Left Atrial Dissection After Mitral and Aortic Valve Replacement: The Importance of Early Diagnosis of a Rare Entity
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TTE | Transthoracic echocardiography |
| TEE | Transesophageal echocardiography |
| LVIDd | Left ventricular internal end diastolic diameter |
| IVSd | Linear dichroism |
| LVPWd | Left Ventricular Posterior Wall thickness in diastole |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| ICU | Intensive care unit |
| CPB | Cardiopulmonary bypass |
References
- Fukuhara, S.; Dimitrova, K.R.; Geller, C.M.; Hoffman, D.M.; Tranbaugh, R.F. Left atrial dissection: An almost unknown entity. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Mishra, M.; Mehta, Y.; Trehan, N. A case of left atrial dissection after mitral valve replacement. Ann. Card. Anaesth. 2018, 21, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.G.; Kumar, B.; Singh, H.; Biswas, I. Left atrial dissection and rupture following excision of left atrial myxoma: Role of transesophageal echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2823–2826. [Google Scholar] [PubMed]
- Fukuhara, S.; Dimitrova, K.R.; Geller, C.M.; Hoffman, D.M.; Ko, W.; Tranbaugh, R.F. Left atrial dissection: Etiology and treatment. Ann. Thorac. Surg. 2013, 95, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Katahira, S.; Hoshino, T.; Hanzawa, K.; Tomioka, H. Rapidly vanishing left atrial dissection following mitral valve replacement: A case report. J. Cardiothorac. Surg. 2020, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, M.; Takamoto, S.; Kotsuka, Y.; Ohtsuka, T. Left atrial dissection after double valve replacement. Ann. Thorac. Surg. 2003, 75, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Suraci, N.; Mihos, C.G.; Volsky, A.; Santana, O. Left atrial dissection: A rare entity. Echocardiography 2019, 36, 1598–1600. [Google Scholar] [CrossRef]
- Barnes, T.J.; Hockstein, M.A.; Jabaley, C.S. Vasoplegia after cardiopulmonary bypass: A narrative review of pathophysiology and emerging targeted therapies. Ther. Adv. Cardiovasc. Dis. 2020, 14, 1753944720935466. [Google Scholar] [CrossRef]
- Hamada, Y.; Kameyama, Y.; Narita, H.; Benson, K.T.; Goto, H. Protamine after heparin produces hypotension resulting from decreased sympathetic outflow secondary to increased nitric oxide in the central nervous system. Anesth. Analg. 2005, 100, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Denault, A.Y.; Deschamps, A.; Couture, P. Intraoperative hemodynamic instability during and after separation from cardiopulmonary bypass. Semin. Cardiothorac. Vasc. Anesth. 2010, 14, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.E.; Rochon, A.; Cogan, J.; Ased, H.; Desjardins, G.; Deschamps, A.; Gavra, P.; Lebon, J.-S.; Couture, P.; Ayoub, C.; et al. Acute right ventricular failure in cardiac surgery during cardiopulmonary bypass separation: A retrospective case series of 12 years’ experience with intratracheal milrinone administration. J. Cardiothorac. Vasc. Anesth. 2019, 33, 651–660. [Google Scholar] [CrossRef] [PubMed]


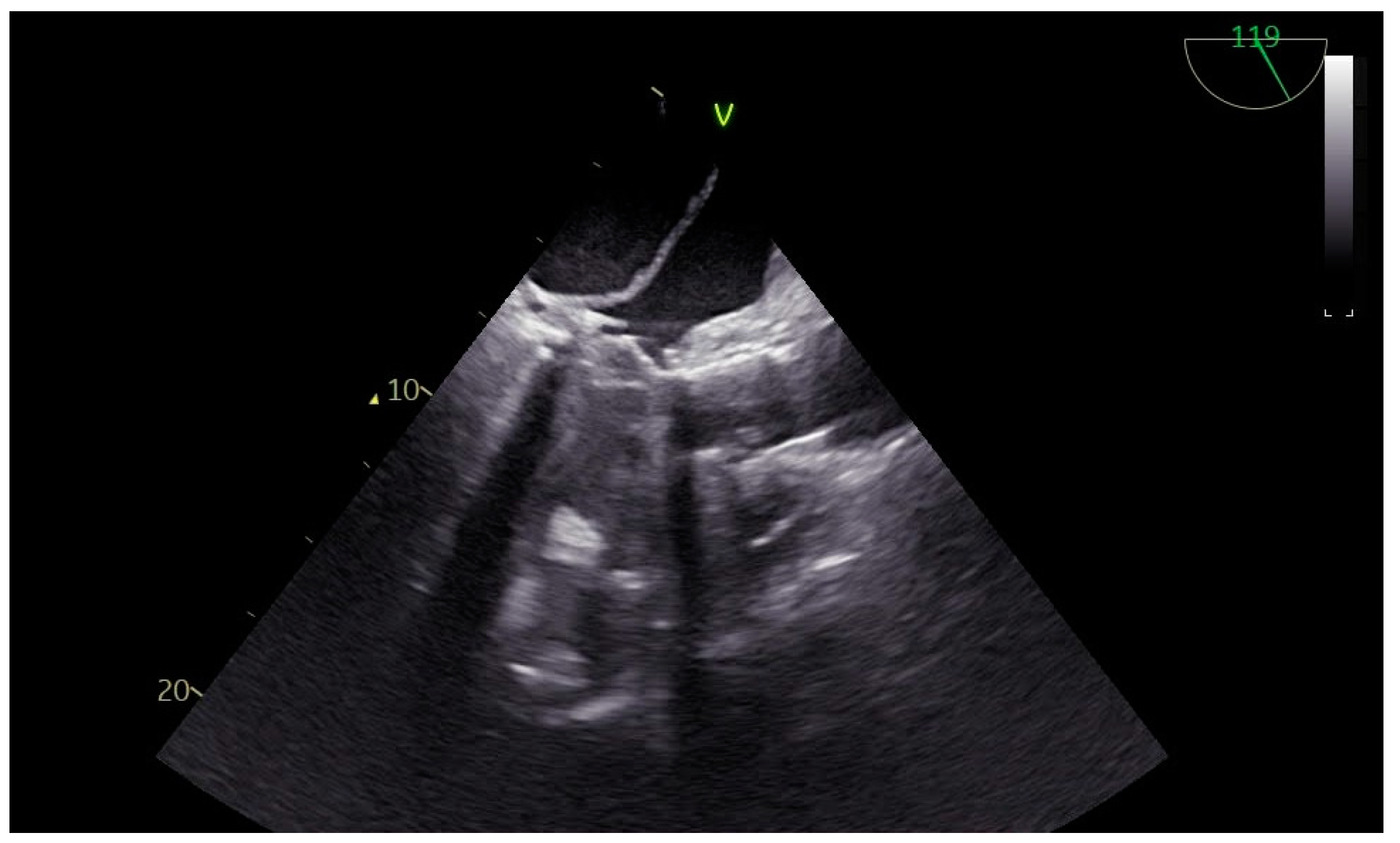
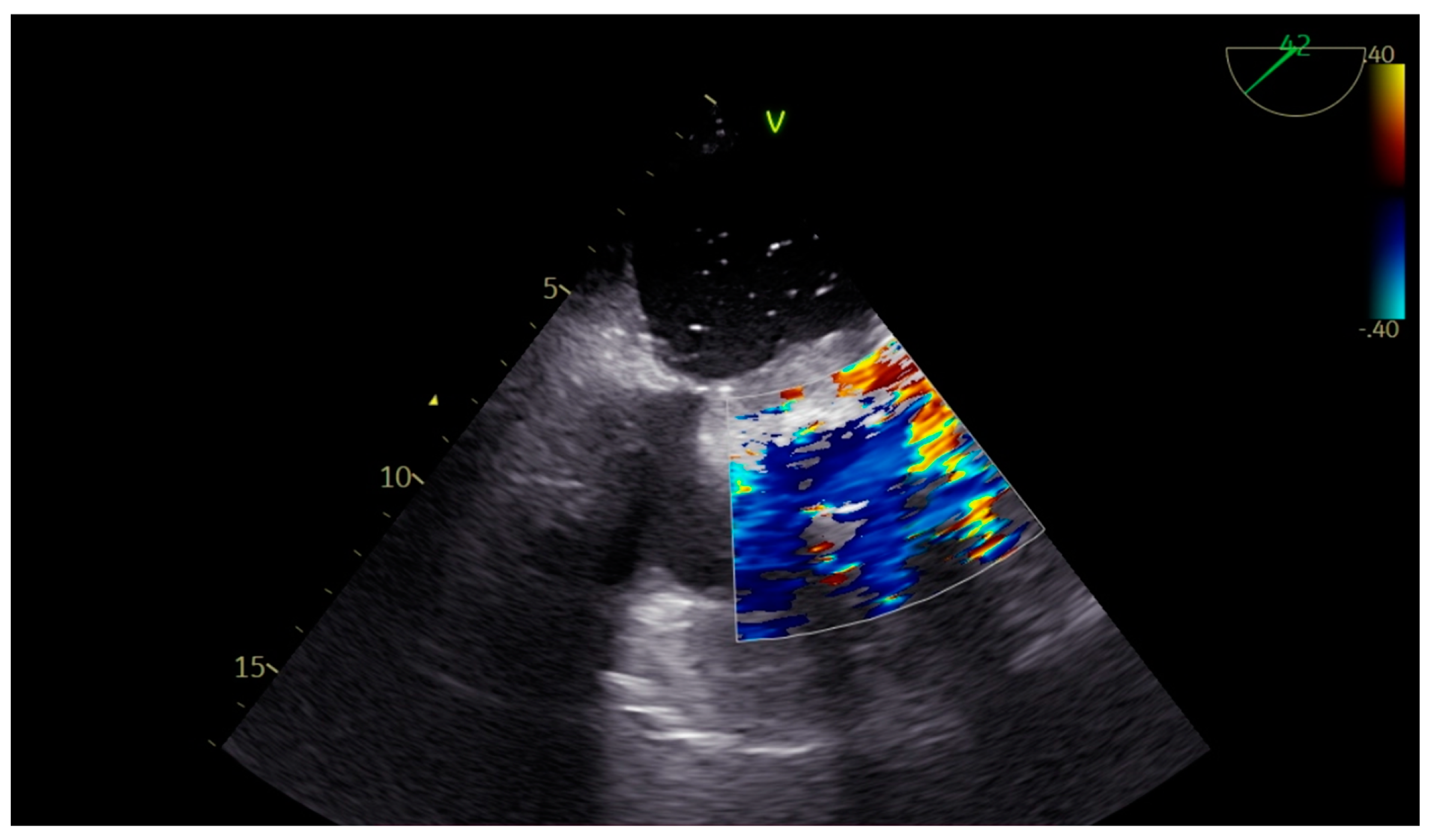
| Left Atrium Dilated | >6.5 × 5.2 mm (No difficulty in visualization of the left atrial roof). No spontaneous echocardiography contrast seen. Left atrial appendage: No signs of clots. |
| Mitral valve annulus | 38 mm |
| Aortic Valve Annulus | 23 mm with heavy calcification |
| Sinotubular Junction | 26 mm |
| Ascending Aorta | 32 mm |
| LVEF | 55% |
| LVIDd | 55 mm |
| IVSd | 13 mm |
| LVPWd | 15 mm |
| TAPSE | 19 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarridou, D.; Mouratoglou, S.A.; Ioannidis, R.; Amaniti, A.; Mitos, G.; Argiriadou, E. Left Atrial Dissection After Mitral and Aortic Valve Replacement: The Importance of Early Diagnosis of a Rare Entity. Reports 2025, 8, 205. https://doi.org/10.3390/reports8040205
Sarridou D, Mouratoglou SA, Ioannidis R, Amaniti A, Mitos G, Argiriadou E. Left Atrial Dissection After Mitral and Aortic Valve Replacement: The Importance of Early Diagnosis of a Rare Entity. Reports. 2025; 8(4):205. https://doi.org/10.3390/reports8040205
Chicago/Turabian StyleSarridou, Despoina, Sophia Anastasia Mouratoglou, Rafail Ioannidis, Aikaterini Amaniti, Giakoumis Mitos, and Eleni Argiriadou. 2025. "Left Atrial Dissection After Mitral and Aortic Valve Replacement: The Importance of Early Diagnosis of a Rare Entity" Reports 8, no. 4: 205. https://doi.org/10.3390/reports8040205
APA StyleSarridou, D., Mouratoglou, S. A., Ioannidis, R., Amaniti, A., Mitos, G., & Argiriadou, E. (2025). Left Atrial Dissection After Mitral and Aortic Valve Replacement: The Importance of Early Diagnosis of a Rare Entity. Reports, 8(4), 205. https://doi.org/10.3390/reports8040205






