Abstract
The neurological manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are underrecognized. Ischemic stroke and thrombotic complications have been documented in patients with SARS-CoV-2 infection. Acute disseminated encephalomyelitis (ADEM) associated with coronavirus disease 2019 (COVID-19) is rare but can occur; the incidence of COVID-19-associated ADEM is still not clear due to the lack of reporting of cases. ADEM may have atypical stroke-like manifestations, such as hemiparesis, hemiparesthesia and dysarthria. The treatment strategies for ADEM and acute stroke are different. Early identification and prompt management may prevent further potentially life-threatening complications. We report a patient with SARS-CoV-2 infection presenting with stroke-like manifestations. We also make a comparison between demyelinating diseases, COVID-19-associated ADEM and acute stroke. This case can prompt physicians to learn about the clinical manifestations of SARS-CoV-2-associated ADEM.
1. Introduction
Respiratory illness with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is most commonly described, and some neurological manifestations may occur. A systematic review and meta-analysis indicated that more than one-third of COVID-19 patients exhibited at least one neurological manifestation, including fatigue, headache, dizziness, disturbance of consciousness and so on [1].
Neurological complications may be caused by direct viral effects on neurons and glial cells, the immune-mediated response to virus and a hypercoagulable state, which may lead to systemic disease with potential life-threatening complications, such as acute stroke and demyelination [2,3,4].
Demyelinating diseases caused by SARS-CoV-2 are rare but include acute disseminated encephalomyelitis (ADEM), multiple sclerosis, and neuromyelitis optica [3,5]. ADEM is defined as acute and fulminant encephalopathy with multifocal neurologic findings, typically following a prodromal viral illness. ADEM is a diagnosis of exclusion and should be differentiated from other demyelinating diseases through cerebrospinal fluid (CSF) oligoclonal band analysis, brain magnetic resonance imaging (MRI) and some biomarkers [4]. Several reports have described a possible association between ADEM and SARS-CoV-2 infection [4,6,7,8]. Except COVID-19 infection, COVID-19 vaccinations have been reported to induce severe neurological adverse effects, such as ADEM, cerebrovascular stroke, cerebral venous sinus thrombosis and encephalitis [9,10,11,12]. However, the neuropathological mechanism of COVID-19-associated ADEM or ADEM after COVID-19 vaccinations is still not clear [4,13]. Similarly, the case presentation is a patient with SARS-CoV-2 infection presenting stroke-like manifestations, such as right hemiparesis, hemiparesthesia and dysarthria. In addition, we make a comparison between COVID-19-associated ADEM and acute stroke. This case may provide an understanding of the different manifestations between SARS-CoV-2-associated ADEM and acute stroke to physicians.
2. Detailed Case Description
In January 2023, a healthy 19-year-old man (height 167 cm, weight 47 cm, BMI 16.8 kg/m2) presented to our emergency department with right hemiparesis, hemiparesthesia and dysarthria 8 days after coronavirus disease 2019 (COVID-19) infection. Initially, he had only a slight respiratory illness, including symptoms such as cough and runny nose. Fever, headache and drowsiness developed gradually. He performed an at-home COVID-19 rapid antigen test on day 4 after the upper respiratory infection symptoms had begun, and the test confirmed COVID-19 infection. However, the neurological symptoms of slurred speech, weakness and numbness of the right upper and lower limbs came on suddenly after he took a nap in the afternoon 4 days later. There was no known neurological history and no family history of neurological disorders such as cerebrovascular accident, amyotrophic lateral sclerosis and multiple sclerosis. He received two doses of BNT-162b2 vaccination without obvious side effects 1 year prior.
On arrival, his initial vital signs were as follows: temperature, 37.4 °C; heart rate, 85 beats per minute; respiratory rate, 18 breaths per minute; blood pressure, 142/92 mmHg; and pulse oximetry, 97% on room air. He was lethargic, and the neurological examination (Figure 1) showed slurred speech and right upper and lower limb weakness in the absence of other neurologic signs (altered mental status, facial droop, aphasia, disorientation, gaze palsy, visual deficit, ataxia and inattention) and decreased muscle power of approximately three to four points. Weakness of the left upper and lower limbs was also reported, but the muscle power was normal. Although the patient had right upper and lower limb numbness, the pinprick test revealed no sensory loss. The score of the National Institutes of Health Stroke Scale was 3. The results of the complete blood count and blood biochemistry tests showed no significant abnormalities. A chest X-ray and electrocardiography also showed no obvious abnormalities, such as widened mediastinum or atrial fibrillation. A brain computed tomography (CT) revealed no focal hypodense lesions or intracranial hemorrhage.
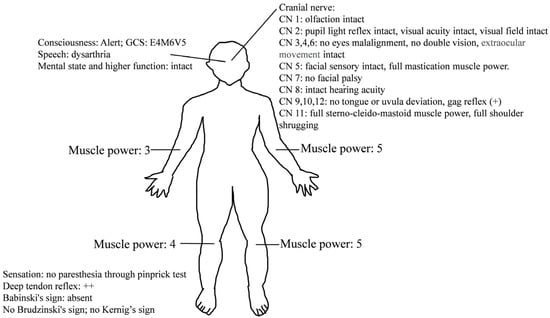
Figure 1.
Neurological examinations of our patient.
Dual antiplatelet therapy (DAPT) with 300 mg aspirin and 300 mg clopidogrel was administered because this case was highly suspicious for presumed stroke in a patient with no known risk factors. Then, the patient was admitted for a workup.
After admission, the blood test results (Table 1), including autoimmune panels (ANA, anti-ds DNA, C3, C4, cANCA, pANCA and rheumatoid factor) and hypercoagulable states tests (protein C, protein S, antithrombin III, antiphospholipid antibody), were all negative. A lumbar puncture was performed, and the CSF analysis (Table 2) presented no pleocytosis, a normal total protein level of 30.5 (normal range: 15–45) mg/dL and a normal IgG index of 0.62 (normal range: 0–0.7). The CSF results showed negative findings for viral infections, bacteria, fungi and mycobacterium tuberculosis complex cultures. A repeated brain CT on admission day 2 still revealed no focal hypodense regions. A brain MRI demonstrated bilateral subcortical white matter and corpus callosum hyperintensity signal lesions on T2-weighted and fluid-attenuated inversion recovery (FLAIR). The demyelinating lesions also showed increased diffusivity on diffusion-weighted imaging (DWI) and decreased apparent diffusion coefficient (ADC) values (Figure 2). No evidence of large vessel occlusion or vascular territory involvement was noted.

Table 1.
The results of blood examinations.

Table 2.
The results of cerebrospinal fluid examinations.
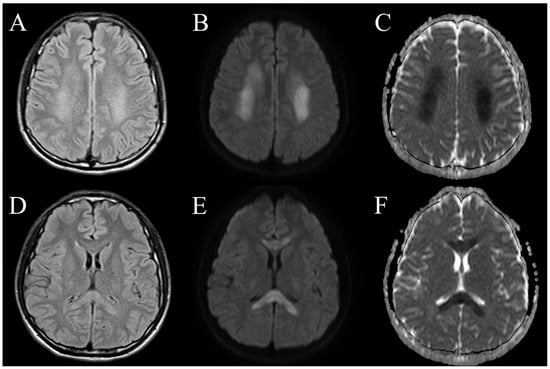
Figure 2.
Axial brain MRI sequences of the patient with COVID-19-associated ADEM. Subcortical white matter lesions displayed bilateral diffuse, hyperintense signals on T2-weighted FLAIR images (A), increased diffusivity on DWI (B) and decreased ADC values (C). Corpus callosum lesions demonstrated bilateral T2-weighted FLAIR hyperintensities (D), increased diffusivity on DWI (E) and subtle restricted diffusion on ADC (F).
The final diagnosis of COVID-19-associated ADEM was made according to the clinical features and MRI image findings. We then discontinued the DAPT prescription immediately. Medical treatment with a 3-day course of intravenous dexamethasone (10 mg/day) and a 3-day course of intravenous remdesivir (200 mg loading dose, then 100 mg/day) was administered. Although we tried relatively low-dose intravenous glucocorticoid therapy, the patient had a dramatically good response after 2 days. We did not titrate up to the high-dose glucocorticoid regimen because our treatment achieved a clinical response, and possible side effects were a concern. The patient had significant improvement after glucocorticoid and antiviral therapy during the hospital stay.
The patient was discharged and had a 7-day course of oral prednisolone 40 mg, a 5-day course of oral prednisolone 20 mg and then a 5-day course of oral prednisolone 10 mg. He fully recovered after a 3-month follow-up. Figure 3 shows the timeline of the patient from the day of COVID-19 infection and symptom onset to the 3-month follow-up.
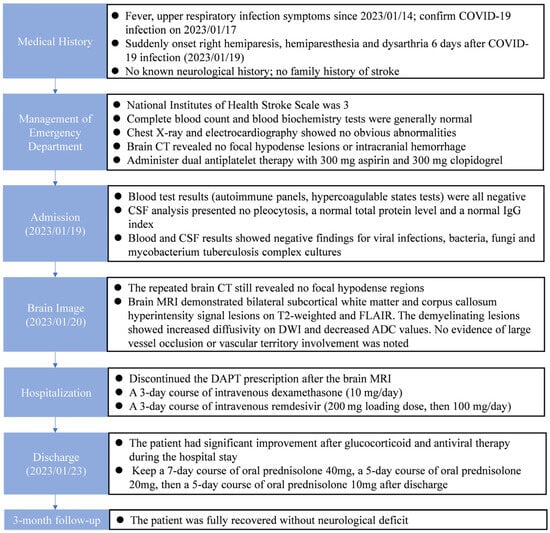
Figure 3.
Timeline of the patient from the day of COVID-19 infection and symptom onset to the 3-month follow-up.
3. Discussion
ADEM is an uncommon central nervous system (CNS) inflammatory demyelinating disease commonly associated with preceding viral infections. The duration from viral infection symptom onset to the development of ADEM varies from days to 6 weeks, with the majority occurring within 15–30 days in COVID-19 patients [7,8]. Our patient developed neurological deficits approximately one week after the onset of COVID-19 symptoms. The estimated annual incidence is approximately 1 to 3 per million in children [14]. ADEM is a very rare illness in adults, and the precise incidence is still unknown [4,14]. COVID-19-associated ADEM has been reported, but the incidence is higher in adults than in children [7,8]. ADEM is characterized by fulminant encephalopathy or neurological deficits. The typical presentations usually involve coma, paraparesis, quadriparesis, oculomotor deficits and dysarthria [7,8,14]. A brain MRI typically shows bilateral and asymmetric T2-weighted and FLAIR hyperintense lesions in the subcortical, periventricular white matter and deep gray matter, including the basal ganglia and thalamus [15]. In the acute stage, the MRI images usually demonstrate increased diffusivity on DWI and decreased ADC values [16]. The diagnostic guidelines for pediatric patients are based upon the clinical and radiologic features that were proposed by the International Pediatric Multiple Sclerosis Study Group [17]. The two major features are a first multifocal CNS demyelinating disease and encephalopathy, accompanied by an abnormal brain MRI during the acute phase. It is challenging to make a diagnosis of ADEM in adults due to the lack of established diagnostic criteria and a distinctive biological marker for adults. Encephalopathy is still an unclear diagnostic feature in adults. A retrospective study reported that only 56% of adult ADEM cases presented encephalopathy [18]. In our case, the typical features of encephalopathy, such as altered consciousness, confusion and irritability, were absent. Lethargy was considered to be a symptom of COVID-19 infection rather than encephalopathy. Acute onset neurological deficits, such as right hemiparesis, hemiparesthesia and dysarthria, were presented. It is intriguing that the brain MRI revealed bilateral demyelinating lesions that predominantly influenced the limbs on one side of the body. The treatment of ADEM includes supportive care and immune modulation therapy. Administration of high-dose glucocorticoids with a regimen of intravenous methylprednisolone at a dose of 1000 mg/day for 3 to 5 days is the first-line therapy. To reduce the risk of relapses, oral corticosteroid therapy should be continued and tapered gradually over 6 weeks [19]. Concurrent, empiric antibiotics and antiviral drugs can be considered. Intravenous immunoglobulin treatment (0.4 gm/kg/day for a 5-day course) and plasma exchange are options in patients who have a poor response to glucocorticoids or in some severe cases [4,6,12,19].
Ischemic stroke is also one of the most serious neurological complications in COVID-19 patients [3,20]. Coagulation dysfunctions may be caused by vascular endothelial damage that is secondary to direct viral damage, hypoxemia and cytokine storm [3,21,22]. It can lead to thrombosis and a hypercoagulable state. An estimated incidence of 1.4% was reported in the study by Nannoni S et al. [20]. They pooled 1106 patients with COVID-19 complicated by acute cerebrovascular disease and suggested that individuals with COVID-19 who developed stroke were more likely to be older and have preexisting cardiovascular comorbidities. However, the patients with COVID-19 infection and acute cerebrovascular disease were younger (approximately 6 years) than those presenting with stroke without COVID-19 [20]. Furthermore, Oxley TJ et al. reported several cases of large-vessel stroke in young COVID-19 patients and indicated that younger COVID-19 patients were also at risk for acute cerebrovascular disease [23]. The clinical characteristics between COVID-19-associated ADEM and acute stroke are shown in Table 3. Antiplatelet therapy, intravenous thrombolysis and endovascular thrombectomy are therapies for ischemic stroke [21]. In our case, the patient had no potential risk of stroke, such as hypercoagulable states, thrombosis or cardioembolism. The brain MRI did not demonstrate vascular territory infarction. However, based on the initial history and symptoms of our case, COVID-19-associated acute stroke may be easily misdiagnosed in the emergency department.

Table 3.
Comparison between COVID-19-associated ADEM and acute stroke.
Except for COVID-19-associated acute stroke, it is challenging to differentiate ADEM from various inflammatory and demyelinating disorders, such as multiple sclerosis (MS), myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease and neuromyelitis optica spectrum disorder (NMOSD) (Table 4) [5]. Our case study is limited by the lack of a CSF oligoclonal band analysis, serum or CSF anti-MOG-IgG and anti-aquaporin 4 (AQP4) antibody studies, repeated brain MRI and a long-term follow up. Although our patient did not present optic neuritis or transverse myelitis, it would be beneficial to consider running tests for anti-MOG-IgG and serum AQP4 antibodies, which are potentially compatible with MOG-IgG-mediated disease or NMOSD. Oligoclonal bands in CSF may point to a diagnosis of multiple sclerosis, although they may be present in some ADEM patients [8,17]. A repeated MRI image and a long-term follow-up are appropriate to differentiate ADEM from a first attack of MS, CNS inflammatory disorders or other demyelinating diseases. However, our patient denied any further tests, including blood studies (anti-MOG-IgG and anti-AQP4 antibodies), repeated CSF analysis and brain MRI because his symptoms resolved completely after the treatment. The limits of our case study result in diagnostic uncertainty.

Table 4.
Differential diagnosis of acute disseminated encephalomyelitis.
4. Conclusions
In conclusion, the clinical presentations of COVID-19-associated ADEM may mimic acute stroke. In adult COVID-19 patients, classic symptoms of ADEM, such as encephalopathy, may not be present. This can cause misdiagnosis or delay treatment. However, the treatment strategy between these two diseases is different. The treatment of stroke mainly involves anticoagulants, IV thrombolysis and endovascular thrombectomy. Considering the potential coagulation dysfunctions in COVID-19 patients, a misdiagnosis and corresponding treatments may increase the risks of cerebral hemorrhage. Early detection of ADEM and timely administration of an immune modulation therapy, such as IV steroids, may reduce permanent disability in these patients. Early identification and well-timed therapy may help to achieve favorable clinical outcomes.
Author Contributions
Conceptualization, Y.-X.J., M.-H.C., Y.-Y.L., C.-C.C. and P.-J.H.; writing—original draft, Y.-X.J., M.-H.C., Y.-Y.L., Y.-H.K., T.-W.L., C.-C.C. and P.-J.H.; writing—review and editing, Y.-H.K., C.-C.C. and P.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. It was reviewed and approved by the Institutional Review Board of the Tri-service General Hospital. TSGHIRB No.: C202315059 on 2 June 2023.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the staff and researchers at the Taoyuan Armed Forces General Hospital, Taiwan. They also thank the patient for consenting to publish his clinical information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Misra, S.; Misra, S.; Kolappa, K.; Kolappa, K.; Prasad, M.; Prasad, M.; Radhakrishnan, D.; Radhakrishnan, D.; Thakur, K.T.; Thakur, K.T.; et al. Frequency of Neurologic Manifestations in COVID-19: A Systematic Review and Meta-analysis. Neurology 2021, 97, e2269–e2281. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Anyfanti, P.; Gavriilaki, M.; Lazaridis, A.; Douma, S.; Gkaliagkousi, E. Endothelial Dysfunction in COVID-19: Lessons Learned from Coronaviruses. Curr. Hypertens. Rep. 2020, 22, 63. [Google Scholar] [CrossRef]
- Dai, X.; Cao, X.; Jiang, Q.; Wu, B.; Lou, T.; Shao, Y.; Hu, Y.; Lan, Q. Neurological complications of COVID-19. Qjm Int. J. Med. 2023, 116, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Bajko, Z.; Stoian, M.; Cioflinc, R.A.; Niculescu, R.; Arbănași, E.M.; Russu, E.; Botoncea, M.; Bălașa, R. The Occurrence of Acute Disseminated Encephalomyelitis in SARS-CoV-2 Infection/Vaccination: Our Experience and a Systematic Review of the Literature. Vaccines 2023, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Feizi, P.; Sharma, K.; Pasham, S.R.; Nirwan, L.; Joseph, J.; Jaiswal, S.; Sriwastava, S. Central nervous system (CNS) inflammatory demyelinating diseases (IDDs) associated with COVID-19: A case series and review. J. Neuroimmunol. 2022, 371, 577939. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Manzano, G.S.; McEntire, C.R.S.; Martinez-Lage, M.; Mateen, F.J.; Hutto, S.K. Acute Disseminated Encephalomyelitis and Acute Hemorrhagic Leukoencephalitis Following COVID-19: Systematic Review and Meta-synthesis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, 1080. [Google Scholar] [CrossRef]
- Parsons, T.; Banks, S.; Bae, C.; Gelber, J.; Alahmadi, H.; Tichauer, M. COVID-19-associated acute disseminated encephalomyelitis (ADEM). J. Neurol. 2020, 267, 2799–2802. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Noori, M.; Rahmani, S.; Hosseini, H. Acute disseminated encephalomyelitis (ADEM) following COVID-19 vaccination: A systematic review. J. Clin. Neurosci. 2023, 111, 57–70. [Google Scholar] [CrossRef]
- Kakovan, M.; Shirkouhi, S.G.; Zarei, M.; Andalib, S. Stroke Associated with COVID-19 Vaccines. J. Stroke Cerebrovasc. Dis. 2022, 31, 106440. [Google Scholar] [CrossRef]
- Wang, R.L.; Chiang, W.F.; Shyu, H.Y.; Chen, M.H.; Lin, C.I.; Wu, K.A.; Yang, C.C.; Huang, L.Y.; Hsiao, P.J. COVID-19 vaccine-associated acute cerebral venous thrombosis and pulmonary artery embolism. Qjm Int. J. Med. 2021, 114, 506–507. [Google Scholar] [CrossRef]
- Fan, H.T.; Lin, Y.Y.; Chiang, W.F.; Lin, C.Y.; Chen, M.H.; Wu, K.A.; Chan, J.S.; Kao, Y.H.; Shyu, H.Y.; Hsiao, P.J. COVID-19 vaccine-induced encephalitis and status epilepticus. Qjm Int. J. Med. 2022, 115, 91–93. [Google Scholar] [CrossRef]
- Chen, W.-P.; Chen, M.-H.; Shang, S.-T.; Kao, Y.-H.; Wu, K.-A.; Chiang, W.-F.; Chan, J.-S.; Shyu, H.-Y.; Hsiao, P.-J. Investigation of Neurological Complications after COVID-19 Vaccination: Report of the Clinical Scenarios and Review of the Literature. Vaccines 2023, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Pohl, D.; Alper, G.; Van Haren, K.; Kornberg, A.J.; Lucchinetti, C.F.; Tenembaum, S.; Belman, A.L. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology 2016, 87 (Suppl. S2), S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Bhawna, S.; Rahul, H.; Kadam, N.; Swayam, P.; Gupta, P.K.; Agrawal, R.; Sisodiya, M.S. Transient splenial diffusion-weighted image restriction mimicking stroke. Am. J. Emerg. Med. 2014, 32, 1156.e1–1156.e2. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanya, K.S.; Kovoor, J.M.E.; Jayakumar, P.N.; Ravishankar, S.; Kamble, R.B.; Panicker, J.; Nagaraja, D. Diffusion-weighted imaging and proton MR spectroscopy in the characterization of acute disseminated encephalomyelitis. Neuroradiology 2007, 49, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Tardieu, M.; Amato, M.P.; Banwell, B.; Chitnis, T.; Dale, R.C.; Ghezzi, A.; Hintzen, R.; Kornberg, A.; Pohl, D.; et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: Revisions to the 2007 definitions. Mult. Scler. J. 2013, 19, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Koelman, D.L.; Chahin, S.; Mar, S.S.; Venkatesan, A.; Hoganson, G.M.; Yeshokumar, A.K.; Barreras, P.; Majmudar, B.; Klein, J.P.; Chitnis, T.; et al. Acute disseminated encephalomyelitis in 228 patients: A retrospective, multicenter US study. Neurology 2016, 86, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Murthy, J.M.K. Acute disseminated encephalomyelitis: Treatment guidelines. Ann. Indian. Acad. Neurol. 2011, 14 (Suppl. S1), S60–S64. [Google Scholar] [CrossRef]
- Nannoni, S.; de Groot, R.; Bell, S.; Markus, H.S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke. 2021, 16, 137–149. [Google Scholar] [CrossRef]
- Kits, A.; Pantalone, M.R.; Illies, C.; Antovic, A.; Landtblom, A.-M.; Iacobaeus, E. Fatal Acute Hemorrhagic Encephalomyelitis and Antiphospholipid Antibodies following SARS-CoV-2 Vaccination: A Case Report. Vaccines 2022, 10, 2046. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).