Severe Bushmaster Snakebite Envenoming: Case Report and Overview
Abstract
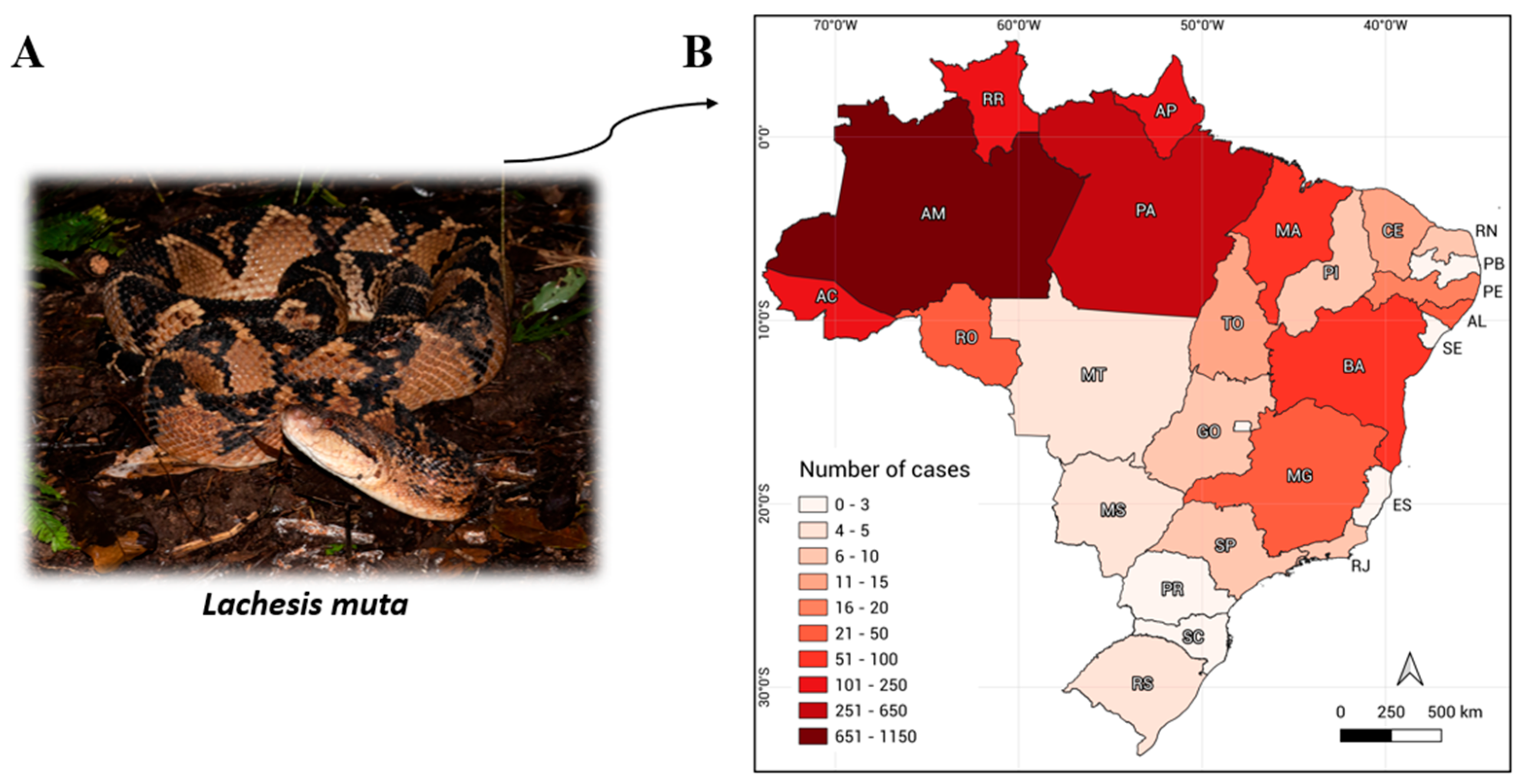
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.-P. Snakebite Envenomation Turns Again into a Neglected Tropical Disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- WHO. Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 18 January 2024).
- DATASUS. Doenças e Agravos de Notificação. Available online: https://datasus.saude.gov.br/acesso-a-informacao/doencas-e-agravos-de-notificacao-de-2007-em-diante-sinan/ (accessed on 17 October 2023).
- Da Siva, A.M.; Monteiro, W.M.; Bernarde, P.S. Popular Names for Bushmaster (Lachesis muta) and Lancehead (Bothrops atrox) Snakes in the Alto Juruá Region: Repercussions for Clinical-Epidemiological Diagnosis and Surveillance. Rev. Soc. Bras. Med. Trop. 2019, 52, e-20180140. [Google Scholar] [CrossRef] [PubMed]
- De Lima, P.H.S.; Haddad Junior, V. A Snakebite Caused by a Bushmaster (Lachesis muta): Report of a Confirmed Case in State of Pernambuco, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, K.R.; Greene, H.W. Phylogeography of the Bushmaster (Lachesis muta: Viperidae): Implications for Neotropical Biogeography, Systematics, and Conservation. Biol. J. Linn. Soc. 1997, 62, 421–442. [Google Scholar] [CrossRef]
- Malveira, S.K.M.; de Salis, C.R.; Correia, J.M.; Albuquerque, P.L.M.M.; Romeu, G.A. Acidente por Surucucu (Lachesis sp.) no Estado do Ceará: Relato de caso. Rev. Casos Consult. 2021, 12, e23909. [Google Scholar]
- Jorge, M.T.; Sano-Martins, I.S.; Tomy, S.C.; Castro, S.C.; Ferrari, R.A.; Ribeiro, L.A.; Warrell, D.A. Snakebite by the Bushmaster (Lachesis muta) in Brazil: Case Report and Review of the Literature. Toxicon 1997, 35, 545–554. [Google Scholar] [CrossRef] [PubMed]
- De O. Pardal, P.P.; Souza, S.M.; de C. da C. Monteiro, M.R.; Fan, H.W.; Cardoso, J.L.C.; França, F.O.S.; Tomy, S.C.; Sano-Martins, I.S.; de Sousa-e-Silva, M.C.C.; Colombini, M.; et al. Clinical Trial of Two Antivenoms for the Treatment of Bothrops and Lachesis Bites in the North Eastern Amazon Region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Manual de Diagnóstico e Tratamento de Acidentes Por Animais Peçonhentos; Ministério da Saúde: Brasília, Brazil, 2001.
- Wiezel, G.A.; dos Santos, P.K.; Cordeiro, F.A.; Bordon, K.C.F.; Selistre-de-Araújo, H.S.; Ueberheide, B.; Arantes, E.C. Identification of Hyaluronidase and Phospholipase B in Lachesis Muta Rhombeata Venom. Toxicon 2015, 107, 359–368. [Google Scholar] [CrossRef]
- Stransky, S.; Costal-Oliveira, F.; Lopes-de-Souza, L.; Guerra-Duarte, C.; Chávez-Olórtegui, C.; Braga, V.M.M. In Vitro Assessment of Cytotoxic Activities of Lachesis muta muta Snake Venom. PLoS Negl. Trop. Dis. 2018, 12, e0006427. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.S.; de Almeida, D.E.G.; Santos-Filho, N.A.; Sartim, M.A.; de Almeida Baldo, A.; Brasileiro, L.; Albuquerque, P.L.; Oliveira, S.S.; Sachett, J.A.G.; Monteiro, W.M.; et al. Crosstalk of Inflammation and Coagulation in Bothrops Snakebite Envenoming: Endogenous Signaling Pathways and Pathophysiology. Int. J. Mol. Sci. 2023, 24, 11508. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Franco, M.V.S.; Medeiros, J.M.; Oliveira, I.S.; Ahmadi, S.; Cerni, F.A.; Zottich, U.; Bassoli, B.K.; Monteiro, W.M.; Laustsen, A.H. Chronic Kidney Failure Following Lancehead Bite Envenoming: A Clinical Report from the Amazon Region. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200083. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Monteiro, W.; Houcke, S.; Pujo, J.M.; Mathien, C.; Mayence, C.; Neviere, R.; Hommel, D.; de Almeida Gonçalves Sachett, J.; Mehdaoui, H.; et al. Bothrops Snakebite Envenomings in the Amazon Region. Curr. Trop. Med. Rep. 2020, 7, 48–60. [Google Scholar] [CrossRef]
- De A. G. Sachett, J.; Marinho, A.P.S.; de O. Santos, M.M.; Fan, H.W.; Bernarde, P.S.; Monteiro, W.M. When to Think about a Lachesis muta Envenomation in the Western Brazilian Amazon: Lessons from a Case Report. Rev. Soc. Bras. Med. Trop. 2022, 55, e0027. [Google Scholar] [CrossRef]
- Cañas, C.A.; Castaño-Valencia, S.; Castro-Herrera, F. The Colombian Bushmasters Lachesis acrochorda (García, 1896) and Lachesis muta (Linnaeus, 1766): Snake Species, Venoms, Envenomation, and Its Management. Toxicon 2023, 230, 107152. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Flores-Sánchez, E.; Franceschi, A.; Magalhaes, A.; Gutiérrez, J.M. Characterization of the Local Tissue Damage Induced by LHF-II, a Metalloproteinase with Weak Hemorrhagic Activity Isolated from Lachesis muta muta Snake Venom. Toxicon 1999, 37, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Wiezel, G.A.; Bordon, K.C.; Silva, R.R.; Gomes, M.S.; Cabral, H.; Rodrigues, V.M.; Ueberheide, B.; Arantes, E.C. Subproteome of Lachesis Muta Rhombeata Venom and Preliminary Studies on LmrSP-4, a Novel Snake Venom Serine Proteinase. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e147018. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Monteiro, M.R.; Magalhães, H.P.; Mares-Guia, M.; Rogana, E. Thrombin-like Enzyme from Lachesis muta muta Venom: Isolation and Topographical Analysis of Its Active Site Structure by Means of the Binding of Amidines and Guanidines as Competitive Inhibitors. Toxicon 1997, 35, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Escolano, J.; Ferretti, M.; Biscoglio, M.J.; Rivera, E.; Crescenti, E.J.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake Venomics of the South and Central American Bushmasters. Comparison of the Toxin Composition of Lachesis muta Gathered from Proteomic versus Transcriptomic Analysis. J. Proteom. 2008, 71, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Bard, R.; de Lima, J.C.R.; de Sa Neto, R.P.; de Oliveira, S.G.; Santos, M.C. dos Ineficácia do antiveneno botrópico na neutralização da atividade coagulante do veneno de Lachesis muta muta: Relato de caso e comprovação experimental. Rev. Inst. Med. Trop. São Paulo 1994, 36, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De O. Pardal, P.P.; Bezerra, I.S.; da S. Rodrigues, L.; de O. Pardal, J.S.; Farias, P.H.S. de Acidente Por Surucucu (Lachesis muta muta) Em Belém-Pará: Relato de Caso. Rev. Para. Med. 2007, 21, 37–42. [Google Scholar] [CrossRef]
- Bateup, M. Spiritual Grief and Loss After an Amputation. Aborig. Isl. Health Work. J. 2010, 34, 20–22. [Google Scholar]
- Franco, M.V.S.; Alexandre-Silva, G.M.; Oliveira, I.S.; Santos, P.L.; Sandri, E.A.; Cerni, F.A.; Pucca, M.B. Physical and Social Consequences of Snakebites in the Yanomami Indigenous Community, Brazil: Report of Two Cases. Toxicon 2022, 214, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Elbey, B.; Baykal, B.; Yazgan, Ü.C.; Zengin, Y. The Prognostic Value of the Neutrophil/Lymphocyte Ratio in Patients with Snake Bites for Clinical Outcomes and Complications. Saudi J. Biol. Sci. 2017, 24, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.A.; Jorge, M.T.; Iversson, L.B. Epidemiologia do acidente por serpentes peçonhentas: Estudo de casos atendidos em 1988. Rev. Saúde Pública 1995, 29, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Hui Wen, F.; Monteiro, W.M.; Moura da Silva, A.M.; Tambourgi, D.V.; Mendonça da Silva, I.; Sampaio, V.S.; dos Santos, M.C.; Sachett, J.; Ferreira, L.C.L.; Kalil, J.; et al. Snakebites and Scorpion Stings in the Brazilian Amazon: Identifying Research Priorities for a Largely Neglected Problem. PLoS Negl. Trop. Dis. 2015, 9, e0003701. [Google Scholar] [CrossRef] [PubMed]
- Portal do Butantan: Soros e Bulas. Available online: https://butantan.gov.br/soros-e-vacinas/soros (accessed on 31 August 2023).
- Júnior, S.; Silva, D. Acidentes Ofídicos na População Pediátrica em um Hospital Universitário no Norte do Brasil: Uma Proposta de Ensino em Saúde. 2022. Available online: https://repositorio.uft.edu.br/handle/11612/4259 (accessed on 6 June 2024).
- De A. G. Sachett, J. Guia Para o Tratamento dos Acidentes Ofídicos; Julio Sampaio: Manaus, AM, Brazil, 2022; ISBN 9786500443394. [Google Scholar]
- Butantan Primeiro Socorros. Available online: https://butantan.gov.br/atendimento-medico/primeiro-socorros (accessed on 26 July 2024).
- Borges, C.C.; Sadahiro, M.; Santos, M.C. Dos Aspectos Epidemiológicos e Clínicos dos Acidentes Ofídicos Ocorridos nos Municípios do Estado do Amazonas. Rev. Soc. Bras. Med. Trop. 1999, 32, 637–646. [Google Scholar] [CrossRef]
- Layfield, H.J.; Williams, H.F.; Ravishankar, D.; Mehmi, A.; Sonavane, M.; Salim, A.; Vaiyapuri, R.; Lakshminarayanan, K.; Vallance, T.M.; Bicknell, A.B.; et al. Repurposing Cancer Drugs Batimastat and Marimastat to Inhibit the Activity of a Group I Metalloprotease from the Venom of the Western Diamondback Rattlesnake, Crotalus Atrox. Toxins 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Muniz, E.G.; Noronha, M.D.D.N.; Saraiva, M.D.G.G.; Monteiro, W.M.; Oliveira, S.S. Neutralization of Hemostatic Disorders Induced by Lachesis muta Venom Using Brazilian Antivenoms. Toxicon 2021, 191, 44–47. [Google Scholar] [CrossRef] [PubMed]


| Analytes * | 10 November | 11 November | 24 November | 28 November | 6 December | 11 December | Reference Range ** |
|---|---|---|---|---|---|---|---|
| Hemoglobin 1 | 17 | 13.7 | 7.8 | 11.2 | 11.8 | 11.5 | 13.5–18.0 g/dL |
| Leucocytes 1 | 32,090 | 28,070 | 12,270 | 9720 | 8640 | 12,730 | 4000–10,000 cells/µL |
| Neutrophils 1 | 89.8% | 88% | 75.8% | 69.2% | 41.1% | 39.8% | 50–70% |
| Platelets 1,a | 233,000 | 182,000 | 234,000 | 545,000 | 539,000 | 386,000 | 150,000–400,000/µL |
| PT 2 | 13.7 | 14.2 | 11.6 | 12.8 | 14.1 | - | 10–14 s |
| INR 2 | 1.01 | 1.08 | 1 | 1 | 1.07 | - | 0.8–1.2 |
| Urea 3 | 58.8 | 117.5 | 29.5 | 26.67 | 37.84 | 36.5 | 16–40 mg/dL |
| Creatinine 4 | 2.29 | 2.97 | 1.1 | 0.87 | 1.13 | 1.1 | 0.7–1.4 mg/dL |
| ALT 3 | 15.95 | 16.5 | 51.9 | 90.3 | 110.2 | 98.7 | 5–48 U/mL |
| AST 5 | 21.2 | 45.4 | 86.6 | 140.6 | 66.1 | 71 | 5–48 U/mL |
| CRP 6 | 162 | - | 138 | 48 | 3.4 | 4 | 0.0–8.0 mg/L |
| Snake | Clinical Manifestation | Type of Serum | Number of Ampoules |
|---|---|---|---|
| Bothrops spp. | Mild: The most common form of envenoming, characterized by mild or no local pain and swelling and mild or absent hemorrhagic manifestations. | 2–4 | |
| Moderate: Characterized by pain and significant swelling that extends beyond the bitten anatomical segment. Moderate envenoming may or may not be accompanied by local or systemic hemorrhagic alterations. | SAB; SABL; SABC | 4–8 | |
| Severe: Characterized by pain and significant swelling that extends beyond the bitten anatomical segment. Moderate envenoming may or may not be accompanied by local or systemic hemorrhagic alterations. | 12 | ||
| Lachesis spp. | Moderate: Local symptoms present, with or without bleeding. Some cases may lack vagal manifestations, making them challenging to differentiate from Bothrops envenoming. | 10 | |
| SABL | |||
| Severe: Intense local symptoms, severe bleeding, and/or vagal manifestations. | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcês-Filho, A.Q.; Santos, H.H.M.; Aguiar, T.K.P.P.; Ramos, D.L.S.; Galan, L.E.B.; Dantas, D.S.M.; Cerni, F.A.; Carbonell, R.C.; Pucca, M.B. Severe Bushmaster Snakebite Envenoming: Case Report and Overview. Reports 2024, 7, 68. https://doi.org/10.3390/reports7030068
Garcês-Filho AQ, Santos HHM, Aguiar TKPP, Ramos DLS, Galan LEB, Dantas DSM, Cerni FA, Carbonell RC, Pucca MB. Severe Bushmaster Snakebite Envenoming: Case Report and Overview. Reports. 2024; 7(3):68. https://doi.org/10.3390/reports7030068
Chicago/Turabian StyleGarcês-Filho, Allan Quadros, Humberto H. M. Santos, Thays K. P. P. Aguiar, Dafnin L. S. Ramos, Luis E. B. Galan, Domingos S. M. Dantas, Felipe A. Cerni, Roberto C. Carbonell, and Manuela B. Pucca. 2024. "Severe Bushmaster Snakebite Envenoming: Case Report and Overview" Reports 7, no. 3: 68. https://doi.org/10.3390/reports7030068
APA StyleGarcês-Filho, A. Q., Santos, H. H. M., Aguiar, T. K. P. P., Ramos, D. L. S., Galan, L. E. B., Dantas, D. S. M., Cerni, F. A., Carbonell, R. C., & Pucca, M. B. (2024). Severe Bushmaster Snakebite Envenoming: Case Report and Overview. Reports, 7(3), 68. https://doi.org/10.3390/reports7030068







