Severe Secondary Atrophic Rhinitis with Extensive Osteomyelitis Following COVID-19-Associated Necrotizing Rhinitis: A Case Report and Microbiological Analysis
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Patient Background and Clinical History
2.2. Clinical and Radiological Findings (2024)
2.3. Microbiological Investigations and Review
2.3.1. Sample Collection, Culture, and Identification
2.3.2. Antimicrobial Susceptibility Testing (AST)
2.4. Therapeutic Intervention and Outcome
- Nasal rest [14]: A structured “nasal rest” protocol was initiated, which involved the placement of cotton tampons to occlude the nasal passages for progressively shorter durations over a period of 17 days. This protocol aimed to reduce airflow, decrease mucosal drying, and promote healing. The patient discontinued this part of the regimen after two months due to difficulty with removing a tampon but diligently continued with the nasal irrigations.
- Intensive Nasal Irrigation: The patient was instructed to perform nasal irrigation at least three times per day. The irrigation solution consisted of isotonic saline mixed with a small amount of baby soap to act as a surfactant.
- Mechanical Debridement: Daily endoscopic removal of all visible crusts and purulent debris was performed for the first two weeks of treatment.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Atrophic Rhinitis |
| AST | Antimicrobial Susceptibility Testing |
| CFU/mL | Colony Forming Units per milliliter |
| CRS | Chronic Rhinosinusitis |
| CT | Computed Tomography |
| ENS | Empty Nose Syndrome |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| I | Susceptible, Increased Exposure |
| R | Resistant |
| S | Susceptible |
References
- Takami, T. Nasal Secretion Insufficiency Syndrome (New Concept of Ozena). J. Lung Pulm. Respir. Res. 2020, 7, 56–60. [Google Scholar] [CrossRef]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 9 January 2025).
- Rodgers, B.C.; Baker, K. A Case of Atrophic Rhinitis Caused by Klebsiella ozaenae. Otolaryngol. Head Neck Surg. 2012, 147, P240. [Google Scholar] [CrossRef]
- Dutt, S.N.; Kameswaran, M. The aetiology and management of atrophic rhinitis. J. Laryngol. Otol. 2005, 119, 843–852. [Google Scholar] [CrossRef]
- Yareshko, A.G.; Kaidashev, I.P.; Loban, G.A.; Kravchenko, V.G.; Vorodyukhina, A.K. To the Differential Diagnostics of Focal Pneumonia, Tuberculosis and Sarcoidosis (Clinical Case). Bull. Probl. Biol. Med. 2024, 1, 294. [Google Scholar] [CrossRef]
- Fong, P.Y.; Lim, K.T.; Gnanam, A.; Charn, T.C. Role of probiotics in Chronic Rhinosinusitis: A systematic review of randomised controlled trials. J. Laryngol. Otol. 2023, 137, 1300–1311. [Google Scholar] [CrossRef]
- Widdicombe, J.H.; Wine, J.J. Airway Gland Structure and Function. Physiol. Rev. 2015, 95, 1241–1319. [Google Scholar] [CrossRef]
- Wu, C.L.; Fu, C.H.; Lee, T.J. Distinct Histopathology Characteristics in Empty Nose Syndrome. Laryngoscope 2021, 131, E14–E18. [Google Scholar] [CrossRef] [PubMed]
- Chegini, Z.; Noei, M.; Hemmati, J.; Arabestani, M.R.; Shariati, A. The Destruction of Mucosal Barriers, Epithelial Remodeling, and Impaired Mucociliary Clearance: Possible Pathogenic Mechanisms of Pseudomonas Aeruginosa and Staphylococcus Aureus in Chronic Rhinosinusitis. Cell Commun. Signal. 2023, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Liva, G.; Karatzanis, A.; Prokopakis, E. Review of Rhinitis: Classification, Types, Pathophysiology. J. Clin. Med. 2021, 10, 3183. [Google Scholar] [CrossRef]
- Burges Watson, D.L.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered Smell and Taste: Anosmia, Parosmia and the Impact of Long Covid-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ko, I.; Kim, M.S.; Yu, M.S.; Cho, B.J.; Kim, D.K. Association of Chronic Rhinosinusitis with Depression and Anxiety in a Nationwide Insurance Population. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 313–319. [Google Scholar] [CrossRef]
- Schlosser, R.J.; Gage, S.E.; Kohli, P.; Soler, Z.M. Burden of illness: A systematic review of depression in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.J.; Hartley, C.; Farrington, W.T. Closure of the nasal vestibule in atrophic rhinitis--a new non-surgical technique. J. Laryngol. Otol. 1998, 112, 543–546. [Google Scholar] [CrossRef]
- Pelle, M.C.; Zaffina, I.; Lucà, S.; Forte, V.; Trapanese, V.; Melina, M.; Giofrè, F.; Arturi, F. Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options. Life 2022, 12, 1605. [Google Scholar] [CrossRef]
- Chen, W.; Pan, J.Y. Anatomical and Pathological Observation and Analysis of SARS and COVID-19: Microthrombosis Is the Main Cause of Death. Biol. Proced. Online 2021, 23, 4. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, M.Y. SARS-CoV-2 Infection (COVID-19) and Rhinologic Manifestation: Narrative Review. J. Pers. Med. 2022, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Magne, F.; Venugopal, G.; Purkait, S.; Mutha, N.V.R.; Maiti, R.; Sharma, P.; Ramadass, B. Altered Nasal Microbiome in Atrophic Rhinitis: A Novel Theory of Etiopathogenesis and Therapy. Microorganisms 2022, 10, 2092. [Google Scholar] [CrossRef] [PubMed]
- Assad, S.K.; Sabah, M.; Kakamad, F.H.; Salih, A.M.; Salih, R.Q.; Mohammed, S.H.; Ali, R.K.; Abdalla, B.A.; Hassan, M.N. Avascular necrosis of femoral head following COVID-19 infection. Ann. Med. Surg. 2023, 85, 4206–4210. [Google Scholar] [CrossRef]
- Mañón, V.A.; Balandran, S.; Young, S.; Wong, M.; Melville, J.C. COVID-Associated Avascular Necrosis of the Maxilla-A Rare, New Side Effect of COVID-19. J. Oral. Maxillofac. Surg. 2022, 80, 1254–1259. [Google Scholar] [CrossRef]
- Tahir, E.; Bilek, H.C.; Demirel, E.; Atmaca, S. Skull Base Osteomyelitis Complicating COVID-19: A Novel Secondary Infection? J. Clin. Pract. Res. 2023, 45, 651–654. [Google Scholar] [CrossRef]
- Angelou, D.; Calder, N. A case of COVID-19-related necrotic nasal ulceration. Clin. Case Rep. 2023, 11, e6944. [Google Scholar] [CrossRef] [PubMed]
- Ergün, S.; Adıyaman, C.; Şensöz, E.; Eceviz, E. Avascular Necrosis of the Hip Triggered by COVID-19 Infection in a Patient with Sickle Cell Disease: A Case Report. J. Orthop. Case Rep. 2022, 12, 10–12. [Google Scholar] [CrossRef]
- Souza, L.; Lessa, B.; Lima, C.; Lessa, M.; Lessa, H.; Verde, R. Clinical and tomography evolution of frontal osteomyelitis: Case report. Arquivos Int. Otorrinol. 2014, 16, 130–134. [Google Scholar] [CrossRef]
- Pincus, D.J.; Armstrong, M.B.; Thaller, S.R. Osteomyelitis of the craniofacial skeleton. Semin. Plast. Surg. 2009, 23, 73–79. [Google Scholar] [CrossRef]
- Verma, A.; Singh, V.; Jindal, N.; Yadav, S. Necrosis of maxilla, nasal, and frontal bone secondary to extensive rhino-cerebral mucormycosis. Natl. J. Maxillofac. Surg. 2013, 4, 249–251. [Google Scholar] [CrossRef]
- Potentas-Policewicz, M.; Fijolek, J. Granulomatosis with polyangiitis: clinical characteristics and updates in diagnosis. Front. Med. 2024, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, M.; Kofonow, J.; Nayak, J.V.; Palmer, J.N.; Chiu, A.G.; Leid, J.G.; Cohen, N.A. Biofilms in Chronic Rhinosinusitis: A Review. Am. J. Rhinol. Allergy 2009, 23, 255–260. [Google Scholar] [CrossRef]
- Sumaily, I.A.; Hakami, N.A.; Almutairi, A.D.; Alsudays, A.A.; Abulqusim, E.M.; Abualgasem, M.M.; Alghulikah, A.A.; Alserhani, A.A. An Updated Review on Atrophic Rhinitis and Empty Nose Syndrome. Ear Nose Throat J. 2023, 1455613231185022. [Google Scholar] [CrossRef] [PubMed]
- Botelho-Nevers, E.; Gouriet, F.; Lepidi, H.; Couvret, A.; Amphoux, B.; Dessi, P.; Raoult, D. Chronic Nasal Infection Caused by Klebsiella Rhinoscleromatis or Klebsiella ozaenae: Two Forgotten Infectious Diseases. Int. J. Infect. Dis. 2007, 11, 423–429. [Google Scholar] [CrossRef]
- Lee, Y.J.; Moore, L.S.P.; Almeyda, J. A Report on a Rare Case of Klebsiella ozaenae Causing Atrophic Rhinitis in the UK. BMJ Case Rep. 2011, 2011, bcr0920114812. [Google Scholar] [CrossRef]
- Bist, S.S.; Bisht, M.; Purohit, J.P. Primary Atrophic Rhinitis: A Clinical Profile, Microbiological and Radiological Study. ISRN Otolaryngol. 2012, 2012, 404075. [Google Scholar] [CrossRef]
- Zhang, Z.; Adappa, N.D.; Doghramji, L.J.; Chiu, A.G.; Cohen, N.A.; Palmer, J.N. Different Clinical Factors Associated with Staphylococcus aureus and Pseudomonas aeruginosa in Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 724–733. [Google Scholar] [CrossRef]
- Moore, E.J.; Kern, E.B. Atrophic Rhinitis: A Review of 242 Cases. Am. J. Rhinol. 2001, 15, 355–361. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.-H.; Han, S.-A.; Kim, W. Compositional Alterations of the Nasal Microbiome and Staphylococcus aureus–Characterized Dysbiosis in the Nasal Mucosa of Patients with Allergic Rhinitis. Clin. Exp. Otorhinolaryngol. 2022, 15, 335–345. [Google Scholar] [CrossRef]
- Vickery, T.W.; Ramakrishnan, V.R.; Suh, J.D. The Role of Staphylococcus aureus in Patients with Chronic Sinusitis and Nasal Polyposis. Curr. Allergy Asthma Rep. 2019, 19, 21. [Google Scholar] [CrossRef]
- Alyousef, M.; Alroqi, A.; AlAmari, N. Atrophic Rhinitis Secondary to an Infection of Unusual Bacteria: A Case Report and Literature Review. Ear Nose Throat J. 2025, 104, 162S–164S. [Google Scholar] [CrossRef]
- Ghallab, A.F.; Hashim, H.F.; Mostafa, M.S.; El Sayed, R.A. The Role of the Bacterial Infections of the Nose in Etiology of Primary Atrophic Rhinitis. Egypt. J. Med. Microbiol. 2020, 29, 117–121. [Google Scholar] [CrossRef]
- Magyar, T.; Lax, A.J. Atrophic Rhinitis. In Polymicrobial Diseases; ASM Press: Washington, DC, USA, 2002; pp. 169–197. [Google Scholar]

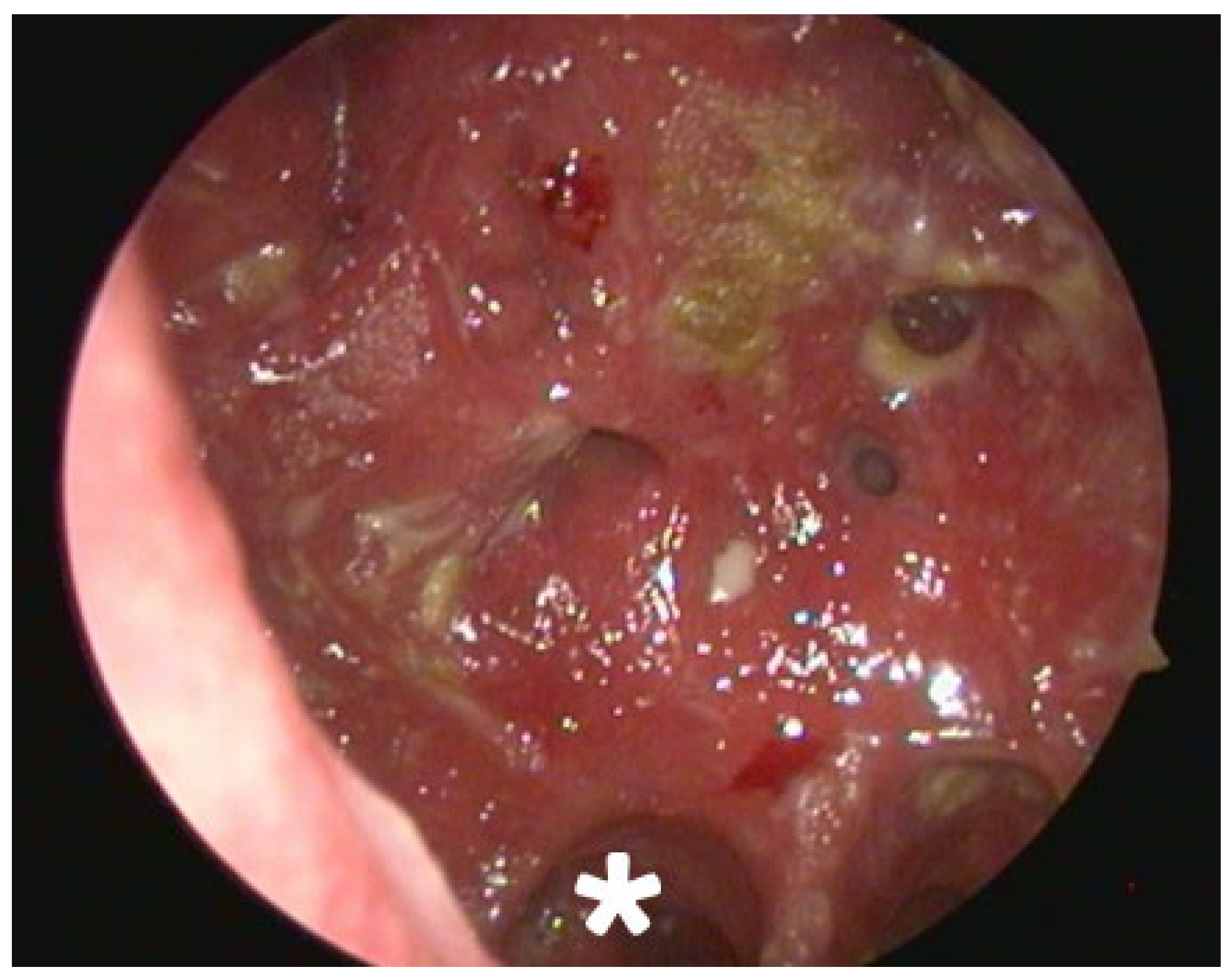
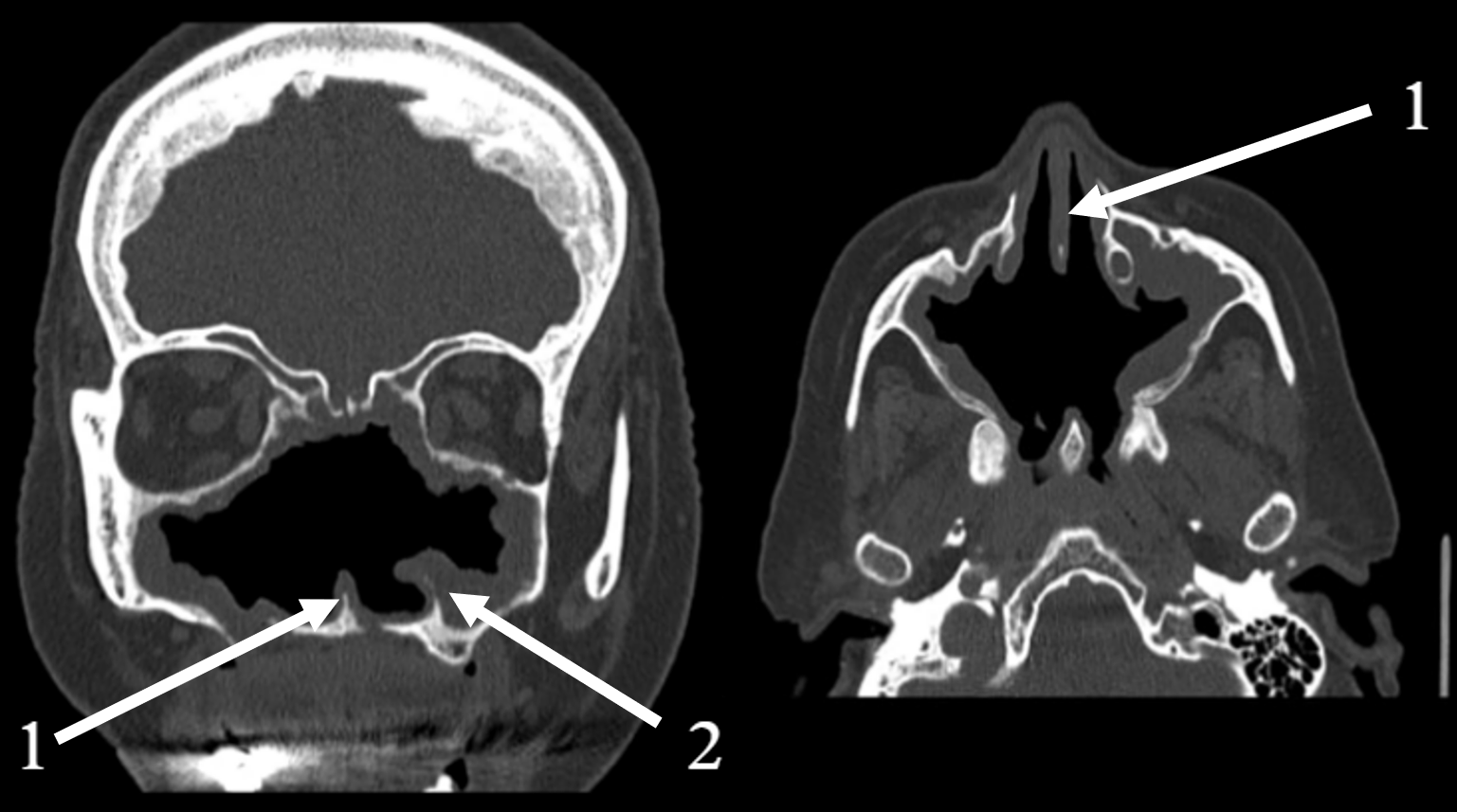
| Date/Time Period | Key Clinical Event | Diagnostic Findings |
|---|---|---|
| October 2021 | Acute SARS-CoV-2 Infection | Onset of persistent nasal symptoms. Continued for 10 months. |
| Late August 2022 | Rapid worsening, onset of necrotizing rhinitis | Clinical evidence of necrosis of nasal cavity structures. Partial debrodement, histopathology: Focally necrotic tissue no fungal invasion identified. Culture: Proteus vulgaris (104 CFU/mL), and Enterococcus sp. (103 CFU/mL). |
| Late August–mid September 2022 | No significant improvement | Conservative treatment with unsatisfactory results. Partial debridement mid Sep due to condition worsening |
| Late September 2022 | Disease progression | CT scan reveals an “osteolytic process”. A major Polysinusotomy with necrosectomy is performed under general anesthesia, involving extensive removal of necrotic bone and tissue. The second histopathology report confirms the findings of necrotic tissue and bone fragments, with no evidence of fungal invasion or granylomatosis. |
| 2022–2024 | Atrophic rhinitis progression | Disease progression with gradual nasal degeneration. Treated elsewhere (occasional mechanical crust removal) |
| 2024 | Hospitalization for Unrelated Condition | Admitted for pneumonia and pulmonary embolism. |
| 2024 | Secondary atrophic rhinitis | Endoscopy, CT scan. Treatment initiation. 3-month follow-up. |
| Antimicrobial Agent | Pseudomonas aeruginosa (2024) | Antimicrobial Agent | Staphylococcus aureus (2024) | Antimicrobial Agent | Proteus vulgaris (2022) |
|---|---|---|---|---|---|
| Beta-lactams | Beta-lactams | Beta-lactams | R | ||
| Ampicillin/sulbactam | - | Penicillin | - | Ampicillin | R |
| Ticarcillin/clavulanic acid | R | Oxacillin (Cefoxitin screen) | S | Meropenem | S |
| Piperacillin | R | Cefoxitin | S | Ceftazidime | S |
| Piperacillin/tazobactam | R | Fluoroquinolones | Ceftriaxon | I | |
| Ceftazidime | R | Ciprofloxacin | I | Cefepime | S |
| Cefepime | R | Levofloxacin | I | Gentamicin | S |
| Aztreonam | R | Moxifloxacin | S | Amikacin | S |
| Imipenem | R | Aminoglycosides | Ceftazidime-avibactam | S | |
| Meropenem | R | Amikacin | S | Cefpirome | S |
| Aminoglycosides | Gentamicin | S | Cefoperazone | S | |
| Amikacin | R | Tobramycin | S | ||
| Gentamicin | - | Macrolides/Lincosamides | |||
| Tobramycin | R | Erythromycin | S | ||
| Fluoroquinolones | Clindamycin | S | |||
| Ciprofloxacin | I | Other Agents | |||
| Levofloxacin | I | Linezolid | S | ||
| Polymyxins | Trimethoprim/Sulfamethoxazole | S | |||
| Colistin | R | Tetracycline | S | ||
| Other Agents | Fusidic acid | S | |||
| Trimethoprim/sulfamethoxazole | - | Rifampicin | S | ||
| Tigecycline | - | ||||
| Chloramphenicol | - |
| Feature | Secondary Atrophic Rhinitis (as in this Case) | Empty Nose Syndrome (ENS) |
|---|---|---|
| Etiology | Severe destruction from infection, trauma, or granulomatous disease. | Iatrogenic, typically from aggressive turbinate resection. |
| Key Symptoms | Extensive crusting, severe foul odor (ozena), anosmia, variable nasal obstruction. | Paradoxical nasal obstruction (sensation of suffocation), nasal dryness, crusting (usually less severe than AR). |
| Histopathology | Squamous metaplasia with profound loss of ciliated epithelium, goblet cells, and submucosal glands. Bony resorption is common. | Ciliated epithelium and goblet cells are often preserved. Submucosal fibrosis and gland reduction may occur, but bony resorption is not a primary feature. |
| Sensory Perception | Anosmia due to destruction of olfactory epithelium. Sensation of airflow is lost. | Paradoxical obstruction is linked to downregulation of trigeminal thermoreceptors (e.g., TRPM8), impairing the sensation of airflow. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danylevych, A.; Tsolko, S.; Tymechko, I.; Korniychuk, O.; Konechnyi, Y. Severe Secondary Atrophic Rhinitis with Extensive Osteomyelitis Following COVID-19-Associated Necrotizing Rhinitis: A Case Report and Microbiological Analysis. Reports 2025, 8, 237. https://doi.org/10.3390/reports8040237
Danylevych A, Tsolko S, Tymechko I, Korniychuk O, Konechnyi Y. Severe Secondary Atrophic Rhinitis with Extensive Osteomyelitis Following COVID-19-Associated Necrotizing Rhinitis: A Case Report and Microbiological Analysis. Reports. 2025; 8(4):237. https://doi.org/10.3390/reports8040237
Chicago/Turabian StyleDanylevych, Anton, Sofiya Tsolko, Iryna Tymechko, Olena Korniychuk, and Yulian Konechnyi. 2025. "Severe Secondary Atrophic Rhinitis with Extensive Osteomyelitis Following COVID-19-Associated Necrotizing Rhinitis: A Case Report and Microbiological Analysis" Reports 8, no. 4: 237. https://doi.org/10.3390/reports8040237
APA StyleDanylevych, A., Tsolko, S., Tymechko, I., Korniychuk, O., & Konechnyi, Y. (2025). Severe Secondary Atrophic Rhinitis with Extensive Osteomyelitis Following COVID-19-Associated Necrotizing Rhinitis: A Case Report and Microbiological Analysis. Reports, 8(4), 237. https://doi.org/10.3390/reports8040237







