Prenatal Diagnosis of Autosomal Dominant Polycystic Kidney Disease: Case Report
Abstract
1. Introduction and Clinical Significance
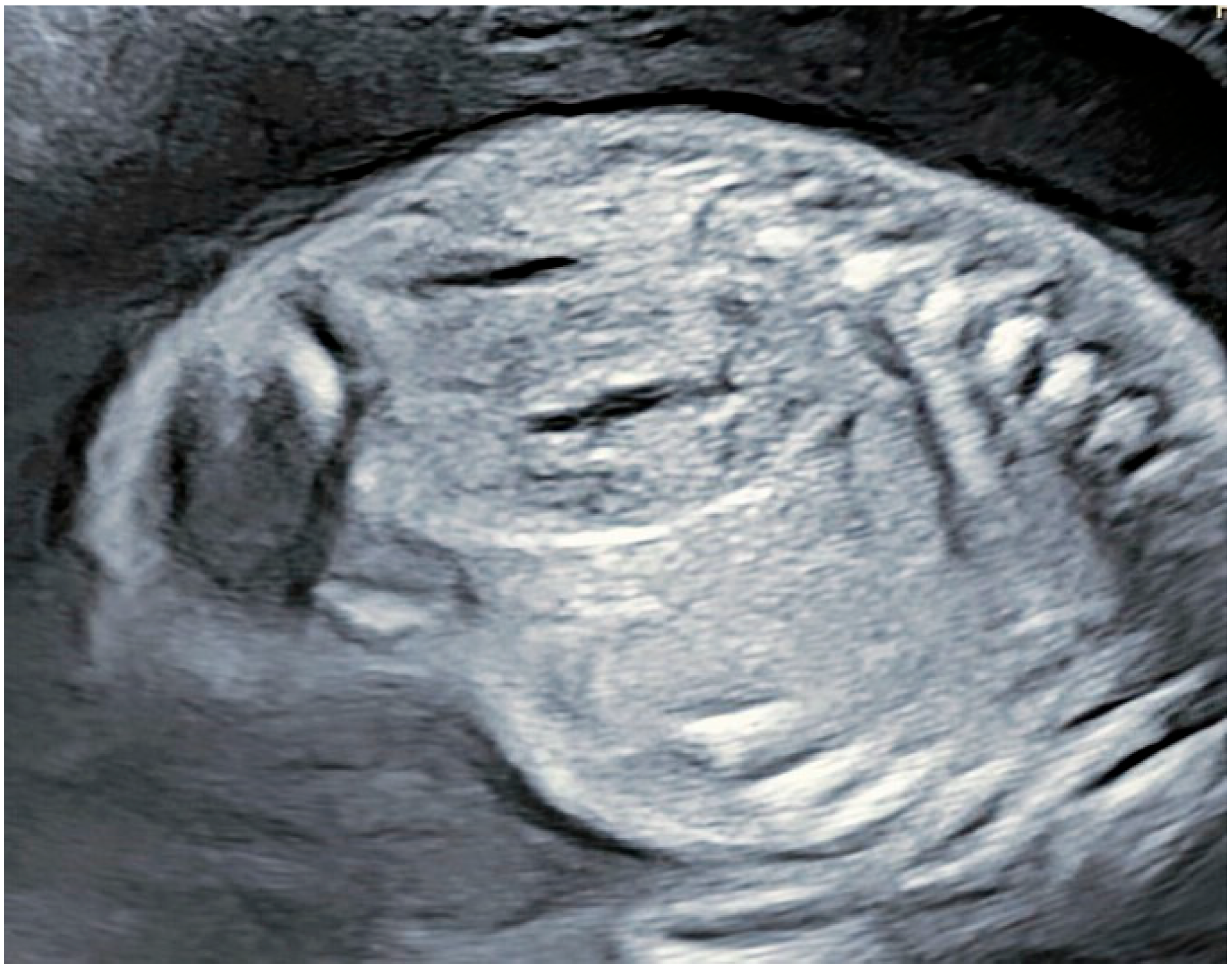
2. Case Presentation
Patient Information
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute of Diabetes and Digestive and Kidney Diseases. What is Polycystic Kidney Disease? Available online: https://www.niddk.nih.gov/health-information/kidney-disease/polycystic-kidney-disease/what-is-pkd (accessed on 10 March 2025).
- The NHS Website. Autosomal Dominant Polycystic Kidney Disease. Available online: https://www.nhs.uk/conditions/autosomal-dominant-polycystic-kidney-disease-adpkd/ (accessed on 10 March 2025).
- Mahboob, M.; Rout, P.; Leslie, S.W.; Bokhari, S.R.A. Autosomal Dominant Polycystic Kidney Disease; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532934/ (accessed on 10 March 2025).
- PKD1 Gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/pkd1/ (accessed on 10 March 2025).
- PKD2 Gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/pkd2/ (accessed on 10 March 2025).
- Steele, C.; You, Z.; Gitomer, B.Y.; Brosnahan, G.M.; Abebe, K.Z.; Braun, W.E.; Chapman, A.B.; Harris, P.C.; Perrone, R.D.; Steinman, T.I.; et al. PKD1 compared with PKD2 genotype and cardiac hospitalizations in the halt progression of polycystic kidney disease studies. Kidney Int. Rep. 2021, 7, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Fung, E. Autosomal Dominant Polycystic Kidney Disease (ADPKD). Available online: https://www.msdmanuals.com/professional/genitourinary-disorders/cystic-kidney-disease/autosomal-dominant-polycystic-kidney-disease-adpkd#Pathophysiology_v1053526 (accessed on 10 March 2025).
- Shaw, C.; Simms, R.J.; Pitcher, D.; Sandford, R. Epidemiology of patients in England and Wales with autosomal dominant polycystic kidney disease and end-stage renal failure. Nephrol. Dial. Transplant. 2014, 29, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Torres, V.E. Autosomal Dominant Polycystic Kidney Disease: Core Curriculum 2016. Am. J. Kidney Dis. 2015, 67, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Holmes, J.; Williams, G.; Chess, J.; Hartfiel, N.; Charles, J.M.; McLauglin, L.; Noyes, J.; Edwards, R.T. Current costs of dialysis modalities: A comprehensive analysis within the United Kingdom. Perit. Dial. Int. 2022, 42, 578–584. [Google Scholar] [CrossRef]
- Zerres, K.; Weiss, H.; Bulla, M.; Roth, B. Prenatal diagnosis of an early manifestation of autosomal dominant adult-type polycystic kidney disease. Lancet 1982, 320, 988. [Google Scholar] [CrossRef]
- Bergmann, C. Genetics of Autosomal Recessive polycystic kidney Disease and its differential diagnoses. Front. Pediatr. 2018, 5, 221. [Google Scholar] [CrossRef]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Bergmann, C. ARPKD and early manifestations of ADPKD: The original polycystic kidney disease and phenocopies. Pediatr. Nephrol. 2014, 30, 15–30. [Google Scholar] [CrossRef]
- Issa, N.; Chedid, M.; Irazabal, M.V.; Dean, P.G.; Chebib, F.T. Twenty-Year survival of kidney transplant from a deceased donor with autosomal dominant polycystic kidney disease. Kidney Int. Rep. 2021, 6, 2240–2242. [Google Scholar] [CrossRef]
- MacDermot, K.D.; Saggar-Malik, A.K.; Economides, D.L.; Jeffery, S. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J. Med. Genet. 1998, 35, 13–16. [Google Scholar] [CrossRef]
- Liebau, M.C.; Mekahli, D.; Bergmann, C. Polycystic Kidney Disease: ADPKD and ARPKD. In Pediatric Kidney Disease; Springer: Cham, The Netherlands, 2023; pp. 317–348. [Google Scholar] [CrossRef]
- Finnegan, C.; Murphy, C.; Breathnach, F. Neonatal polycystic kidney disease: A novel variant. BMJ Case Rep. 2021, 14, e242991. [Google Scholar] [CrossRef]
- Garel, J.; Lefebvre, M.; Cassart, M.; Della Valle, V.; Guilbaud, L.; Jouannic, J.-M.; Pointe, H.D.L.; Blondiaux, E.; Garel, C. Prenatal ultrasonography of autosomal dominant polycystic kidney disease mimicking recessive type: Case series. Pediatr. Radiol. 2019, 49, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abebe, K.Z.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef]
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 2016, 32, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Hussain, N.; Naim, M.; Zayed, M.; Al-Mulla, F.; Kehinde, E.O.; Seaburg, L.M.; Sundsbak, J.L.; Harris, P.C. A novel PKD1 variant demonstrates a disease-modifying role in trans with a truncating PKD1 mutation in patients with Autosomal Dominant Polycystic Kidney Disease. BMC Nephrol. 2015, 16, 26. [Google Scholar] [CrossRef]
- Durkie, M.; Chong, J.; Valluru, M.K.; Harris, P.C.; Ong, A.C.M. Biallelic inheritance of hypomorphic PKD1 variants is highly prevalent in very early onset polycystic kidney disease. Genet. Med. 2020, 23, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Cal, M.; Godinho, I.; Sá, M.S.E.; Cunha, M.; Carvalho, R. P04.03: In utero diagnosis of autosomal dominant polycystic kidney disease. Ultrasound Obstet. Gynecol. 2019, 54 (Suppl. S1), 165. [Google Scholar] [CrossRef]
- Michaud, J.; Russo, P.; Grignon, A.; Dallaire, L.; Bichet, D.; Rosenblatt, D.; Lamothe, E.; Lambert, M. Autosomal dominant polycystic kidney disease in the fetus. Am. J. Med. Genet. 1994, 51, 240–246. [Google Scholar] [CrossRef]
- Thomas, J.; Manjunath, A.; Rai, L.; Kudva, R. Autosomal recessive polycystic kidney disease diagnosed in fetus. Indian J. Urol. 2007, 23, 328. [Google Scholar] [CrossRef]
- Audrézet, M.-P.; Corbiere, C.; Lebbah, S.; Morinière, V.; Broux, F.; Louillet, F.; Fischbach, M.; Zaloszyc, A.; Cloarec, S.; Merieau, E.; et al. Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2015, 27, 722–729. [Google Scholar] [CrossRef]
- Nowak, K.L.; Cadnapaphornchai, M.A.; Chonchol, M.B.; Schrier, R.W.; Gitomer, B. Long-Term Outcomes in Patients with Very-Early Onset Autosomal Dominant Polycystic Kidney Disease. Am. J. Nephrol. 2016, 44, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y. Diagnostic approach in autosomal dominant polycystic kidney Disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Odland, D. A patient Perspective on genetic testing for ADPKD. Clin. J. Am. Soc. Nephrol. 2020, 16, 671–673. [Google Scholar] [CrossRef]
- Shaw, V.; Anderson, C.; Desloovere, A.; Greenbaum, L.A.; Harshman, L.; Nelms, C.L.; Pugh, P.; Polderman, N.; Renken-Terhaerdt, J.; Snauwaert, E.; et al. Nutritional management of the child with chronic kidney disease and on dialysis. Pediatr. Nephrol. 2024, 40, 69–84. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. Autosomal Dominant Polycystic Kidney Disease. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/polycystic-kidney-disease/autosomal-dominant-pkd#:~:text=person%20to%20person.-,What%20are%20the%20most%20common%20complications%20of%20ADPKD?,7 (accessed on 19 April 2025).
- Massella, L.; Mekahli, D.; Paripović, D.; Prikhodina, L.; Godefroid, N.; Niemirska, A.; Ağbaş, A.; Kalicka, K.; Jankauskiene, A.; Mizerska-Wasiak, M.; et al. Prevalence of Hypertension in Children with Early-Stage ADPKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 874–883. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A. Autosomal dominant polycystic kidney disease in children. Curr. Opin. Pediatr. 2015, 27, 193–200. [Google Scholar] [CrossRef]
- Ecder, T.; Schrier, R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 2009, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyokova, E.; Hristova-Atanasova, E.; Odumosu, E.; Andreeva, A. Prenatal Diagnosis of Autosomal Dominant Polycystic Kidney Disease: Case Report. Reports 2025, 8, 56. https://doi.org/10.3390/reports8020056
Gyokova E, Hristova-Atanasova E, Odumosu E, Andreeva A. Prenatal Diagnosis of Autosomal Dominant Polycystic Kidney Disease: Case Report. Reports. 2025; 8(2):56. https://doi.org/10.3390/reports8020056
Chicago/Turabian StyleGyokova, Elitsa, Eleonora Hristova-Atanasova, Elizabeth Odumosu, and Antonia Andreeva. 2025. "Prenatal Diagnosis of Autosomal Dominant Polycystic Kidney Disease: Case Report" Reports 8, no. 2: 56. https://doi.org/10.3390/reports8020056
APA StyleGyokova, E., Hristova-Atanasova, E., Odumosu, E., & Andreeva, A. (2025). Prenatal Diagnosis of Autosomal Dominant Polycystic Kidney Disease: Case Report. Reports, 8(2), 56. https://doi.org/10.3390/reports8020056






