Hidradenitis Suppurativa in Association with Ulcerative Proctitis: Surgical Management in a Refractory Case to Topical and Systemic Treatment
Abstract
1. Introduction
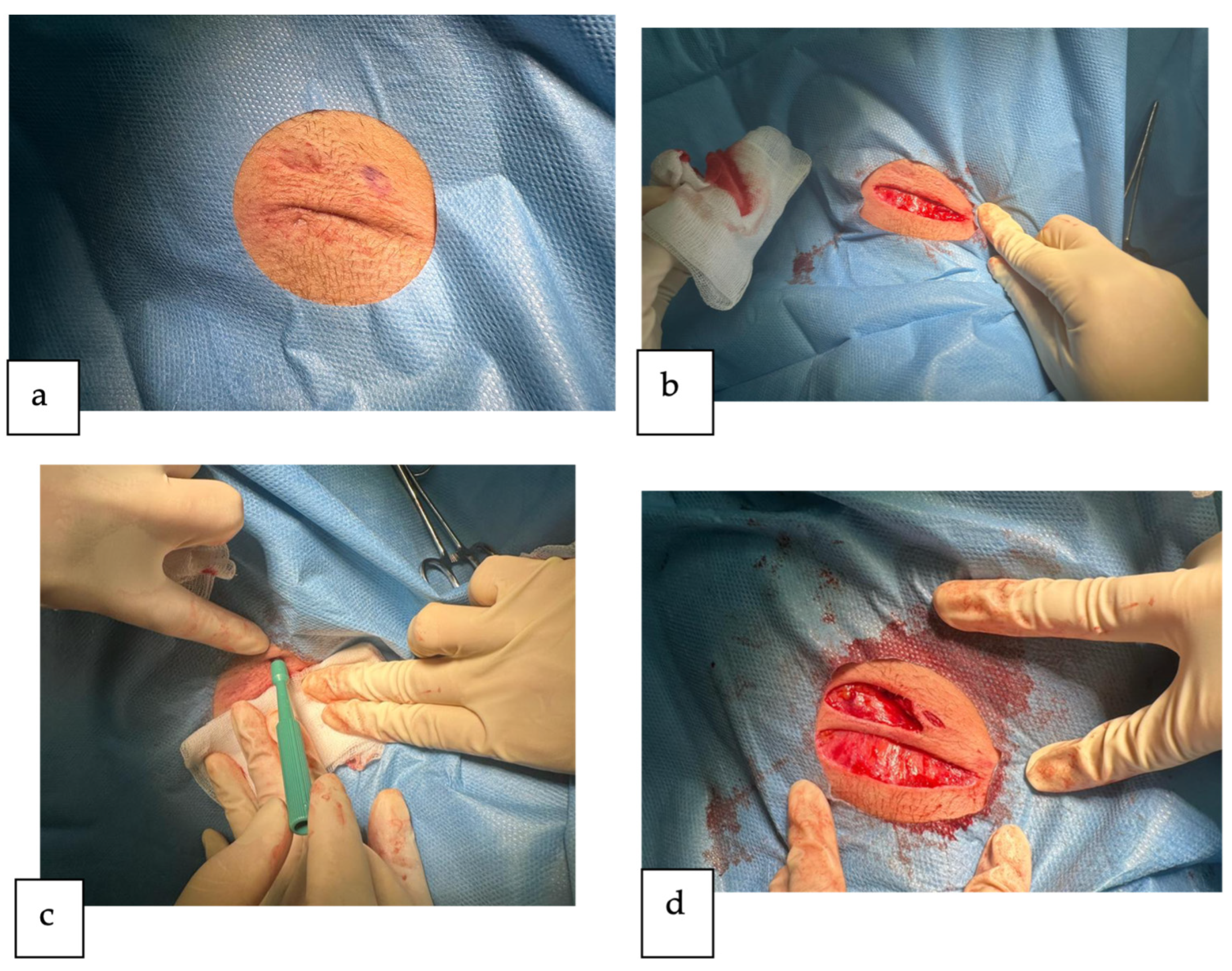
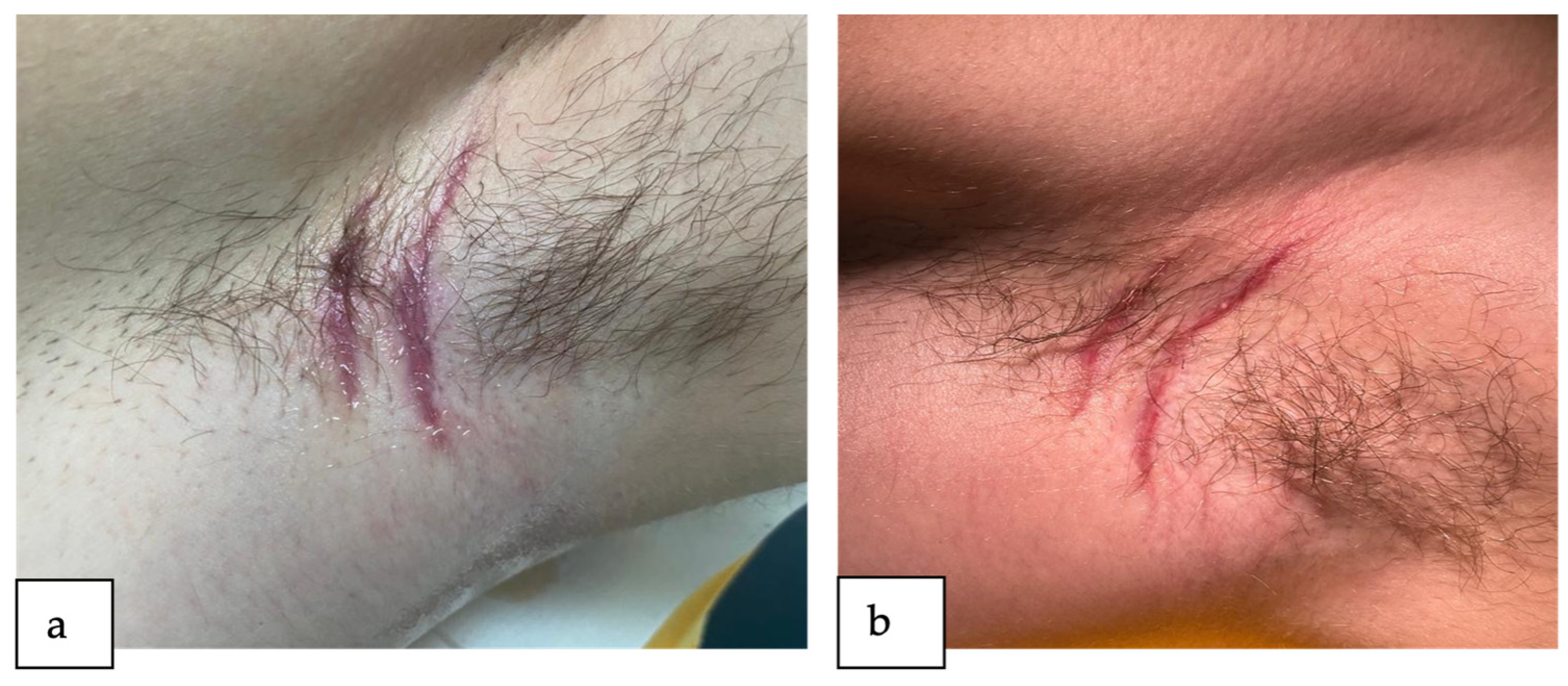
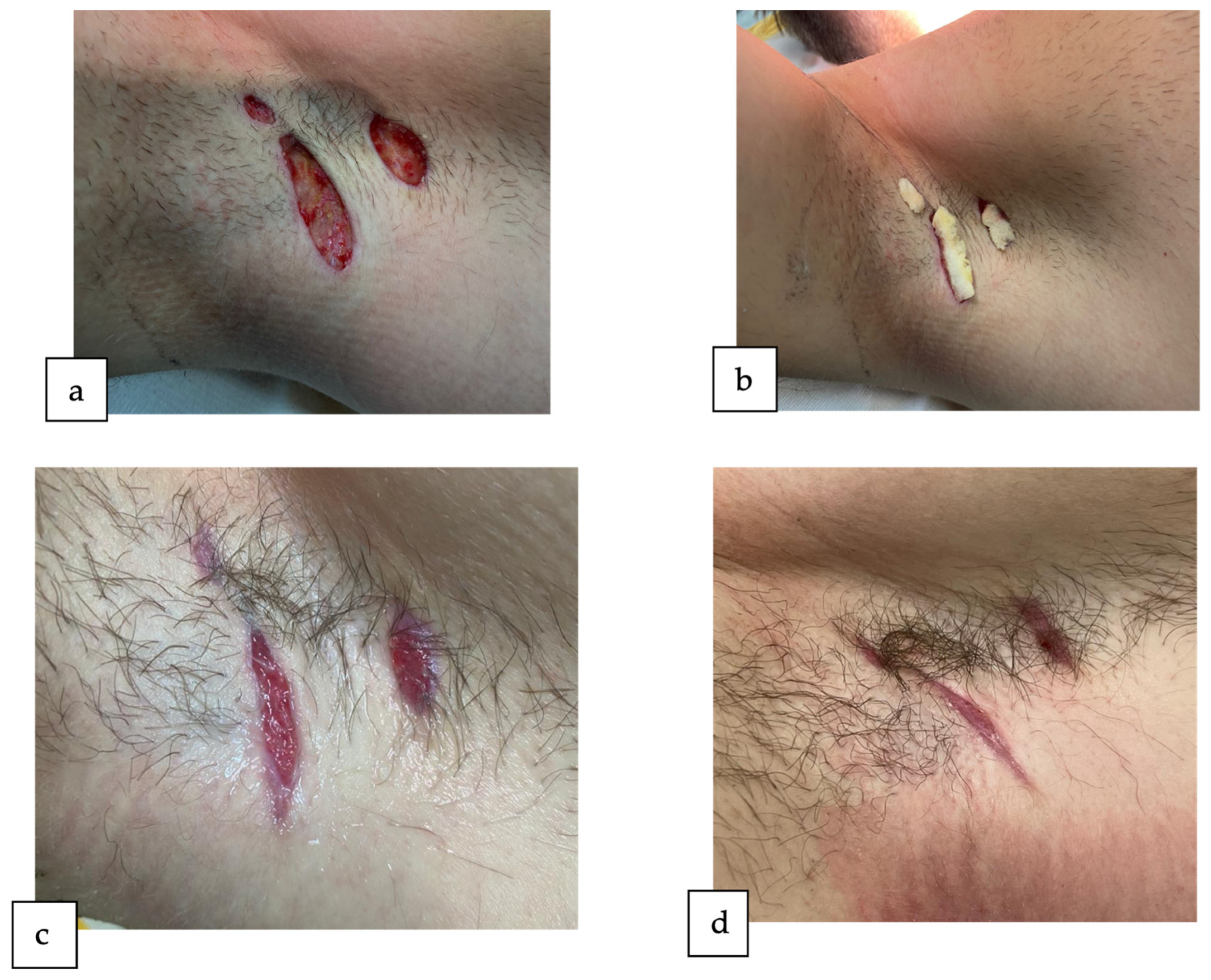
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Hidradenitis suppurativa |
| FPSUs | Folliculopilosebaceous units |
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| IHS4 | International Hidradenitis Suppurativa Severity Score System |
References
- Sellheyer, K.; Krahl, D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int. J. Dermatol. 2005, 44, 535–540. [Google Scholar] [CrossRef]
- Revuz, J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Kouris, A.; Platsidaki, E.; Christodoulou, C.; Efstathiou, V.; Dessinioti, C.; Tzanetakou, V.; Korkoliakou, P.; Zisimou, C.; Antoniou, C.; Kontochristopoulos, G. Quality of Life and Psychosocial Implications in Patients with Hidradenitis Suppurativa. Dermatology 2016, 232, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R. The epidemiology of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Lavian, J.; Lin, G.; Strunk, A.; Alloo, A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J. Am. Acad. Dermatol. 2017, 77, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Kurzen, H.; Kurokawa, I.; Jemec, G.B.E.; Emtestam, L.; Sellheyer, K.; Giamarellos-Bourboulis, E.J.; Giamarellos-Bourboulis, E.J.; Nagy, I.; Bechara, F.G.; Sartorius, K.; et al. What causes hidradenitis suppurativa? Exp. Dermatol. 2008, 17, 455–456. [Google Scholar]
- Ingram, J.R.; Dellavalle, R.P.; Owen, C.; Ofori, A.O. Hidradenitis Suppurativa: Pathogenesis, Clinical Features, and Diagnosis; UpToDate; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Revuz, J.E.; Canoui-Poitrine, F.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Roujeau, J.C.; Bonnelye, G.; et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J. Am. Acad. Dermatol. 2008, 59, 596–601. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chi, C.-C. Association of Hidradenitis Suppurativa with Inflammatory Bowel Disease. JAMA Dermatol. 2019, 155, 1022–1027. [Google Scholar] [CrossRef]
- Van Der Zee, H.H.; Van Der Woude, C.J.; Florencia, E.F.; Prens, E.P. Hidradenitis suppurativa and inflammatory bowel disease: Are they associated? Results of a pilot study. Br. J. Dermatol. 2010, 162, 195–197. [Google Scholar] [CrossRef]
- Slade, D.E.M.; Powell, B.W.; Mortimer, P.S. Hidradenitis suppurativa: Pathogenesis and management. Br. J. Plast. Surg. 2003, 56, 451–461. [Google Scholar] [CrossRef]
- Deckers, I.E.; van der Zee, H.H.; Boer, J.; Prens, E.P. Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J. Am. Acad. Dermatol. 2015, 72, 485–488. [Google Scholar] [CrossRef]
- Saunte, D.M.; Boer, J.; Stratigos, A.; Szepietowski, J.C.; Hamzavi, I.; Kim, K.H.; Zarchi, K.; Antoniou, C.; Matusiak, L.; Lim, H.W.; et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br. J. Dermatol. 2015, 173, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, R.G. Natural history, presentation, and diagnosis of hidradenitis suppurativa. Semin. Cutan. Med. Surg. 2014, 33, S51–S53. [Google Scholar] [CrossRef]
- Canoui-Poitrine, F.; Revuz, J.E.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Bastuji-Garin, S. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J. Am. Acad. Dermatol. 2009, 61, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Simonart, T. Hidradenitis suppurativa and smoking. J. Am. Acad. Dermatol. 2010, 62, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Nazary, M.; van der Zee, H.H.; Prens, E.P.; Folkerts, G.; Boer, J. Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur. J. Pharmacol. 2011, 672, 1–8. [Google Scholar] [CrossRef]
- Ingram, J.R.; Dellavalle, R.P.; Owen, C.; Ofori, A.O. Hidradenitis Suppurativa: Management; UpToDate; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Ellis, L.Z. Hidradenitis suppurativa: Surgical and other management techniques. Dermatol. Surg. 2012, 38, 517–536. [Google Scholar] [CrossRef]
- Deckers, I.E.; Benhadou, F.; Koldijk, M.J.; del Marmol, V.; Horváth, B.; Boer, J.; van der Zee, H.H.; Prens, E.P. Inflammatory bowel disease is associated with hidradenitis suppurativa: Results from a multicenter cross-sectional study. J. Am. Acad. Dermatol. 2017, 76, 49–53. [Google Scholar] [CrossRef]
- Zee HH van der Horvath, B.; Jemec, G.B.E.; Prens, E.P. The Association between Hidradenitis Suppurativa and Crohn’s Disease: In Search of the Missing Pathogenic Link. J. Investig. Dermatol. 2016, 136, 1747–1748. [Google Scholar] [CrossRef]
- Bao, B.; Zhu, C.; Shi, J.; Lu, C. Causal association between inflammatory bowel disease and hidradenitis suppurativa: A two-sample bidirectional Mendelian randomization study. Front. Immunol. 2023, 14, 1071616. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1071616 (accessed on 27 December 2023). [CrossRef] [PubMed]
- Kamal, N.; Cohen, B.L.; Buche, S.; Delaporte, E.; Colombel, J.-F. Features of Patients with Crohn’s Disease and Hidradenitis Suppurativa. Clin. Gastroenterol. Hepatol. 2016, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Orgill, D.P.; Jeschke, M.G.; Owen, C. Surgical Management of Hidradenitis Suppurativa; UpToDate; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Kohorst, J.J.; Baum, C.L.; Otley, C.C.; Roenigk, R.K.; Schenck, L.A.; Pemberton, J.H.; Dozois, E.J.; Tran, N.V.; Senchenkov, A.; Davis, M.D.P. Surgical Management of Hidradenitis Suppurativa: Outcomes of 590 Consecutive Patients. Dermatol. Surg. 2016, 42, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, E.; Clements, S.; Driscoll, M. A concise clinician’s guide to therapy for hidradenitis suppurativa. Int. J. Women’s Dermatol. 2020, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Riis, P.T.; Boer, J.; Prens, E.P.; Saunte, D.M.L.; Deckers, I.E.; Emtestam, L.; Sartorius, K.; Jemec, G.B.E. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): A case series. J. Am. Acad. Dermatol. 2016, 75, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Danby, F.W.; Hazen, P.G.; Boer, J. New and traditional surgical approaches to hidradenitis suppurativa. J. Am. Acad. Dermatol. 2015, 73, S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Jemec, G.B.E. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin. Exp. Dermatol. 2010, 35, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef]
- PubMed. Four-Weekly Infliximab in the Treatment of Severe Hidradenitis Suppurativa. Available online: https://pubmed.ncbi.nlm.nih.gov/24641123/ (accessed on 12 December 2023).
- Kimball, A.B.; Jemec, G.B.E.; Alavi, A.; Reguiai, Z.; Gottlieb, A.B.; Bechara, F.G.; Paul, C.; Bourboulis, E.J.G.; Villani, A.P.; Schwinn, A.; et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): Week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet 2023, 401, 747–761. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Prens, E.P.; Boer, J. Deroofing: A tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J. Am. Acad. Dermatol. 2010, 63, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.M.; Hamaguchi, R.; Kramer, K.M.; Kimball, A.B.; Orgill, D.P. Plastic Surgical Management of Hidradenitis Suppurativa. Plast. Reconstr. Surg. 2021, 147, 479. [Google Scholar] [CrossRef] [PubMed]
- Janse, I.; Bieniek, A.; Horváth, B.; Matusiak, Ł. Surgical Procedures in Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 97–109. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoenică, I.-V.; Dumitrașcu, M.C.; Petca, A.; Petca, R.-C.; Șandru, F. Hidradenitis Suppurativa in Association with Ulcerative Proctitis: Surgical Management in a Refractory Case to Topical and Systemic Treatment. Reports 2024, 7, 13. https://doi.org/10.3390/reports7010013
Stoenică I-V, Dumitrașcu MC, Petca A, Petca R-C, Șandru F. Hidradenitis Suppurativa in Association with Ulcerative Proctitis: Surgical Management in a Refractory Case to Topical and Systemic Treatment. Reports. 2024; 7(1):13. https://doi.org/10.3390/reports7010013
Chicago/Turabian StyleStoenică, Ioana-Valentina, Mihai Cristian Dumitrașcu, Aida Petca, Răzvan-Cosmin Petca, and Florica Șandru. 2024. "Hidradenitis Suppurativa in Association with Ulcerative Proctitis: Surgical Management in a Refractory Case to Topical and Systemic Treatment" Reports 7, no. 1: 13. https://doi.org/10.3390/reports7010013
APA StyleStoenică, I.-V., Dumitrașcu, M. C., Petca, A., Petca, R.-C., & Șandru, F. (2024). Hidradenitis Suppurativa in Association with Ulcerative Proctitis: Surgical Management in a Refractory Case to Topical and Systemic Treatment. Reports, 7(1), 13. https://doi.org/10.3390/reports7010013






