Diphtheria-like Pseudomembranous Corynebacterium striatum Chronic Infection of Left Ventricular Assist Device Driveline Bridged to Heart Transplantation with Dalbavancin Treatment
Abstract
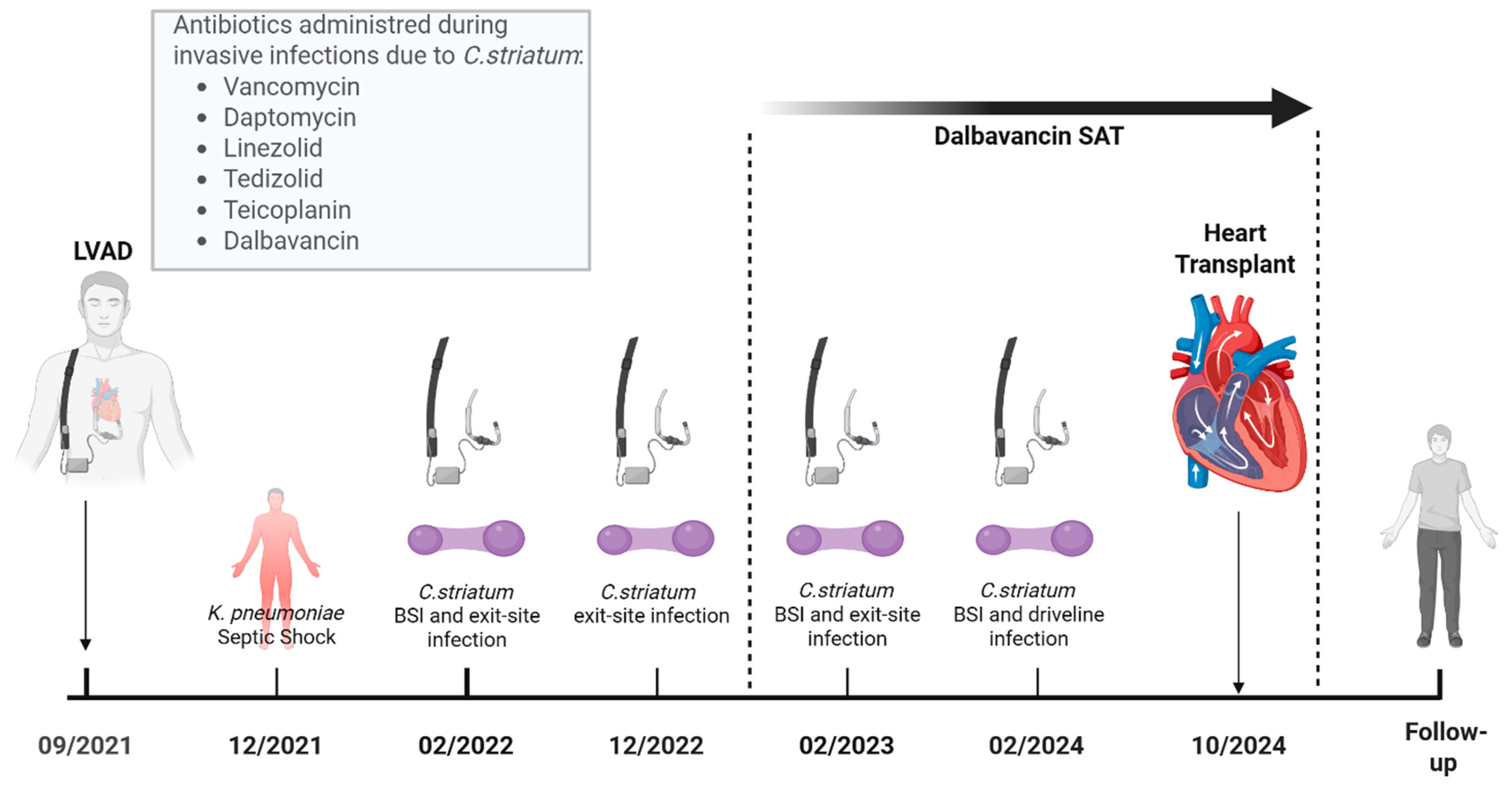
1. Introduction and Clinical Significance
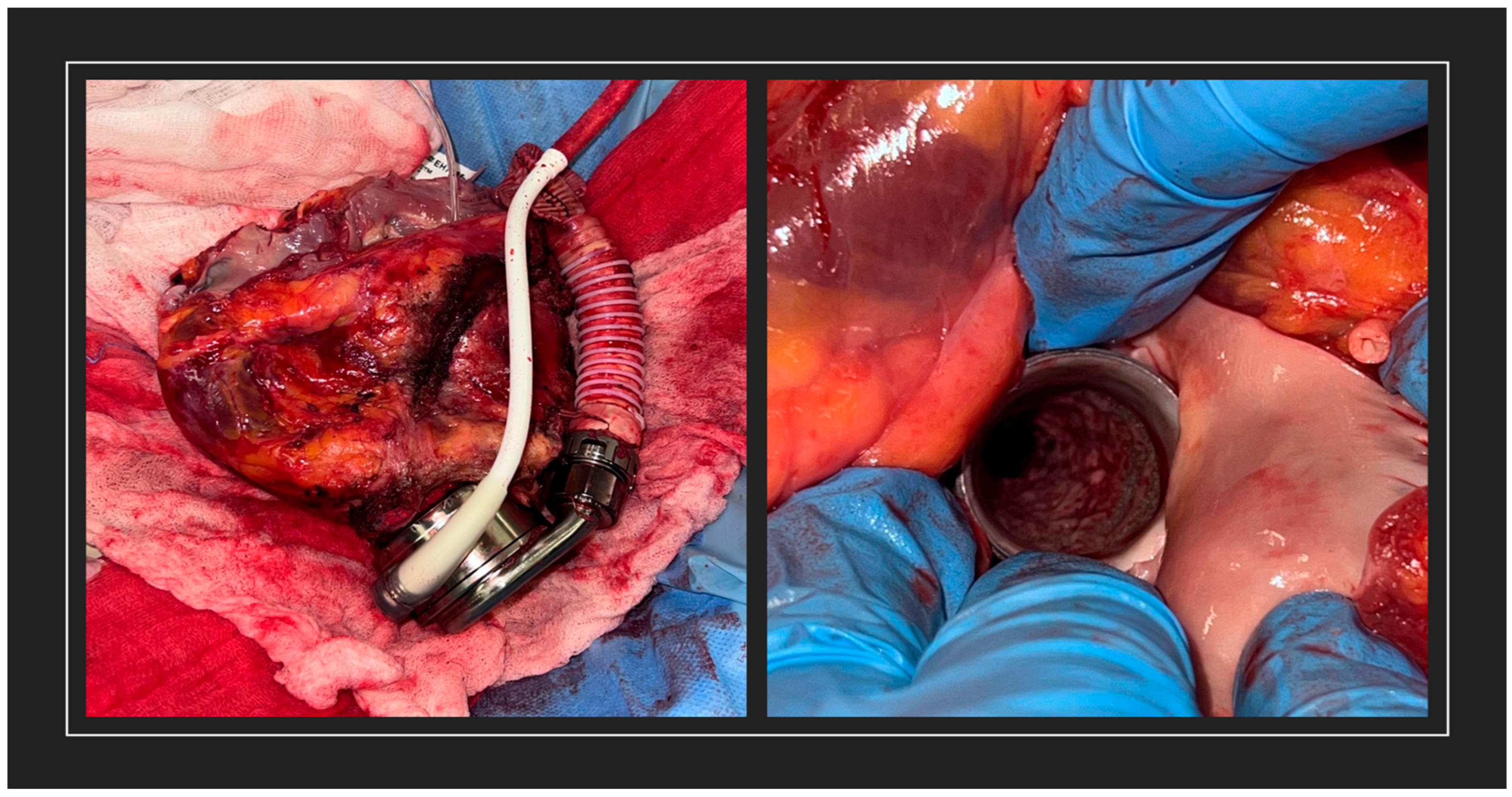
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, J.G.; Wiist, J.; Krech, T.; Hany, A. Classical pseudomembranous diphtheria caused by Corynebacterium ulcerans. Schweiz. Med. Wochenschr. 1990, 120, 1812. (In German) [Google Scholar] [PubMed]
- Reddy, K.; Gericke, S.; Rabie, H.; Pienaar, C.; Maloba, M. Exudative pharyngitis and Corynebacterium pseudodiphtheriticum: A case report and review of the literature. S. Afr. J. Infect. Dis. 2021, 36, 225. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Hasegawa, M.; Akutsu, Y.; Okudaira, T. Pseudomembranous bronchitis (non-diphtherial) resulting in sudden death: An autopsy report. Intern. Med. 1992, 31, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Charley, E.; Vaccarello, A.; Talon, A.; Irandost, M.; Varda, B.; Saeed, A.I. Rare case of Corynebacterium infection of the lower respiratory tract causing pseudomembrane formation in lung transplant recipient. Am. Thorac. Soc. 2021, TP97, A3966. [Google Scholar]
- Costerton, J.W.; Veeh, R.; Shirtliff, M. The application of biofilm science to the study of and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1446–1477. [Google Scholar] [CrossRef]
- Souza, C.d.; Faria, Y.V.; Sant’Anna, L.d.O.; Viana, V.G.; Seabra, S.H.; Souza, M.C.; Vieira, V.V.; Hirata, R., Jr.; Moreira, L.d.O.; Mattos-Guaraldi, A.L. Biofilm production by multiresistant Corynebacterium striatum associated with nosocomial outbreak. Mem. Inst. Oswaldo Cruz 2015, 110, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.N.; Souza, C.; Faria, Y.V.; da Silva, E.C.; Veras, J.F.C.; Baio, P.V.P.; Seabra, S.H.; Moreira, L.d.O.; Hirata, R., Jr.; Mattos-Guaraldi, A.L.; et al. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect. Dis. 2019, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, Z.; Du, X.; Liu, R.; Wang, J. Antibiofilm effects of extracellular matrix degradative agents on the biofilm of different strains of multidrug-resistant Corynebacterium striatum. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- O’Horo, J.C.; Abu Saleh, O.M.; Stulak, J.M.; Wilhelm, M.P.; Baddour, L.M.; Sohail, M.R. Left ventricular assist device infections: A systematic review. Asaio J. 2018, 64, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Werth, B.J.; Hahn, W.O.; Butler-Wu, S.M.; Rakita, R.M. Emergence of high-level daptomycin resistance in Corynebacterium striatum in two patients with left ventricular assist device infections. Microb. Drug Resist. 2016, 22, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.W.; Puttagunta, S.; Sprenger, C.R.; Rubino, C.; Van Wart, S.; Baldassarre, J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob. Agents Chemother. 2015, 59, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.M.; Preslaski, C.R.; Shihadeh, K.C.; Hawkins, K.L.; Jenkins, T.C. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: A scoping review. Open Forum Infect. Dis. 2021, 8, ofab486. [Google Scholar] [CrossRef] [PubMed]
- Bergey’s Manual Trust. Bergey’s Manual of Systematic Bacteriology; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Milosavljevic, M.N.; Milosavljevic, J.Z.; Kocovic, A.G.; Stefanovic, S.M.; Jankovic, S.M.; Djesevic, M.; Milentijevic, M.N. Antimicrobial treatment of Corynebacterium striatum invasive infections: A systematic review. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, W.; Luo, Z. A case of dyspnea due to pathogenic Corynebacterium striatum in a patient with a bronchial stent. Am. J. Respir. Crit. Care Med. 2020, 201, A3223. [Google Scholar] [CrossRef]
- Fornari, V.; Accardo, G.; Lupia, T.; De Rosa, F.G.; Corcione, S. Suppressive antibiotic treatment (SAT) in the era of MDRO infections: A narrative review. Expert Rev. Anti Infect. Ther. 2025, 23, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Söderquist, B.; Henningsson, T.; Stegger, M. Corynebacterium striatum prosthetic joint infection successfully treated with long-term dalbavancin. Microorganisms 2023, 11, 550. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupia, T.; Casarotto, M.; Mornese Pinna, S.; Corcione, S.; Bondi, A.; Boffini, M.; Rinaldi, M.; De Rosa, F.G. Diphtheria-like Pseudomembranous Corynebacterium striatum Chronic Infection of Left Ventricular Assist Device Driveline Bridged to Heart Transplantation with Dalbavancin Treatment. Reports 2025, 8, 208. https://doi.org/10.3390/reports8040208
Lupia T, Casarotto M, Mornese Pinna S, Corcione S, Bondi A, Boffini M, Rinaldi M, De Rosa FG. Diphtheria-like Pseudomembranous Corynebacterium striatum Chronic Infection of Left Ventricular Assist Device Driveline Bridged to Heart Transplantation with Dalbavancin Treatment. Reports. 2025; 8(4):208. https://doi.org/10.3390/reports8040208
Chicago/Turabian StyleLupia, Tommaso, Marco Casarotto, Simone Mornese Pinna, Silvia Corcione, Alessandro Bondi, Massimo Boffini, Mauro Rinaldi, and Francesco Giuseppe De Rosa. 2025. "Diphtheria-like Pseudomembranous Corynebacterium striatum Chronic Infection of Left Ventricular Assist Device Driveline Bridged to Heart Transplantation with Dalbavancin Treatment" Reports 8, no. 4: 208. https://doi.org/10.3390/reports8040208
APA StyleLupia, T., Casarotto, M., Mornese Pinna, S., Corcione, S., Bondi, A., Boffini, M., Rinaldi, M., & De Rosa, F. G. (2025). Diphtheria-like Pseudomembranous Corynebacterium striatum Chronic Infection of Left Ventricular Assist Device Driveline Bridged to Heart Transplantation with Dalbavancin Treatment. Reports, 8(4), 208. https://doi.org/10.3390/reports8040208






