1. Introduction and Clinical Significance
Acute pressure injuries can rapidly become chronic over time, particularly within patient populations suffering from age-related health issues, mobility ailments, diagnosed with chronic conditions, surgical patients, and those who reside within hospitals, long-term care facilities, nursing homes, etc. [
1]. Many individuals in such situations encounter both economic and logistical barriers that block access to appropriate care, thereby complicating regular wound management and decreasing regimen compliance, ultimately leading to diminished patient outcomes. As susceptibility arises, the need for appropriate wound management techniques arises.
Emerging advanced wound care technologies have focused on cellular, acellular and matrix-like products (CAMPs) to support wound management. Among these CAMPs are placental allografts, which have arisen as a promising option due to the amniotic membrane’s rich, native composition of extracellular matrix (ECM) components [
2]. Currently, placental-derived allografts are widely used in wound care settings and are commonly processed as single- or multi-layer constructs [
3]. For the patient presentation within this case study, an established dual-amnion graft technology, barrera
TM, was applied after conservative treatments failed.
2. Case Presentation
The retrospective data presented is from a single wound care center (Compassionate Concierge Physicians, CO, USA), dating from February 2024 to April 2024. This report is centered on a 65-year-old, white, non-Hispanic, non-Latino male with no known drug allergens. He is a non-smoker, has a medical history of multiple sclerosis with significant immobility, potential for infection and is wheelchair bound. Progress reports note that the patient had a wound history of bilateral chronic upper-thigh and buttocks decubitus ulcers that have been undergoing wound care for many months. His wounds had been previously managed using a preferred wound care cleanser, non-adherent, petroleum-based gauze dressings, and absorbent foam dressings.
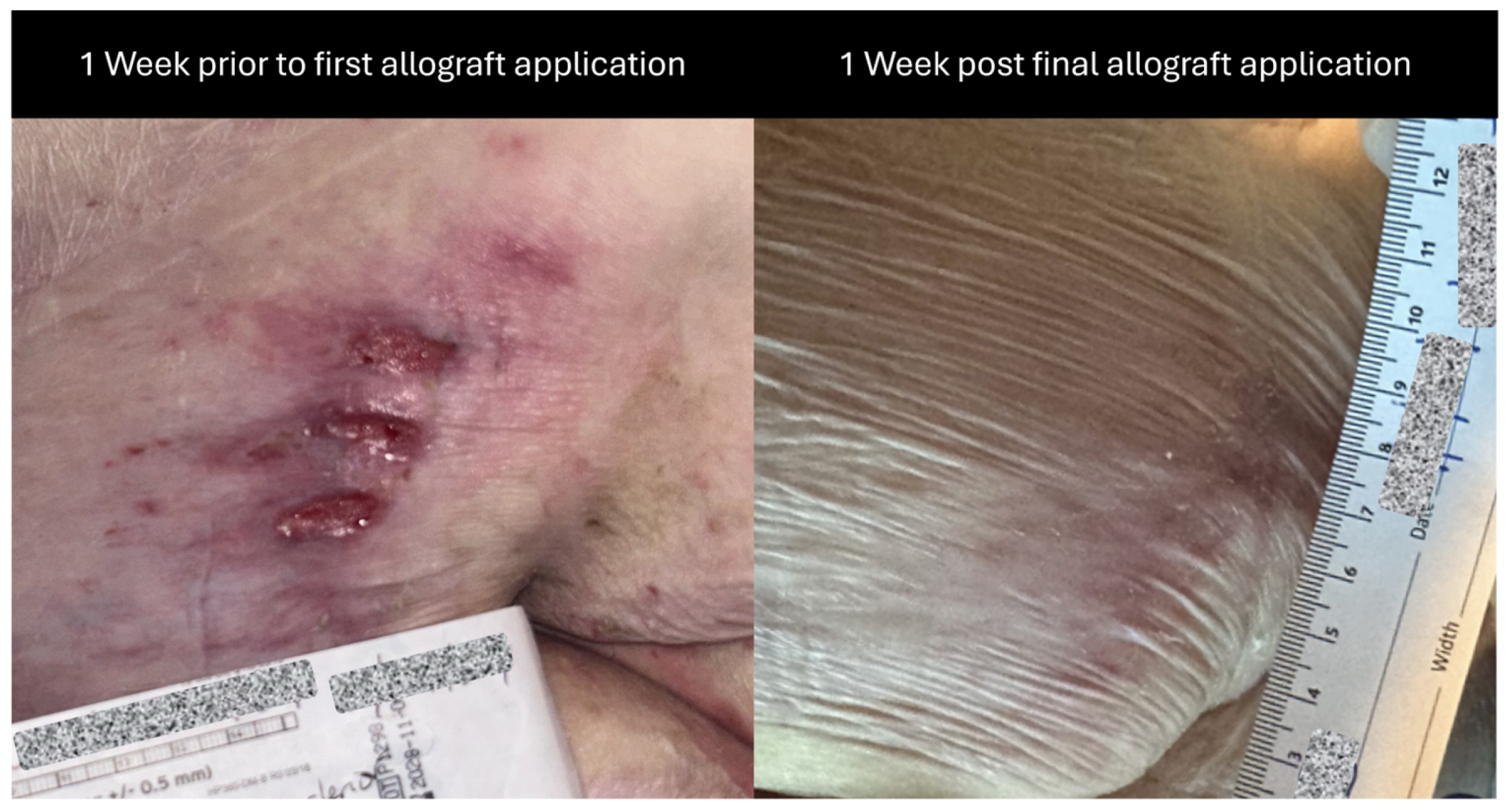
As a result of continued immobility, the patient developed a category 2 pressure ulcer on his left posterior upper thigh, initially separated into three oval-shaped wounds. At 1 week prior to allograft application for this wound, the measurement was presented as 4.2 cm × 2 cm (length × width), with mild, occasional pain and mild tenderness, especially when seated in his wheelchair. Although the wound is divided into three parts, measurements were recorded collectively as there was minimal spacing between the three parts. Additionally, the collective measurement encapsulates the total wound burden area.
Imaging with a point-of-care fluorescence wound imaging device was determined medically necessary to evaluate for bacterial bioburden that could inhibit wound size regression and to target debridement within high-bacterial-burden areas. The MolecuLight device was placed at an appropriate distance (approximately 10 cm) from the left posterior upper-thigh wound with the required dark conditions to ensure proper ambient lighting. A standard image and a fluorescent image were taken of the wound. Results depicted that no significant bacterial burden was evident, thus confirming the wound care plan.
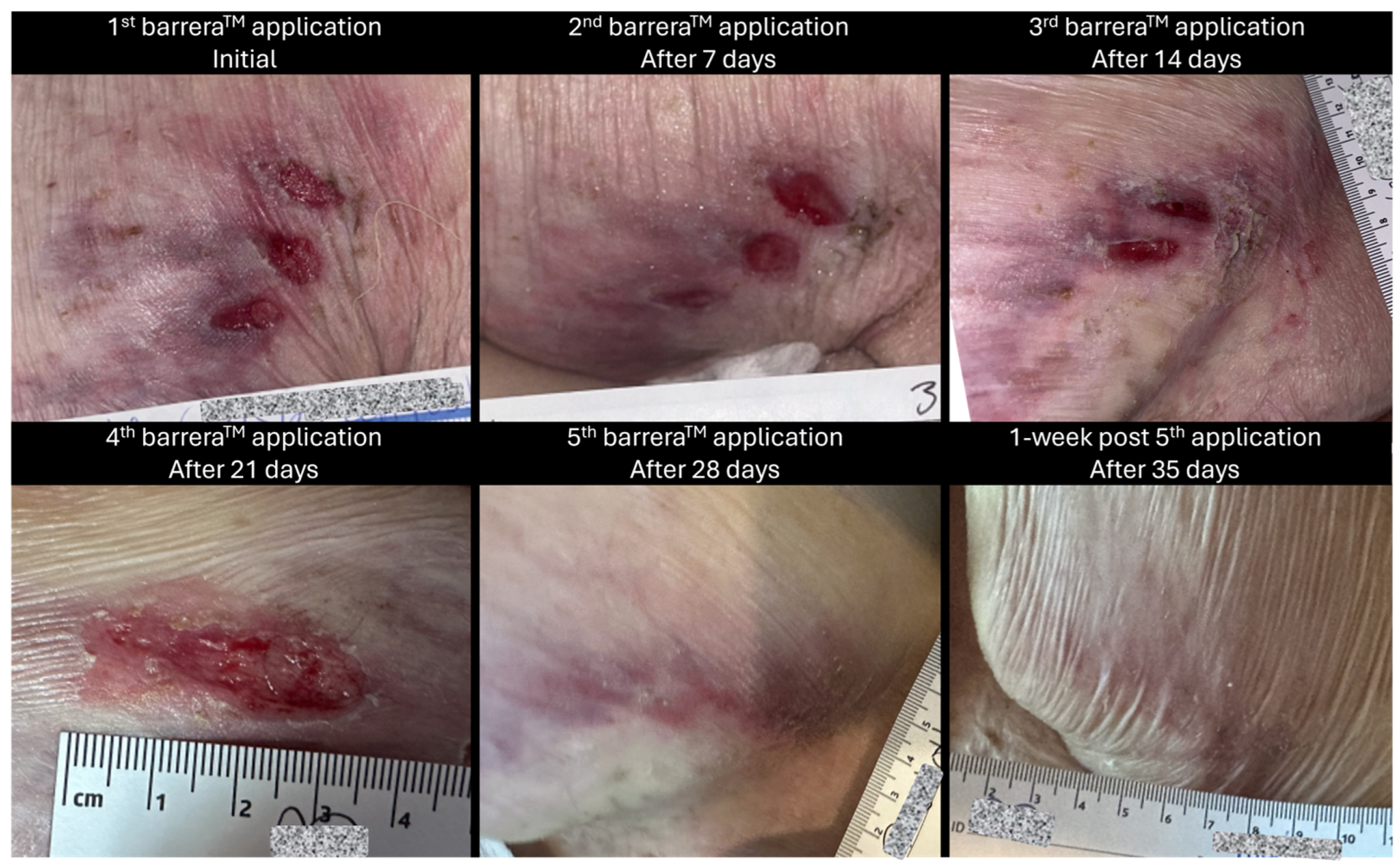
Immediately prior to the initial barreraTM (RegenTX Partners, LLC, an affiliate company of Tiger BioSciences, San Antonio, TX, USA) allograft application, the ulcer measured 3.5 cm × 3 cm (length × width), was still separated into three oval-shaped wounds, had positive mild tenderness, no discharge and minimal erythema. The wound was debrided for 15 min by soaking with a wound care cleansing solution; additionally, surrounding tissue and wounds were scrubbed and cleansed using gauze soaked with the wound care solution for >60 s to remove devitalized tissue. After this, barreraTM amniotic membrane allografts were applied to the wound bed of the posterior thigh with the assistance of saline to cover the entire wound bed. The wound was then dressed with a non-adherent, petroleum-based gauze dressing and an absorbent foam dressing which were secured with a thin border of clear tattoo tape. It was noted that the patient tolerated the procedure well, and there were no complications reported throughout the procedure.
Weekly wound care and barrera
TM allograft placements were provided by qualified providers. In addition, the patient’s daily aide was instructed to perform wound care and dress changes every other day. The patient received a total of five applications of barrera
TM between February 2024 and April 2024 as a part of their care regimen. A pictorial representation of the wound at different time intervals throughout the course of care can be seen in
Figure 1 and
Figure 2.
Quantitative Measures
An average 2.1 cm
2 change in the wound area per week, measured as the rate of healing, was seen from the first application of barrera
TM (immediately before allograft application, wound area: 10.5 cm
2) to 1 week post final allograft application (wound area: 0 cm
2). A corresponding change in the amount of drainage was also observed, going from moderate drainage to no drainage, suggesting a positive, healthy progression as seen in
Figure 3.
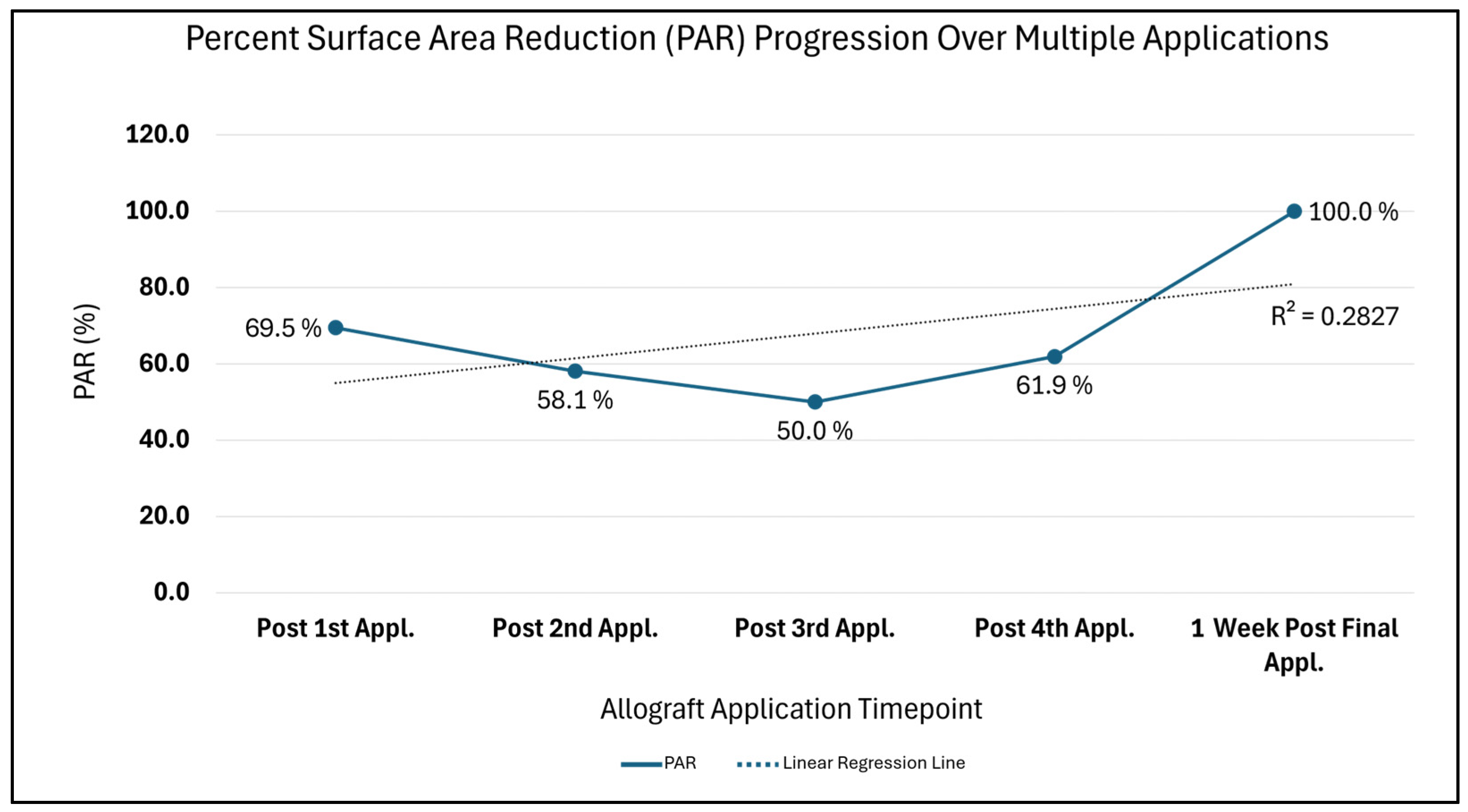
Additionally, the Percent Surface Area Reduction (PAR = [(Initial surface area − Current surface area) ÷ Initial surface area] × 100), with the initial wound area comparison timepoint being 10.5 cm
2, depicts trends synonymous with a wound advancing towards 0 cm
2 closure over time (
Figure 4). A drastic 69.5% PAR immediately after the first allograft application is noted, after which a continued percent improvement trend is seen until 100% size reduction is reached. Likewise, in
Figure 5, the comparison of the initial surface area (SA = length × width) to weekly SA measures also depicts a size attenuation trend. The slight upward trend seen at the third and fourth allograft application timepoints may be associated with the three oval-shaped wounds combining into one. The consolidation of the adjacent wounds may be attributed to friction or sheer associated with movement; however, the timepoint of this occurrence is unknown. Of note, there were no signs of adverse events nor infection during the course of care.
3. Discussion
Yearly, more than 2.5 million people in the United States develop pressure ulcers, which not only increases physical distress, discomfort and risks for infection, but also adds to an overall increase in health care utilization [
4]. Recent hospital metrics and the literature have shown pressure ulcers to be the third most costly disease after cancers and cardiovascular diseases, with mortality rates being two to six times higher compared to other maladies [
4]. Given their increasing emergence, it is not surprising that approximately 60,000 deaths annually are due to this complication, and risk factors such as diminished physical activity, decreased consciousness, incontinence, malnutrition and/or advanced age further contribute to the prevalence of these ulcers [
4]. Once developed, pressure ulcers are commonly associated with decreased mobility, and, in more critical cases, severe infection or limb amputation—all negative outcomes that providers and patients aim to mitigate early on. Amniotic placental allografts act as supportive wound coverings, which have been shown to provide encouraging results as adjuncts to standard of care (SOC) for wounds such as pressure ulcers. When applied to ulcers of varying etiologies, dual-layer amniotic placental allografts may provide supplemental support during the body’s natural healing process. In addition, based on the prevailing scientific literature, they may offer anti-inflammatory effects, anti-microbial effects, minimize the need for more invasive interventions and prevent unnecessary hospitalization [
5,
6].
The human amniotic membrane is a thin collagenous membrane derived from the submucosa of the placenta. It is composed of collagen and the extracellular stromal matrix, both of which are critical for providing structural integrity in allograft-based wound coverings [
5,
7]. barrera™ is one such terminally sterile, dual-layer amniotic placental allograft, which may be used on hard-to-heal, full-thickness wounds. Through the manner in which barrera™ is minimally processed, the native physical integrity of the amnion layers is maintained. Its dual-layer configuration contributes to greater durability relative to single-layer amniotic allografts. In addition, the layering is associated with a slower degradation rate, such that the allograft remains intact longer than a single amnion layer [
8].
This case highlights the clinical utility of barreraTM in the management of a recalcitrant stage 2 pressure injury in a patient with complex care needs. The 65-year-old patient presented with a medical history of multiple sclerosis with severe immobility. While multiple sclerosis is not directly related to the causation of the wounds, the immobility and diminished body sensation complications associated with the disease substantially increased susceptibility to the development of pressure ulcers for this patient. Based on clinical progress notes, the patient’s predisposition to the development of chronic wounds, plus potential presence of external factors such as sheer forces due to repositioning movements, significantly hindered advancement towards wound size regression. In order to avoid future hospitalization, decrease risks of exacerbated infections, and to circumvent more aggressive forms of wound mitigation techniques, placental amniotic allograft application was preferred by both the patient and the patient’s provider team. In this context, the favorable response observed with the application of barreraTM suggests it may offer meaningful clinical benefits as a valuable wound covering, which supports wound regression targets for recalcitrant wounds commonly encountered in immobile, high-risk geriatric populations.
This interval-based, objective analysis of wounds, albeit presented within this paper for an N of 1, contributes to the evidence-based allograft application approach. The SA and PAR measurements are key, complementary wound evaluation metrics, which are used herein to provide empirical clinical insight for the application of barrera
TM. Surface area calculations provide a static snapshot of wound dimensions at particular time intervals, thereby depicting the absolute change over time, which can be compared to initial measurements, whereas dynamic PAR size metrics capture the percentage of change seen for the wound at varying time intervals, depicting a measure of responsiveness. This case study reflects trends supported by the previously published literature, which demonstrate that a 20–30% PAR change in wound size within the first 3 to 4 weeks following amniotic allograft application is a viable benchmark for wound response and prediction of progress towards favorable outcomes [
9,
10]. Additionally, the observed reduction in wound SA over time and decrease in wound drainage for the patient presented herein are indicative of progressive amelioration post application of barrera
TM to the care regimen.
It is acknowledged that limitations of this study include its retrospective design and the small, single-patient sample size. Larger patient cohort studies with SOC control groups are warranted to further define and assess the results of utilizing a dual-layer placental allograft.
4. Conclusions
The intent of this paper was to report on the clinical experience of using a dual-layer amniotic placental tissue allograft, barreraTM, in the management of a hard-to-heal, stage 2 pressure ulcer. This clinical presentation depicts the potential wound-protective properties of dual-layer allografts when applied to complex, recalcitrant wounds within a high-risk patient population, such as the elderly and those with mobility restraints. The paper further expounds upon the potential of placental allografts to facilitate positive clinical endpoint responses, which may improve a patient’s quality of life, decrease infection risks and overall limit the need for hospitalization.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is a retrospective case study involving a single patient, which does not meet the federal definition of research as outlined in 45 CFR 46.102(l). This definition states that research is a systematic investigation designed to develop or contribute to generalizable knowledge. Since the intent of this activity is not to produce generalizable knowledge, but rather to describe a single clinical case, it does not qualify as human subjects research requiring IRB review. The collection and evaluation of all protected patient health information were performed in a HIPAA-compliant manner. To protect the privacy and confidentiality of the patient, all identifiable information was anonymized.
Informed Consent Statement
General patient consent (which encompasses the use of data and imaging) was obtained by the patient’s provider. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data that supports the findings of the manuscript may be available from the corresponding author upon reasonable request that takes into account patient privacy requirements.
Acknowledgments
The author would like to extend gratitude to the entire team at Compassionate Concierge Physicians who brought this work to fruition through their collective support and dedication. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAMPs | Cellular, Acellular and Matrix-like Products |
| ECM | Extracellular Matrix |
| PAR | Percent Area Reduction |
| SA | Surface Area |
| SOC | Standard of Care |
References
- Bhattacharya, S.; Mishra, R.K. Pressure Ulcers: Current Understanding and Newer Modalities of Treatment. Indian J. Plast. Surg. 2015, 48, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.; Hill, S.; Marquez, J.; McDonald, A.G.; Kaur, A. A Retrospective, Observational Case Series of Lower-Extremity Wound Management Using CompleteFT. Int. J. Tissue Repair 2025, 1, 1–9. [Google Scholar] [CrossRef]
- Singh, P.; Easley, A.; Menchaca, K.T.; Fanniel, V.; Gomez, R.; Marquez, J.; Hill, S. Comparative Study of Placental Allografts with Distinct Layer Composition. Int. J. Mol. Sci. 2025, 26, 3406. [Google Scholar] [CrossRef] [PubMed]
- Afzali Borojeny, L.; Albatineh, A.N.; Hasanpour Dehkordi, A.; Ghanei Gheshlagh, R. The Incidence of Pressure Ulcers and Its Associations in Different Wards of the Hospital: A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2020, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Schmiedova, I.; Dembickaja, A.; Kiselakova, L.; Nowakova, B.; Slama, P. Using of Amniotic Membrane Derivatives for the Treatment of Chronic Wounds. Membranes 2021, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.; Fetterolf, D.E. Dehydrated Amniotic Membrane Allografts for the Treatment of Chronic Wounds: A Case Series. J. Wound Care 2012, 21, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic Membrane: From Structure and Functions to Clinical Applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Bonvallet, P.P.; Damaraju, S.M.; Modi, H.N.; Stefanelli, V.L.; Lin, Q.; Saini, S.; Gandhi, A. Biophysical Characterization of a Novel Tri-Layer Placental Allograft Membrane. Adv. Wound Care 2022, 11, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Berlin, J.A.; Strom, B.L. A Comparison of Sensitivity Analyses of the Effect of Wound Duration on Wound Healing. J. Clin. Epidemiol. 1999, 52, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Khoo, R.; Jansen, S. The Evolving Field of Wound Measurement Techniques: A Literature Review. Wounds 2016, 28, 175–181. [Google Scholar] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).