A Conundrum of Colliding Conditions: A Histopathological Case Report of Chiari Type III with Complete Spina Bifida Aperta
Abstract
1. Introduction and Clinical Significance
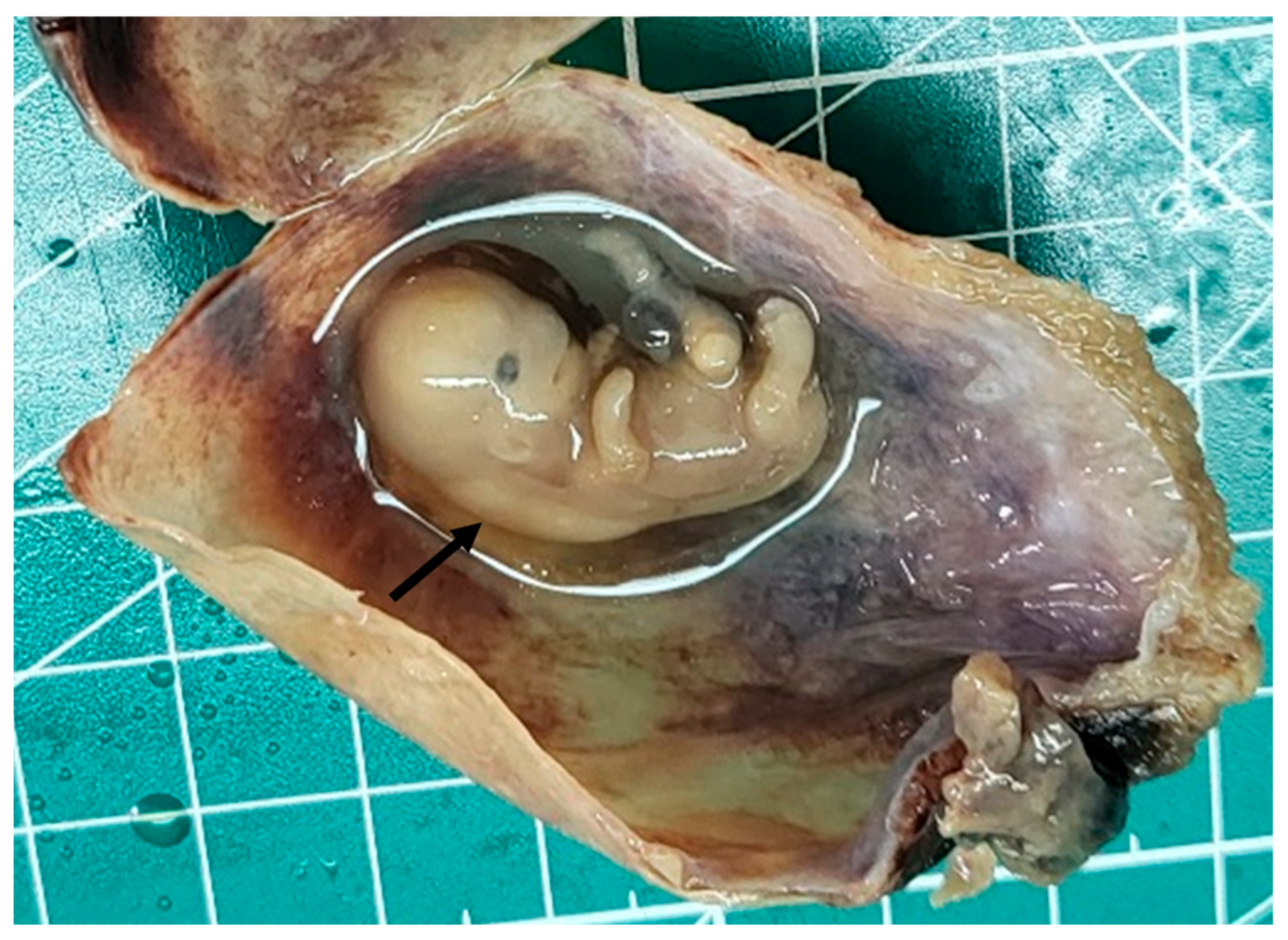
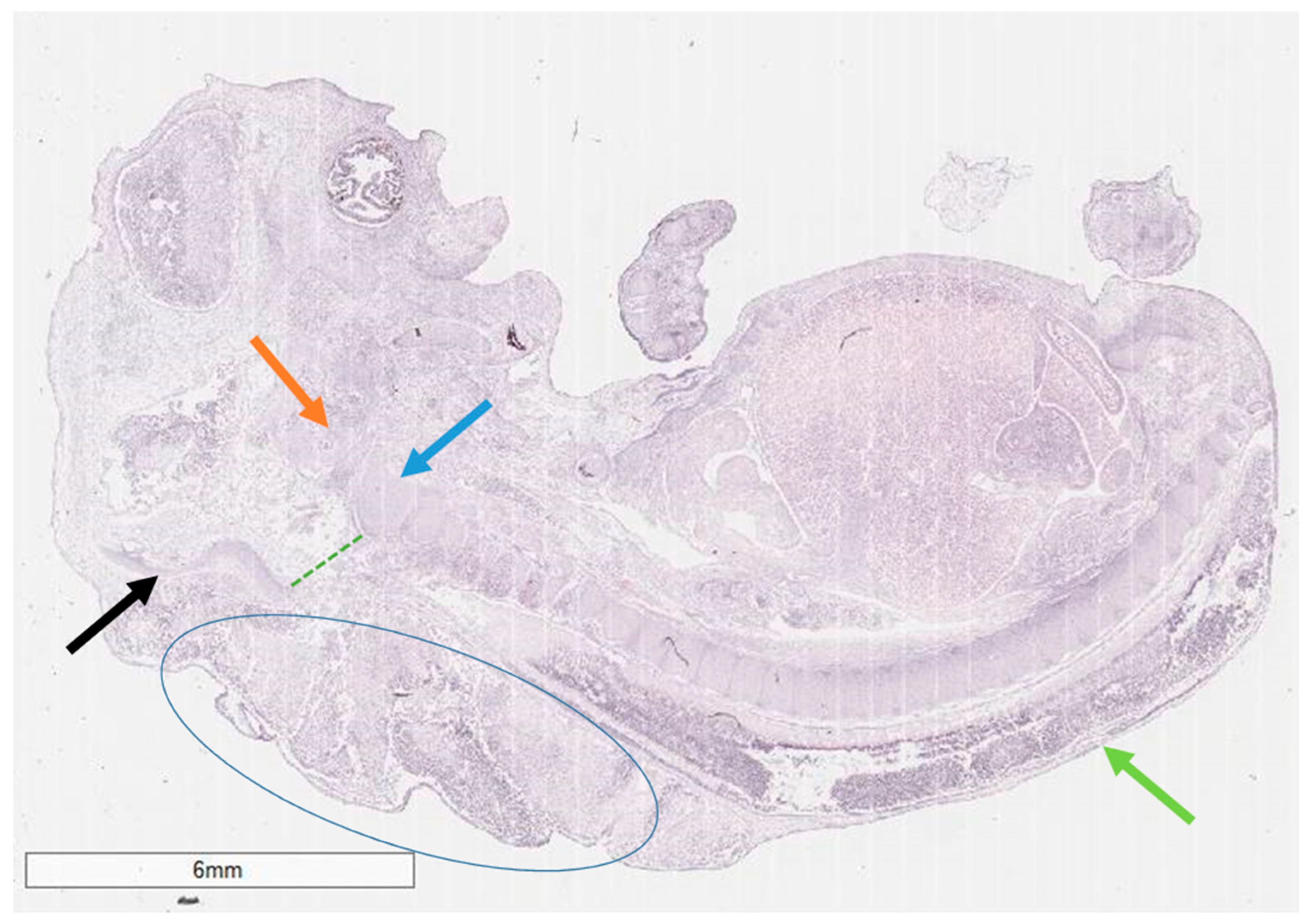
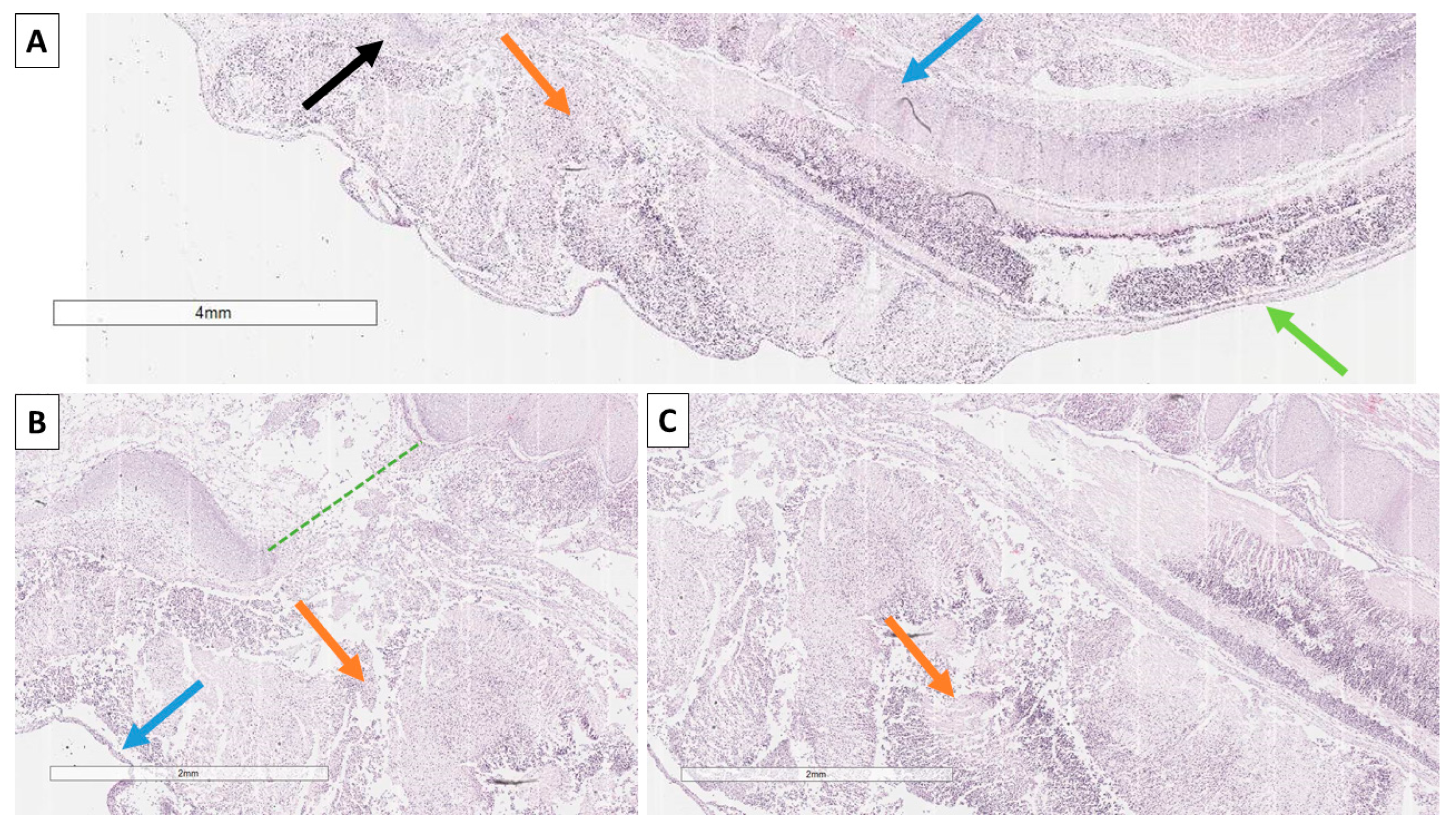
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Copp, A.J.; Adzick, N.S.; Chitty, L.S.; Fletcher, J.M.; Holmbeck, G.N.; Shaw, G.M. Spina Bifida. Nat. Rev. Dis. Prim. 2015, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Oumer, M.; Taye, M.; Aragie, H.; Tazebew, A. Prevalence of Spina Bifida among Newborns in Africa: A Systematic Review and Meta-Analysis. Scientifica 2020, 2020, 4273510. [Google Scholar] [CrossRef] [PubMed]
- Atta, C.A.M.; Fiest, K.M.; Frolkis, A.D.; Jette, N.; Pringsheim, T.; St Germaine-Smith, C.; Rajapakse, T.; Kaplan, G.G.; Metcalfe, A. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am. J. Public Health 2016, 106, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Sahmat, A.; Gunasekaran, R.; Mohd-Zin, S.W.; Balachandran, L.; Thong, M.K.; Engkasan, J.P.; Ganesan, D.; Omar, Z.; Azizi, A.B.; Ahmad-Annuar, A.; et al. The Prevalence and Distribution of Spina Bifida in a Single Major Referral Center in Malaysia. Front. Pediatr. 2017, 5, 301795. [Google Scholar] [CrossRef] [PubMed]
- Taskaynatan, M.A.; Izci, Y.; Ozgul, A.; Hazneci, B.; Dursun, H.; Kalyon, T.A. Clinical Significance of Congenital Lumbosacral Malformations in Young Male Population with Prolonged Low Back Pain. Spine 2005, 30, E210–E213. [Google Scholar] [CrossRef] [PubMed]
- Avrahami, E.; Frishman, E.; Fridman, Z.; Azor, M. Spina Bifida Occulta of S1 Is Not an Innocent Finding. Spine 1994, 19, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Steinbok, P. Dysraphic Lesions of the Cervical Spinal Cord. Neurosurg. Clin. N. Am. 1995, 6, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Akyol, M.E.; Arabaci, O. Cervical Meningomyelocele–Single Center Experience. Van Med. J. 2023, 30, 72–77. [Google Scholar] [CrossRef]
- Mirzaei, S.; Khoshkholghsima, M.; sabaghzadeh, A.; Kurdkandi, H.Z. Cervicothoracic (C6, C7 & T1) Spina Bifida Occulta–A Case Report. Int. J. Surg. Case Rep. 2024, 117, 109477. [Google Scholar] [CrossRef] [PubMed]
- Vannemreddy, P.; Nourbakhsh, A.; Willis, B.; Guthikonda, B. Congenital Chiari Malformations: A Review. Neurol. India 2010, 58, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Fisahn, C.; Shoja, M.M.; Turgut, M.; Oskouian, R.J.; Oakes, W.J.; Tubbs, R.S. The Chiari 3.5 Malformation: A Review of the Only Reported Case. Child’s Nerv. Syst. 2016, 32, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Andica, C.; Soetikno, R.D. Chiari Malformation Type III: Case Report and Review of the Literature. Radiol. Case Rep. 2015, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Sufuentes, S.; Esteban García, J.; Casado Pellejero, J.; Curto Simón, B.; Fustero de Miguel, D. Chiari Malformation Type III and Its Viability. Case Report and Literature Review. Neurochirurgie 2024, 70, 101585. [Google Scholar] [CrossRef] [PubMed]
- Williams, H. A Unifying Hypothesis for Hydrocephalus, Chiari Malformation, Syringomyelia, Anencephaly and Spina Bifida. Cerebrospinal Fluid Res. 2008, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Sagayaraj, B.M.; Barua, R.K.; Sharma, N.; Ranga, U. Arnold Chiari Malformation with Spina Bifida: A Lost Opportunity of Folic Acid Supplementation. J. Clin. Diagn. Res. 2014, 8, OD01. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.S.; Du, Y.L.; Lee, S.Y.; Wang, A.; Farmer, D.L. Spina Bifida: A Review of the Genetics, Pathophysiology and Emerging Cellular Therapies. J. Dev. Biol. 2022, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Zin, S.W.; Marwan, A.I.; Abou Chaar, M.K.; Ahmad-Annuar, A.; Abdul-Aziz, N.M. Spina Bifida: Pathogenesis, Mechanisms, and Genes in Mice and Humans. Scientifica 2017, 2017, 5364827. [Google Scholar] [CrossRef] [PubMed]
- Ivashchuk, G.; Loukas, M.; Blount, J.P.; Tubbs, R.S.; Oakes, W.J. Chiari III Malformation: A Comprehensive Review of This Enigmatic Anomaly. Child’s Nerv. Syst. 2015, 31, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Altunkeser, A.; Kara, T. Fetal Ultrasound of Type 2 and 3 Chiari Malformation. Clin. Case Rep. Rev. 2018, 4, 1–3. [Google Scholar] [CrossRef]
- Gabr, M.; Elmataeshy, M.; Abdullah, A.A. Chiari Type III: Experience of Outcome for 15 Cases. J. Korean Neurosurg. Soc. 2022, 65, 841–845. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanov, G.; Balabanov, I.; Zhivkova, S.; Popov, H. A Conundrum of Colliding Conditions: A Histopathological Case Report of Chiari Type III with Complete Spina Bifida Aperta. Reports 2025, 8, 202. https://doi.org/10.3390/reports8040202
Stoyanov G, Balabanov I, Zhivkova S, Popov H. A Conundrum of Colliding Conditions: A Histopathological Case Report of Chiari Type III with Complete Spina Bifida Aperta. Reports. 2025; 8(4):202. https://doi.org/10.3390/reports8040202
Chicago/Turabian StyleStoyanov, George, Ivaylo Balabanov, Svetoslava Zhivkova, and Hristo Popov. 2025. "A Conundrum of Colliding Conditions: A Histopathological Case Report of Chiari Type III with Complete Spina Bifida Aperta" Reports 8, no. 4: 202. https://doi.org/10.3390/reports8040202
APA StyleStoyanov, G., Balabanov, I., Zhivkova, S., & Popov, H. (2025). A Conundrum of Colliding Conditions: A Histopathological Case Report of Chiari Type III with Complete Spina Bifida Aperta. Reports, 8(4), 202. https://doi.org/10.3390/reports8040202





