossRef]
- George, S.; Neal, T.W.; Schlieve, T. Does the Addition of Platelet-Rich Fibrin in the Surgical Treatment of Medication-Related Osteonecrosis of the Jaws Impact Postoperative Disease Resolution? J. Oral Maxillofac. Surg. 2022, 80, S88–S89. [Google Scholar] [CrossRef]
- Szentpeteri, S.; Schmidt, L.; Restar, L.; Csaki, G.; Szabo, G.; Vaszilko, M. The Effect of Platelet-Rich Fibrin Membrane in Surgical Therapy of Medication-Related Osteonecrosis of the Jaws. J. Oral Maxillofac. Surg. 2020, 78, 738–748. [Google Scholar] [CrossRef]
- Valente, N.A.; Chatelain, S.; Alfonsi, F.; Mortellaro, C.; Barone, A. Medication-Related Osteonecrosis of the Jaws. The Use of Leucocyte- Platelet-Rich Fibrin as an Adjunct in the Treatment. J. Craniofac. Surg. 2019, 30, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Nørholt, S.E.; Hartlev, J. Surgical Treatment of osteonecrosis of the jaw with the use of the platelet -rich fibrin: A prospective study of 15 patients. Int. J. Oral Maxillofac. Surg. 2016, 45, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.J.; Kim, M.R. Leucocyte-rich and platelet -rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: A prospective feasibility study. Br. J. Oral Maxillofac. Surg. 2014, 52, 854–858. [Google Scholar] [CrossRef]
- Giudice, A.; Antonelli, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar] [CrossRef]
- Blatt, S.; Krüger, M.; Kämmerer, P.W.; Thiem, D.G.E.; Matheis, P.; Eisenbeiß, A.-K.; Wiltfang, J.; Al-Nawas, B.; Naujokat, H. Non-Interventional Prospective Observational Study of Platelet Rich Fibrin as a Therapy Adjunctive in Patients with Medication-Related Osteonecrosis of the Jaw. J. Clin. Med. 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Adamska, P.; Stasiak, M.; Kobusinska, N.; Bartmanski, M.; Zedler, A.; Studniarek, M. Treatment of Medication-Related Osteonecrosis of the Jaws Without and With the Use of Advanced Platelet-Rich Fibrin: A Retrospective Clinical Study. J. Funct. Biomater. 2025, 16, 180. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiļčenko, I.; Čēma, I.; Bāgante, I. Adjuvant A-PRF Application in Patients with Various Stages of Medication-Related Osteonecrosis of the Jaw (MRONJ): Case Series. Reports 2025, 8, 240. https://doi.org/10.3390/reports8040240
Vasiļčenko I, Čēma I, Bāgante I. Adjuvant A-PRF Application in Patients with Various Stages of Medication-Related Osteonecrosis of the Jaw (MRONJ): Case Series. Reports. 2025; 8(4):240. https://doi.org/10.3390/reports8040240
Chicago/Turabian StyleVasiļčenko, Irina, Ingrīda Čēma, and Ieva Bāgante. 2025. "Adjuvant A-PRF Application in Patients with Various Stages of Medication-Related Osteonecrosis of the Jaw (MRONJ): Case Series" Reports 8, no. 4: 240. https://doi.org/10.3390/reports8040240
APA StyleVasiļčenko, I., Čēma, I., & Bāgante, I. (2025). Adjuvant A-PRF Application in Patients with Various Stages of Medication-Related Osteonecrosis of the Jaw (MRONJ): Case Series. Reports, 8(4), 240. https://doi.org/10.3390/reports8040240






