Case Report: Scalpel Sign and Dorsal Arachnoid Cyst—The Importance of an Accurate Diagnosis
Abstract
1. Introduction and Clinical Significance
- A transverse extramedullary dorsal arachnoid band;
- A focal indentation of the spinal cord.
- Forced CSF flow through a congenital dural defect;
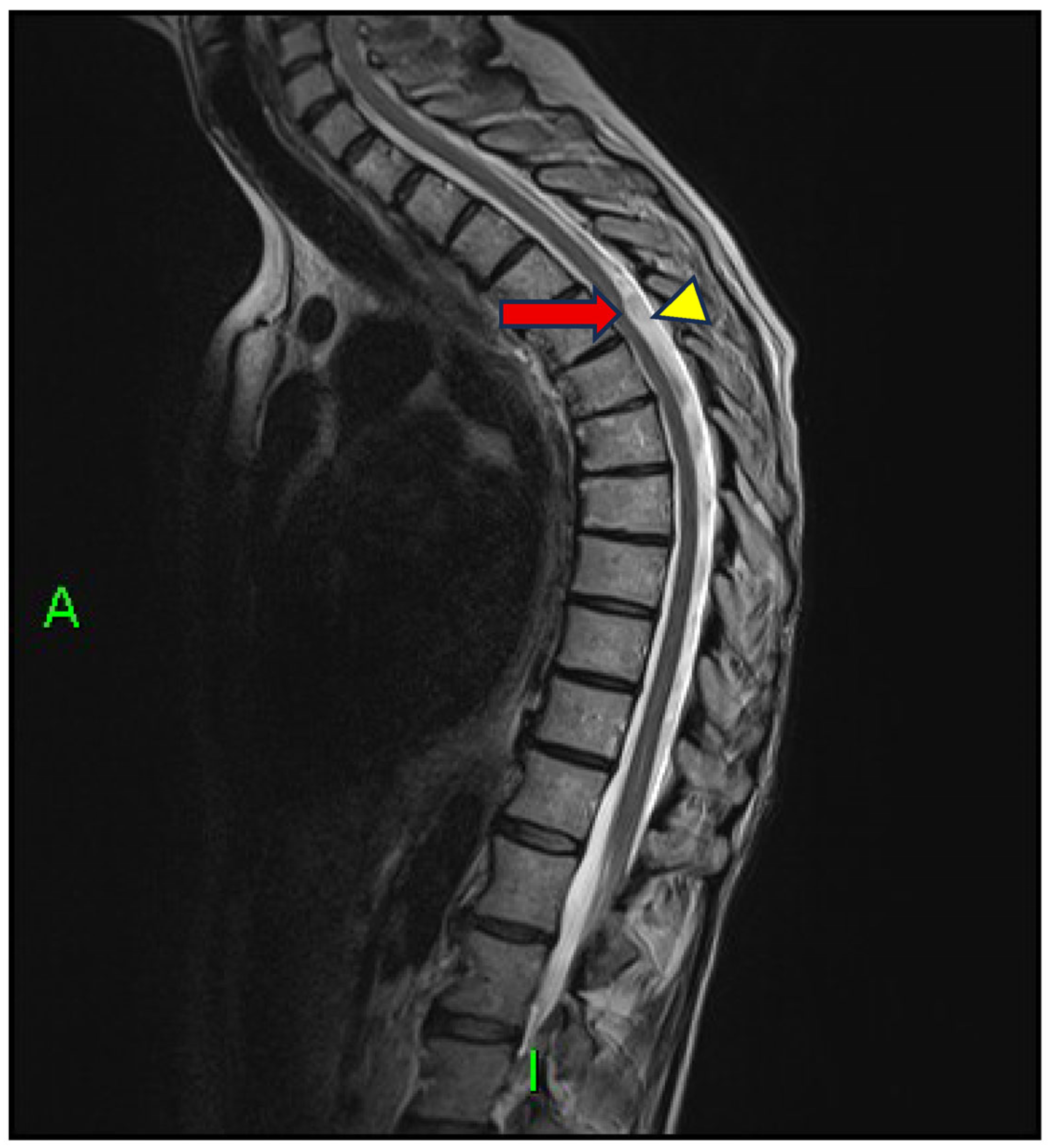
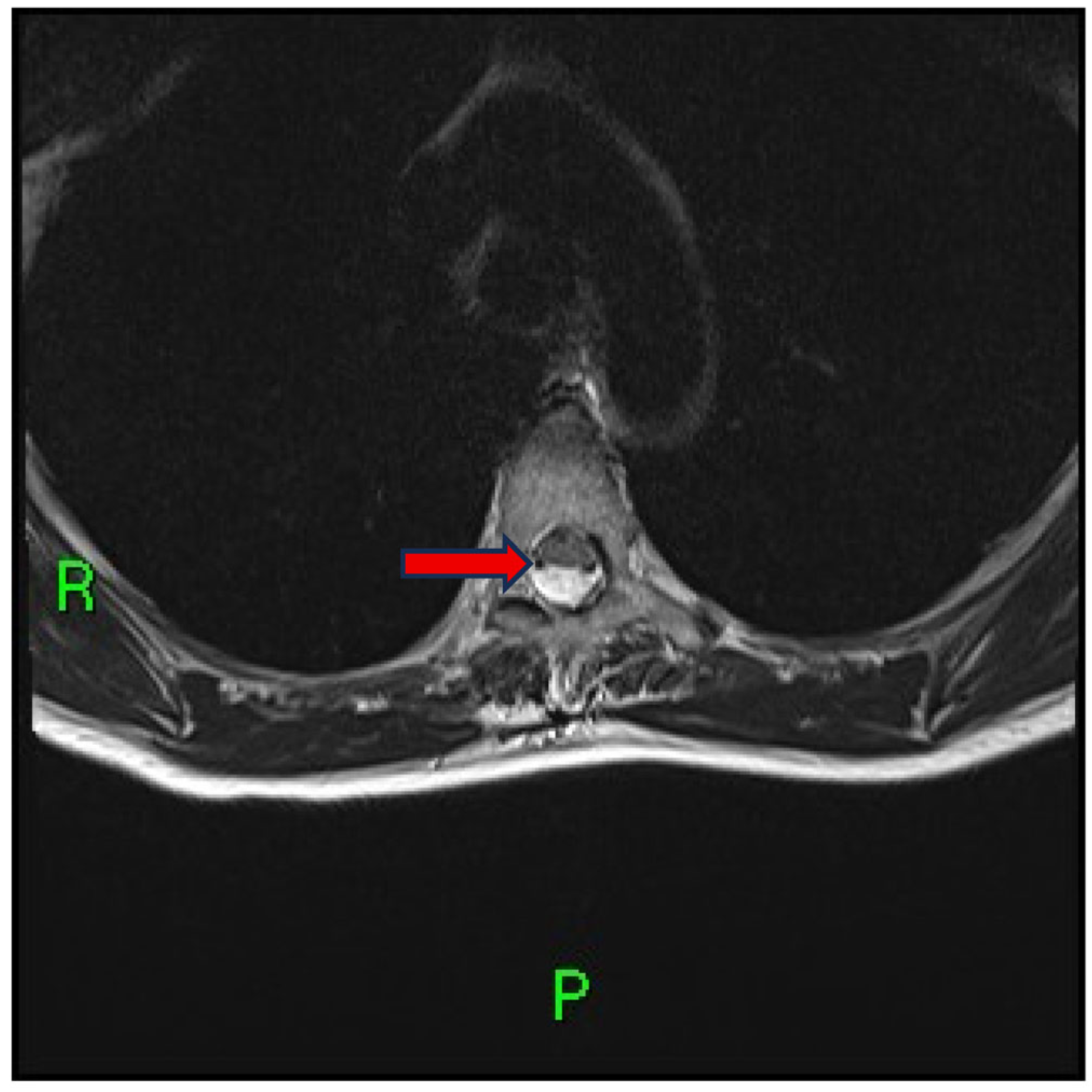
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-CISS | Three-Dimensional Constructive Interference in Steady State |
| DAW | Dorsal Arachnoid Web |
| CSF | Cerebrospinal Fluid |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
References
- Reardon, M.A.; Raghavan, P.; Carpenter-Bailey, K.; Mukherjee, S.; Smith, J.S.; Matsumoto, J.A.; Yen, C.P.; Shaffrey, M.E.; Lee, R.R.; Shaffrey, C.I.; et al. Dorsal thoracic arachnoid web and the “scalpel sign”: A distinct clinical-radiologic entity. AJNR Am. J. Neuroradiol. 2013, 34, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Verma, H.; Kamal, R.; Lone, N.A. Dorsal arachnoid web: The ‘scalpel’ sign—A case report and differential diagnosis. Egypt. J. Radiol. Nucl. Med. 2022, 53, 163. [Google Scholar] [CrossRef]
- Schultz, R.; Steven, A.; Wessell, A.; Fischbein, N.; Sansur, C.A.; Gandhi, D.; Ibrahimi, D.; Raghavan, P. Differentiation of idiopathic spinal cord herniation from dorsal arachnoid webs on MRI and CT myelography. J. Neurosurg. Spine 2017, 26, 754–759. [Google Scholar] [CrossRef]
- Ruschel, L.G.; Agnoletto, G.J.; Aurich, L.A.; Vosgerau, R.P. Dorsal arachnoid web and scalpel sign: A diagnostic imaging entity. Turk. Neurosurg. 2018, 28, 689–690. [Google Scholar] [CrossRef]
- Nisson, P.L.; Hussain, I.; Härtl, R.; Kim, S.; Baaj, A.A. Arachnoid web of the spine: A systematic literature review. J. Neurosurg. Spine 2019, 31, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, R.; El-Sherif, Y.; Voutsinas, L. Dorsal thoracic arachnoid web presenting as neuropathic pain: ‘Scalpel’ sign found on MRI. Neuroradiol. J. 2016, 29, 393–395. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hida, K.; Takeda, M.; Mitsuhara, T.; Morishige, M.; Yamada, N.; Kurisu, K. Visualization of regional cerebrospinal fluid flow with a dye injection technique in focal arachnoid pathologies. J. Neurosurg. Spine 2015, 22, 554–557. [Google Scholar] [CrossRef]
- Dua, S.G.; Jhaveri, M.D. Scalpel sign of dorsal arachnoid web. Neurol. India 2016, 64, 1092–1093. [Google Scholar] [CrossRef]
- Hines, T.; Wang, C.; Duttlinger, C.; Thompson, J.; Watford, K.; Motley, B.; Wheeler, G. Thoracic dorsal arachnoid web with rapid onset of symptoms: A report of two cases and brief review of the literature. Surg. Neurol. Int. 2021, 12, 323. [Google Scholar] [CrossRef]
- Ali, H.B.; Hamilton, P.; Zygmunt, S.; Yakoub, K.M. Spinal arachnoid web—A review article. J. Spine Surg. 2018, 4, 446–450. [Google Scholar] [CrossRef]
- Aljuboori, Z.; Boakye, M. Surgical release of a dorsal thoracic arachnoid web. World Neurosurg. 2020, 143, 289. [Google Scholar] [CrossRef]
- Hirai, T.; Taniyama, T.; Yoshii, T.; Mizuno, K.; Okamoto, M.; Inose, H.; Yuasa, M.; Otani, K.; Shindo, S.; Nakai, O.; et al. Clinical outcomes of surgical treatment for arachnoid web: A case series. Spine Surg. Relat. Res. 2019, 3, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.E.; Hunt, M.A.; Jones, K.E.; Polly, D.W. Thoracic spinal cord impingement by an arachnoid web at the level of a hemivertebra: Case report. J. Neurosurg. Spine 2017, 27, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Inoue, J.; Miyakoshi, N.; Hongo, M.; Kobayashi, T.; Abe, T.; Kikuchi, K.; Abe, E.; Kasukawa, Y.; Ishikawa, Y.; Kudo, D.; et al. Diagnosis and surgical treatment of thoracic dorsal arachnoid web: A report of two cases. Case Rep. Orthop. 2020, 2020, 8816598. [Google Scholar] [CrossRef]
- Vergara, P.; Barone, D.G. Minimally invasive excision of thoracic arachnoid web. World Neurosurg. 2018, 109, e81–e87. [Google Scholar] [CrossRef]
- Zhang, D.; Papavassiliou, E. Spinal intradural arachnoid webs causing spinal cord compression with inconclusive preoperative imaging: A report of 3 cases and a review of the literature. World Neurosurg. 2017, 99, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Naggar, A.; El Ouali, I.; Aidi, S.; Melhaoui, A.; el Kettani, N.E.-C.; Fikri, M.; Jiddane, M.; Touarsa, F. Spinal arachnoid web: A systematic review of a rare entity, with two illustrative case reports. Egypt. J. Radiol. Nucl. Med. 2024, 55, 178. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; Rodrigues, D.B.; Queiroz, J.W.M.; Laube, K.A.C.; Braga, M.C.M.; Kita, W.S.; De Luna, A.A.A.N.F.; De Souza, R.W.; Netto, R.H.D. Surgical treatment of spinal arachnoid web: Report of two cases and literature review. Surg Neurol Int. 2021, 12, 316. [Google Scholar] [CrossRef]
- Delgardo, M.; Higgins, D.; McCormick, K.L.; Reid, P.; Canoll, P.; McCormick, P.C. Clinical characteristics, outcomes, and pathology analysis in patients with dorsal arachnoid web. Neurosurgery 2022, 90, 581–587. [Google Scholar] [CrossRef]
- Voglis, S.; Romagna, A.; Germans, M.R.; Carreno, I.; Stienen, M.N.; Henzi, A.; Frauenknecht, K.; Rushing, E.; Molliqaj, G.; Tung, K.; et al. Spinal arachnoid web-a distinct entity of focal arachnopathy with favorable long-term outcome after surgical resection: Analysis of a multicenter patient population. Spine J. 2022, 22, 126–135. [Google Scholar] [CrossRef]
- Ruella, M.; Marco Del Pont, F.; Martin, A.; María Giovannini, S.J.; Centeno, T.R.; Cervio, A. Scalpel sign in spine pathology: Presentation in 3 different rare diagnoses. World Neurosurg. 2022, 157, e77–e87. [Google Scholar] [CrossRef]
- Weng, W.; Cheng, F.; Zhang, J. The occult spinal arachnoid web with inimitable imaging: A case report with 5-year follow-up. Int. J. Neurosci. 2023, 135, 13–17. [Google Scholar] [CrossRef]
- Na, C.-H.; Ridwan, H.; Neuloh, G.; Schubert, G.A.; Nolte, K.; Prescher, A.; Clusmann, H.; Blume, C. Arachnoid web—A rare but surgically effectively treatable cause of spinal cord compression and syringomyelia. Brain Spine 2025, 5, 104336. [Google Scholar] [CrossRef]
- Asghar, M.R.; Verma, H.; Bawaneh, S.; Kamal, R. Unraveling the Mystery: A case of dorsal arachnoid web of the spine: Clinical and imaging findings. Neurology 2025, 104 (Suppl. S1), 1968. [Google Scholar] [CrossRef]
- Zahoor, A.; Ali, S.; Lakhani, D.; Butcho, E.; Marsh, R.; Kassar, M. A rare case of spinal cord compression due to thoracic arachnoid cyst. Neurology 2025, 104 (Suppl. S1), 4183. [Google Scholar] [CrossRef]
- Capo, G.; Calvanese, F.; Tahhan, N.; Creatura, D.; Zaed, I.; Belli3a, E.; Baram, A.; Cotton, F.; Barrey, C.Y. Prediction of MRI in intra-operative findings for spinal meningeal diseases. Neurochirurgie 2025, 71, 101661. [Google Scholar] [CrossRef]
- Chang, H.S.; Nagai, A.; Oya, S.; Matsui, T. Dorsal spinal arachnoid web diagnosed with the quantitative measurement of cerebrospinal fluid flow on magnetic resonance imaging. J. Neurosurg. Spine 2014, 20, 227–233. [Google Scholar] [PubMed]
- Grewal, S.S.; Pirris, S.M.; Vibhute, P.G.; Gupta, V. Identification of arachnoid web with a relatively novel magnetic resonance imaging technique. Spine J. 2015, 15, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.; Mahdi, E.; Mahmoud, E.; Cousins, J.; Ahsan, H.; Leiva-Salinas, C. Multi-modality imaging evaluation of the dorsal arachnoid web. Neuroradiol. J. 2020, 33, 508–516. [Google Scholar] [CrossRef]
- Mauer, U.M.; Freude, G.; Danz, B.; Kunz, U. Cardiac-gated phase-contrast magnetic resonance imaging of cerebrospinal fluid flow in the diagnosis of idiopathic syringomyelia. Neurosurgery 2008, 63, 1139–1144. [Google Scholar]
- Brasil, P.; Pereira, L.; Távora, D.; Camara, A.; Filho, C.M.; Coimbra, P. Imaging findings in dorsal thoracic arachnoid web and the differential diagnosis of “Scalpel Sign”. Neurographics 2020, 10, 96–102. [Google Scholar] [CrossRef]
- Mukherjee, S.; Reardon, M.A.; Raghavan, P. Dorsal thoracic arachnoid web: Another intradural entity with ventral cord displacement. RadioGraphics 2015, 35, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, R.; Voutsinas, L.; El-Sherif, Y. An overview of arachnoid webs. J. Neurol. 2016, 1, 66–68. [Google Scholar]
- Wali, A.R.; Birk, H.S.; Martin, J.; Santiago-Dieppa, D.R.; Ciacci, J. Neurosurgical management of a thoracic dorsal arachnoid web: Case illustration. Cureus 2019, 11, e4945. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Nisson, P.L.; Kim, S.; Baaj, A.A. Intradural extramedullary surgical lysis of an arachnoid web of the spine. Oper. Neurosurg. 2020, 18, E131. [Google Scholar] [PubMed]
- Paramore, C.G. Dorsal arachnoid web with spinal cord compression: Variant of an arachnoid cyst? J. Neurosurg. Spine 2000, 93, 287–290. [Google Scholar]
- Heiss, J.D.; Snyder, K.; Peterson, M.M.; Patronas, N.J.; Butman, J.A.; Smith, R.K.; DeVroom, H.L.; Sansur, C.A.; Eskioglu, E.; Kammerer, W.A.; et al. Pathophysiology of primary spinal syringomyelia. J. Neurosurg. Spine 2012, 17, 367–380. [Google Scholar]
- Hingwala, D.; Chatterjee, S.; Kesavadas, C.; Thomas, B.; Kapilamoorthy, T.R. Applications of 3D CISS sequence for problem solving in neuroimaging. Indian J. Radiol. Imaging 2011, 21, 90–97. [Google Scholar] [CrossRef]
- Fakhr, F.S.; Kanaan, S.V.; Youness, F.M.; Hourani, M.H.; Haddad, M.C. Thoracic spinal intradural arachnoid cyst: Report of two cases and review. Eur. Radiol. 2002, 12, 877–882. [Google Scholar]
- DeGroot, A.L.; Treffy, R.W.; Bakhaidar, M.; Palmer, P.; Rahman, M.; Shabani, S. Minimally invasive management of a spinal arachnoid cyst with ultrasound-assisted catheter placement: Illustrative case. J. Neurosurg. Case Lessons 2024, 8, CASE24461. [Google Scholar]
- Derouen, K.; Shelvin, K.B.; Payton, T.; Crabill, G.A.; Wilson, J.M.; Tender, G. Arachnoid webs with spinal cord compression: Insights from three cases. J. Surg. Case Rep. 2023, 2023, rjad662. [Google Scholar] [CrossRef] [PubMed]
- Chellathurai, A.; Balasubramaniam, S.; Gnanasihamani, S.; Ramasamy, S.; Durairajan, J. Pathophysiology and Grading of the Ventral Displacement of Dorsal Spinal Cord Spectrum. Asian Spine J. 2018, 12, 224–231. [Google Scholar] [CrossRef]
- Greitz, D. Unraveling the riddle of syringomyelia. Neurosurg. Rev. 2006, 29, 251–264. [Google Scholar] [CrossRef]
- Sridharan, A.; Heilman, C.B. Transverse dorsal arachnoid web and syringomyelia: Case report. Neurosurgery 2009, 65, E216–E217. [Google Scholar]
- Brodbelt, A.R.; Stoodley, M.A.; Klekamp, J. Syringomyelia and the arachnoid web. Acta Neurochir. 2003, 145, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Sayal, P.P.; Zafar, A.; Carroll, T. Syringomyelia secondary to “occult” dorsal arachnoid webs: Report of two cases with review of literature. J. Craniovertebral Junction Spine 2016, 7, 101–104. [Google Scholar] [CrossRef] [PubMed]

| Year | First Author | Journal | n (Type) | Key Remarks | Reference |
|---|---|---|---|---|---|
| 2021 | Hines | Surg Neurol Int | 2 (DAW, scalpel sign) | Rapid onset, syrinx, resection | [9] |
| 2021 | Rodrigues | Surg Neurol Int | 2 (DAW, scalpel sign) | Thoracic DAW, resection | [18] |
| 2022 | Delgardo | Neurosurgery | 17 (DAW, scalpel sign) | 17 pts, 3D-CISS, surgery | [19] |
| 2022 | Voglis | Spine J | 12 (DAW, scalpel sign) | Multicenter, favorable outcomes | [20] |
| 2022 | Ruella | World Neurosurg | 7 (scalpel sign) | Mixed DAW/cyst/herniation | [21] |
| 2023 | Weng | Int J Neurosci | 1 (DAW, scalpel sign) | Occult DAW, 5y follow-up | [22] |
| 2024 | Naggar | Egypt J Radiol Nucl Med | 197 (DAW, scalpel sign predominantly observed) | Systematic review PRISMA Scalpel sign = highly specific imaging marker for DAW, not exclusive; early detection with 3D-CISS improves diagnosis, CSF flow assessment, and surgical planning. | [17] |
| 2025 | Na | Brain Spine | 17 (DAW, scalpel sign) | Cord compression, syrinx, surgery | [23] |
| 2025 | Asghar | Neurology | 1 (DAW, scalpel sign) | DAW case, scalpel sign, resection | [24] |
| 2025 | Zahoor | Neurology | 1 (scalpel sign) | Arachnoid cyst with compression | [25] |
| 2025 | Capo | Neurochirurgie | 1 (scalpel sign) | MRI vs surgery correlation | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, M.; Frigerio, M.; Muto, M.; Maffezzoni, F.; Miglio, S. Case Report: Scalpel Sign and Dorsal Arachnoid Cyst—The Importance of an Accurate Diagnosis. Reports 2025, 8, 198. https://doi.org/10.3390/reports8040198
Bonetti M, Frigerio M, Muto M, Maffezzoni F, Miglio S. Case Report: Scalpel Sign and Dorsal Arachnoid Cyst—The Importance of an Accurate Diagnosis. Reports. 2025; 8(4):198. https://doi.org/10.3390/reports8040198
Chicago/Turabian StyleBonetti, Matteo, Michele Frigerio, Mario Muto, Federico Maffezzoni, and Serena Miglio. 2025. "Case Report: Scalpel Sign and Dorsal Arachnoid Cyst—The Importance of an Accurate Diagnosis" Reports 8, no. 4: 198. https://doi.org/10.3390/reports8040198
APA StyleBonetti, M., Frigerio, M., Muto, M., Maffezzoni, F., & Miglio, S. (2025). Case Report: Scalpel Sign and Dorsal Arachnoid Cyst—The Importance of an Accurate Diagnosis. Reports, 8(4), 198. https://doi.org/10.3390/reports8040198





