Secondary Adrenal Insufficiency Due to Isolated ACTH Deficiency Induced by Pembrolizumab: A Report of Two Cases of Uterine Endometrial Cancer
Abstract
1. Introduction
2. Case Presentation Section
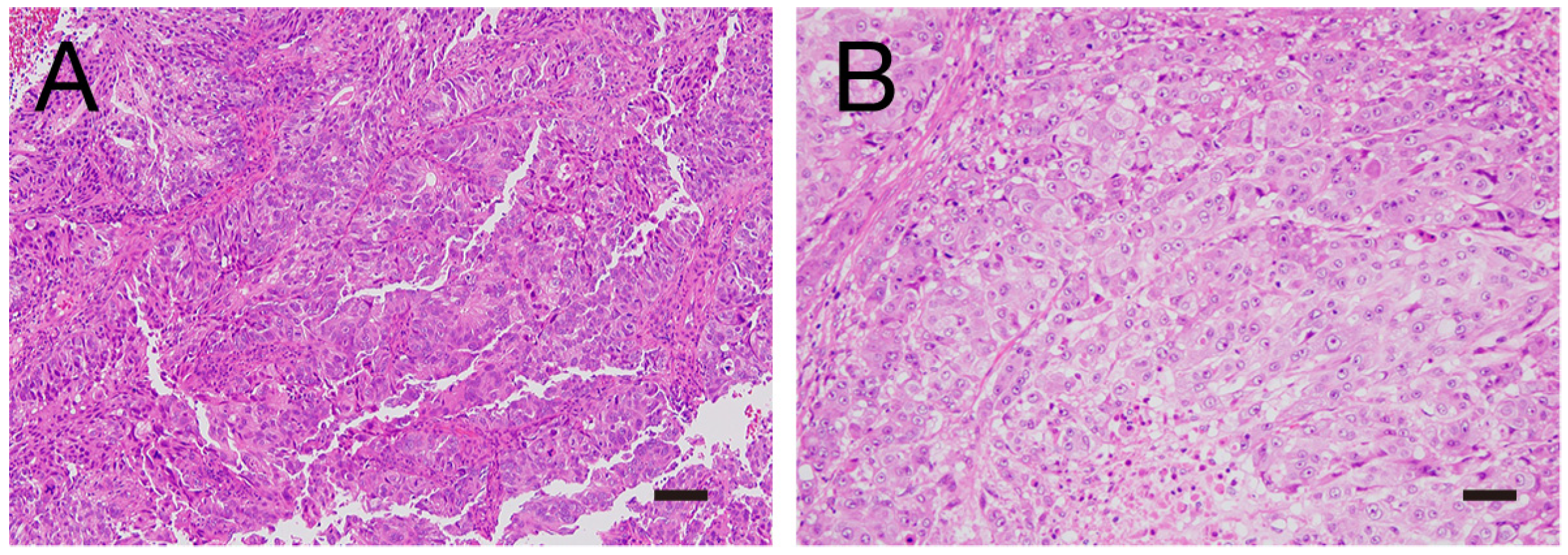
2.1. Patient 1
2.2. Patient 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013, 19, 5300–5309. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kondo, S.; Tada, K.; Kitano, S. Clinical Development of Immune Checkpoint Inhibitors. Biomed. Res. Int. 2015, 2015, 605478. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Yamagami, W.; Nagase, S.; Takahashi, F.; Ino, K.; Hachisuga, T.; Aoki, D.; Katabuchi, H. Clinical statistics of gynecologic cancers in Japan. J. Gynecol. Oncol. 2017, 28, e32. [Google Scholar] [CrossRef]
- Henley, S.J.; Miller, J.W.; Dowling, N.F.; Benard, V.B.; Richardson, L.C. Uterine Cancer Incidence and Mortality—United States, 1999–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1333–1338. [Google Scholar] [CrossRef]
- Hoskins, P.J.; Swenerton, K.D.; Pike, J.A.; Wong, F.; Lim, P.; Acquino-Parsons, C.; Lee, N. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: A phase II study. J. Clin. Oncol. 2001, 19, 4048–4053. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Umeguchi, H.; Takenoshita, H.; Inoue, H.; Kurihara, Y.; Sakaguchi, C.; Yano, S.; Hasuzawa, N.; Sakamoto, S.; Sakamoto, R.; Ashida, K. Autoimmune-Related Primary Hypoparathyroidism Possibly Induced by the Administration of Pembrolizumab: A Case Report. J. Oncol. Pract. 2018, 14, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Peiró, I.; Biagetti, B.; Paja-Fano, M.; Cobo, D.A.; Gómez, C.G.; Mateu-Salat, M.; Genua, I.; Majem, M.; Riudavets, M.; et al. Immunotherapy-induced isolated ACTH deficiency in cancer therapy. Endocr. Relat. Cancer 2021, 28, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.; Reynolds, K.; Zubiri, L.; Lawrence, D.; Cohen, J.V.; Sullivan, R.J.; Nachtigall, L.; Tritos, N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 2019, 181, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Lemiale, V.; Meert, A.P.; Vincent, F.; Darmon, M.; Bauer, P.R.; Van de Louw, A.; Azoulay, E. Severe toxicity from checkpoint protein inhibitors: What intensive care physicians need to know? Ann. Intensive Care 2019, 9, 25. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef]
- López, J.G.; Faria, D.E.; Fernández, M.R. Acute adrenal insufficiency in a patient with panhypopituitarism after vaccination against COVID-19 (BNT162b2 Pfizer-BioNTech). Endocrinol. Diabetes Nutr. (Engl. Ed.) 2022, 69, 762–763. [Google Scholar]
- Maguire, D.; McLaren, D.S.; Rasool, I.; Shah, P.M.; Lynch, J.; Murray, R.D. ChAdOx1 SARS-CoV-2 vaccination: A putative precipitant of adrenal crises. Clin. Endocrinol. 2021. [Google Scholar] [CrossRef]
- Kanie, K.; Iguchi, G.; Bando, H.; Urai, S.; Shichi, H.; Fujita, Y.; Matsumoto, R.; Suda, K.; Yamamoto, M.; Fukuoka, H.; et al. Mechanistic insights into immune checkpoint inhibitor-related hypophysitis: A form of paraneoplastic syndrome. Cancer Immunol. Immunother. 2021, 70, 3669–3677. [Google Scholar] [CrossRef]
- Mitchell, E.; Ciccone, M.; Zhang, B.; Tsai, A.; Brunette, L.L. Paraneoplastic Cushing’s syndrome and hypercalcemia arising from metastatic endometrioid endometrial adenocarcinoma: A case report. Gynecol. Oncol. Rep. 2019, 29, 58–60. [Google Scholar] [CrossRef]
- Yano, S.; Ashida, K.; Sakamoto, R.; Sakaguchi, C.; Ogata, M.; Maruyama, K.; Sakamoto, S.; Ikeda, M.; Ohe, K.; Akasu, S.; et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur. J. Cancer 2020, 130, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Okuji, T.; Ito, M.; Onoue, T.; Goto, M.; Sugiyama, M.; Tsunekawa, T.; et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study. J. Immunother. Cancer 2020, 8, e000779. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Hu, J.; Warner, A.B.; Quach, H.T.; Cann, C.G.; Zhang, M.Z.; Si, L.; Tang, B.; Cui, C.; Yang, X.; et al. Early Use of High-Dose Glucocorticoid for the Management of irAE Is Associated with Poorer Survival in Patients with Advanced Melanoma Treated with Anti-PD-1 Monotherapy. Clin. Cancer Res. 2021, 27, 5993–6000. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | 0 | 15 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|
| ACTH (pg/mL) | 6.3 | 5.9 | 7.1 | 6.9 | 4.5 | 4.4 |
| Cortisol (μg/dL) | 1.4 | 1.4 | 1.2 | 1.2 | 1 | 1 |
| TSH (mIU/L) | 1.8 | 9.6 | 12.7 | 10.3 | 7.7 | 5.8 |
| PRL (ng/mL) | 11.2 | 53.9 | 55.1 | 41.5 | 30.7 | 23.3 |
| LH (mIU/L) | 12.6 | 22.2 | 28.7 | 35.4 | 32 | 30.5 |
| FSH (mIU/L) | 33 | 34.4 | 37.8 | 40.4 | 41.9 | 42.1 |
| GH (ng/mL) | 1.2 | 10.8 | 12 | 8.2 | 4.9 | N.E. |
| Time (min) | 0 | 15 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|
| ACTH (pg/mL) | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 |
| Cortisol (μg/dL) | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 |
| TSH (mIU/L) | 2.9 | 10.6 | 12.7 | 9.6 | 7.2 | 6.0 |
| PRL (ng/mL) | 10.9 | 43.4 | 42.1 | 27.1 | 20.5 | 17.3 |
| LH (mIU/L) | 5.5 | 7.7 | 9.6 | 9.4 | 9.2 | 9.7 |
| FSH (mIU/L) | 29.7 | 35.9 | 38.8 | 39.4 | 39.5 | 40.3 |
| GH (ng/mL) | 2.2 | 40.6 | 30.8 | 19.8 | 15.4 | N.E. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onoyama, I.; Kawakami, M.; Hachisuga, K.; Maenohara, S.; Kodama, K.; Yagi, H.; Yasunaga, M.; Ohgami, T.; Asanoma, K.; Yahata, H.; et al. Secondary Adrenal Insufficiency Due to Isolated ACTH Deficiency Induced by Pembrolizumab: A Report of Two Cases of Uterine Endometrial Cancer. Reports 2023, 6, 18. https://doi.org/10.3390/reports6020018
Onoyama I, Kawakami M, Hachisuga K, Maenohara S, Kodama K, Yagi H, Yasunaga M, Ohgami T, Asanoma K, Yahata H, et al. Secondary Adrenal Insufficiency Due to Isolated ACTH Deficiency Induced by Pembrolizumab: A Report of Two Cases of Uterine Endometrial Cancer. Reports. 2023; 6(2):18. https://doi.org/10.3390/reports6020018
Chicago/Turabian StyleOnoyama, Ichiro, Minoru Kawakami, Kazuhisa Hachisuga, Shoji Maenohara, Keisuke Kodama, Hiroshi Yagi, Masafumi Yasunaga, Tatsuhiro Ohgami, Kazuo Asanoma, Hideaki Yahata, and et al. 2023. "Secondary Adrenal Insufficiency Due to Isolated ACTH Deficiency Induced by Pembrolizumab: A Report of Two Cases of Uterine Endometrial Cancer" Reports 6, no. 2: 18. https://doi.org/10.3390/reports6020018
APA StyleOnoyama, I., Kawakami, M., Hachisuga, K., Maenohara, S., Kodama, K., Yagi, H., Yasunaga, M., Ohgami, T., Asanoma, K., Yahata, H., Kitamura, Y., Sakamoto, R., Kiyozawa, D., & Kato, K. (2023). Secondary Adrenal Insufficiency Due to Isolated ACTH Deficiency Induced by Pembrolizumab: A Report of Two Cases of Uterine Endometrial Cancer. Reports, 6(2), 18. https://doi.org/10.3390/reports6020018






