1. Introduction
Cardiac devices that can be implanted to reduce morbidity and mortality include permanent pacemakers (PPM) and implantable cardioverter defibrillators. With rapid population expansion and increasing indications for device implantation, the number of implants has increased [
1,
2]. Any invasive procedure carries a risk of complications, and cardiac-device implantation is no different.
Often quoted complications are pneumothoraxes, infections, bleeding, myocardial perforation, and lead displacement, with overall complication rates being approximately four to five per cent [
3,
4,
5]. Complications are often related to how vascular access was performed (for example subclavian or axillary vein or cephalic vein approaches) [
5,
6]. Specifically, pneumothorax during venous access is a complication of approximately 0.2–3.7% of procedures; however, some approaches, such as the cephalic vein approach, allow for a reduction in pneumothorax rates [
7]. Pneumothorax risk seems to also be increased in female patients with concurrent chronic obstructive pulmonary disease, and is understandably associated with increased costs [
3,
4].
The management of iatrogenic pneumothorax is not well established. In the United Kingdom, the British Thoracic Society (BTS) guidelines are now over a decade old and only mention that most “will resolve with no intervention” [
8]. A PubMed search with the terms “pneumothorax” OR “cardiac device implantation” OR “pacemaker” AND “management” revealed 24,050 results from December 1981 to December 2021 but none were directly related to guidance regarding post cardiac-device implantation pneumothoraxes. Two studies revealed the use of needle aspiration and unidirectional valves in such pneumothoraxes in retrospective case series [
9,
10].
The evidence around pneumothorax management has evolved over time with some large randomised trials, the results of which have been recently published. Brown et al. [
11] have shown that simple observation in primary spontaneous pneumothorax (pneumothorax occurring in patients with no discernible lung disease) can be feasible in a specific subset of patients. In the same vein, Gerhardy et al. [
12] and Walker et al. [
13] have shown that conservative management of traumatic pneumothoraxes is feasible and this concept will be tested in a randomised controlled trial in the United Kingdom [
14]. Observation of selected secondary spontaneous pneumothoraxes is also feasible [
15]. These studies will be further discussed later in this paper.
Northumbria Healthcare NHS Foundation Trust is a large district general hospital in the North East of England. We provide a well-established regional pleural service with advanced procedures, such as medical thoracoscopy, indwelling pleural catheter insertion, and more recently ambulatory pneumothorax and chest trauma pathways [
16,
17,
18]. Our local approach has always been guided by local experience and the literature available at the time. The size of the pneumothorax appears to be less important than the symptoms. We have examined the outcomes of patients with pneumothorax post image-guided biopsy [
19] and demonstrated, in an observational study, that those with large, asymptomatic pneumothoraxes post biopsy can be observed. Our local cardiology and respiratory services agreed that patients with pneumothoraxes post cardiac-device implantation should be assessed in a similar fashion for symptoms of dyspnoea and that pleural interventions be offered only if the symptoms are progressive.
The local incidence of pneumothorax post cardiac-device implantation is not known and an analysis of the subsequent management has not been previously performed.
2. Materials and Methods
2.1. Local Ethical Approval
Local Caldicott approval (Reference: RPI-C3737, obtained on 18 March 2021) was sought and granted from Northumbria HealthCare NHS Foundation Trust Information Governance Department, North Tyneside, United Kingdom for this retrospective study of cardiac-device implantations performed between 1 January 2010 and the 31 December 2020 from the local database kept by the cardiology department.
2.2. Aims of Study
The main aims of the study were to assess if those iatrogenic pneumothoraxes with no symptoms were indeed observed, how many went on to require intervention, and if those patients had any clinical conditions in common.
2.3. Inclusion Criteria and Exclusion Criteria
We logged all cardiac-device implantation devices onto the database. We then reviewed those patient episodes for their venous approach if relevant, in addition to any post-procedural complications. We excluded those without a post-procedure pneumothorax and further reviewed those identified as being complicated by a pneumothorax in the database. Then, we confirmed pneumothorax using contemporary radiology. We further analysed those patient records regarding basic demographics, pleural interventions required, and eventual or immediate outcomes collected from the notes. We collected long-term outcomes from primary care records if available.
2.4. Size Definition for Pneumothorax
We determined the size of pneumothorax by established BTS guidelines, where the differentiation of a “large” from a “small” pneumothorax is the presence of a visible rim of more than two centimetres (cm) between the lung margin and the chest wall. We digitally measured the size on the hospital’s PACS (picture archiving and communication system) system and independently verified it with a respiratory consultant.
2.5. Analysis
We applied a descriptive statistical methodology. We presented continuous data as median and interquartile ranges (IQRs) if outliers were present, or mean and ranges if normally distributed. We presented categorical variables as frequencies or percentages. We performed the analysis on Microsoft Excel 2019 (Microsoft 365).
3. Results and Discussion
A total of 2056 implantation episodes from January 2010 to December 2020 were reviewed.
Seventy pneumothoraxes (3.4%) were identified on post-implantation chest radiographs and clinical notes.
All were related to PPM insertion. A total of 59 (84.2%) of those PPM insertions were performed with a subclavian vein puncture approach and 1 (1.4%) had a cephalic vein puncture. The notes were incomplete in 10 (14.4%) patients, with no mention of the type of puncture employed.
The median age for those 70 patients with pneumothoraxes was 77.5 years (IQR 11). A total of 39 (56%) patients were female and 31 (44%) were male. All pneumothoraxes were on the side of the PPM (three on the right side, 67 on the left side). A total of 36 pneumothoraxes (51%) were small and 34 (49%) were large according to the above-described BTS criteria.
The above-described results are depicted in
Table 1 below.
None of the 70 patients developed hypoxaemia on peripheral oxygen saturation monitoring. Median peripheral oxygen saturations pre-procedure was 96% (IQR 3), and post-procedure it was 95% (IQR 2).
Fifty-six (80%) patients had no initial respiratory symptoms, such as chest pain or worsening breathlessness, from their baseline. A total of 30 (54%) of those were large pneumothoraxes, as defined by BTS criteria. Eighteen (32%) of those patients had concurrent chronic obstructive pulmonary disease. All 56 patients were observed initially with overnight admission. Five (9%) of those underwent intercostal drainage with a small-bore (12 French gauge) drain the next day due to the development of progressive breathlessness. All five of those had chronic obstructive pulmonary disease. Two of those five who underwent intercostal drainage initially had large pneumothoraxes and three were initially small. The median length of stay for those not requiring drainage was 2.1 days (IQR 2).
Fourteen (20%) patients with post-procedural pneumothoraxes were immediately treated with small-bore intercostal drains due to the development of severe symptomatic dyspnoea and/or chest pain. The median age in this particular group was 78 years (IQR 10, range 69–89). Eight of the patients had concurrent chronic obstructive pulmonary disease. Five (36%) of the pneumothoraxes were large at the outset. The median length of stay was 4.2 days (IQR 2).
All the pneumothoraxes were resolved on follow-up chest radiographs. There was no associated mortality. There were no incidents related to intercostal chest drain insertion.
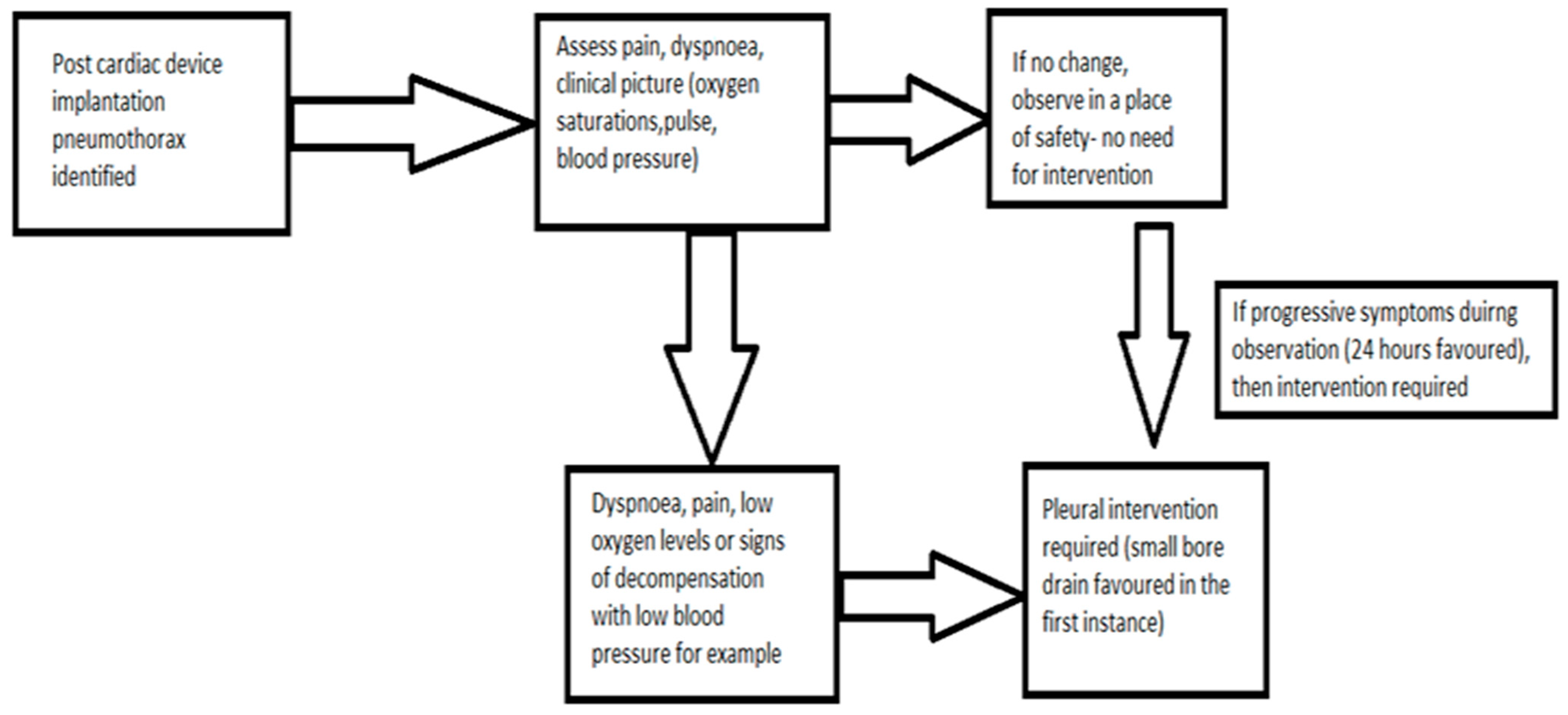
Figure 1 below depicts the treatment pathway of those patients with pneumothoraxes post cardiac-device implantation according to their symptoms.
British Thoracic Society (BTS) criteria for pneumothorax size: differentiation of a “large” from a “small” pneumothorax is the presence of a visible rim of more than two centimetres (cm) between the lung margin and the chest wall on a chest radiograph;
ICD: intercostal drain insertion;
Discussion.
Our single-centre analysis of pneumothoraxes post cardiac-device implantation shows that in the absence of symptoms, or cardiovascular and respiratory decompensation, no pleural intervention is required, irrespective of the size of the pneumothorax. Patients with co-existing chronic obstructive pulmonary disease were more likely to have intercostal drainage after a pneumothorax but the number of patients was too small to infer any statistical significance. Specifically, in this study, the rate of pneumothorax post permanent-pacemaker insertion is within the known incidence percentage, which has a range of 0.2–3.7% [
1,
2,
3].
3.1. Evidence towards Observation of Pneumothorax
The now-outdated 2010 BTS Pleural Disease guidelines suggest that for any pleural intervention, the choice of instrument (needle aspiration, small- or large-bore chest drain insertion and, by extension, any of the now-available ambulatory devices) should be dictated by the expertise available locally [
8]. Locally, the use of needle aspiration for any type of pneumothorax is not commonplace and anecdotally has been associated with an unacceptable failure of pneumothorax re-expansion, with the patients subsequently needing another procedure. Nevertheless, there is evidence from a very small case series that suggests that needle aspiration alone might be feasible in some instances [
9]. This approach needs to be validated in large multi-centre trials before widespread applicability. Thus, we performed small-bore intercostal drain insertion only when an intervention was clearly required. This is reflected in the above findings that only intercostal drains were performed if an intervention was required.
The point about observations regarding primary spontaneous pneumothorax (pneumothorax occurring in patients with no apparent underlying lung disease) is made in the 2010 BTS guidance, where it is recommended that some large primary pneumothoraxes can be safely observed [
8]. Observation for pneumothorax is not a new concept [
20]. Stradling et al. in 1966 described a decade-long case series of 119 pneumothoraxes in 111 patients and approximately 80% of this unselected group of patients were managed conservatively with no apparent ill effects [
20]. Prior to this, Kircher et al in 1957 described the rate of re-absorption of pleural air at 1.25–1.8% of the volume of pneumothorax every 24 h and argued to expect complete re-expansion at 7 weeks [
21]. Expert opinion, which we would agree with, suggests that the patients and their symptoms should be treated rather than waiting for the appearance of the chest radiographs [
22]. Walker et al. have recently eloquently written about intercostal drain insertion potentially exacerbating any air leak. The visceral pleural defect in any pneumothorax might be very self-limiting and intercostal drainage might well increase airflow across that defect; thus, the authors suggest that the avoidance of intervention may be desirable in minimally symptomatic patients [
23]. As mentioned in the introduction, there are some newer clinical studies that have reported favourable outcomes for pneumothorax observation. Despite its limitations (long recruitment period, minimally symptomatic population, and lack of generalisable applicability), Brown et al. have shown that the conservative management of primary spontaneous pneumothorax was non-inferior to interventional management, with a lower risk of serious adverse events [
11]. Only 15% of patients managed conservatively required subsequent chest drain insertion during the study period, and of these patients, only 2% were because of enlarging pneumothorax (other indications were pain and hypoxaemia). In secondary spontaneous pneumothorax, Gerhardy et al. showed that in 64 patients with secondary pneumothoraxes with a greater than one-centimetre pneumothorax, 39% of the managed patients did not require subsequent intervention, and their overall length of stay was shorter [
15].
As also mentioned above in the introduction, there is no specific guidance describing the management of pneumothorax after cardiac-device implantation. An analogy might be made to traumatic pneumothoraxes, where small and/or asymptomatic pneumothoraxes can be observed, although formal randomised trial data needs to be generated [
13,
14]. Our centre has recently described our local experience [
16].
One might argue that the insertion of a small-bore intercostal drain or performing a needle aspiration are simple and safe procedures; however, we would argue that this is only the case in experienced hands and that complications do exist. We have previously published our experience regarding intercostal chest drain and needle aspirations complications, and we described a 3% rate of drain “fall-out”, a 0.5% rate of bleeding, and an approximately 4% rate of surgical emphysema [
24]. These findings are not novel; for example, Sundaralingam et al. performed an elegant narrative review and found even higher rates of drain displacement at nearly 7% [
25].
Our proposed management system is depicted in
Figure 2 below:
3.2. Limitations
The limitations of our study are manifold: it is a single-centre retrospective series with no control arm (a proposed controlled arm would a group without pneumothorax and pre-defined numbers to recruit to each arm for a powered statistical analyses). The initial recommendations for simple observation rather than treatment were based on expert opinion and on local experience rather than a strong evidence base. That evidence base is slowly coming together, as described above. We have successfully applied the same principles to patients with pneumothorax after image-guided biopsies and found that the vast majority of procedural pneumothoraxes can be observed and a symptom-based approach can be employed [
18]. We suggest this as well in this specific patient group. However, all of the above informs the need for further studies in this field, and looking at iatrogenic pneumothoraxes in general. Due to the small numbers of patients, no meaningful statistical analysis was possible.
Furthermore, we do not differentiate between different devices and approaches within the pacemaker-using population (for example a single/dual chamber pacemaker may require full cephalic vein access) versus the population using cardiac resynchronization therapy (CRT) or an implantable cardioverter defibrillator (ICD), which may potentially be associated with severe complications, including pneumo- and haemothorax. As Vogler et al. [
26]. suggest, different approaches, such as a triple-lead cephalic versus subclavian vein approach, can be feasible. Alternative techniques for left-ventricular pacing in cardiac resynchronization therapy may today include his pacing, as described by Senes et al. [
27], or left bundle branch pacing, as described by Liu et al. [
28]., leading to a potentially different vein approach. Furthermore, the age of patients presenting with complex arrhythmias is increasing (clearly demonstrated by Fumagalli et al. [
29]), and this frailty may impact on cardiac-device implantation through a variety of processes. We also did not collect data on the “frailty syndrome”, an emerging clinical problem in the everyday management of clinical arrhythmias. An ageing population with increasing incidences of renal failure, dementia, disability, atrial fibrillation, heart failure, falls, and cancer leads to elderly and frail individuals with enhanced susceptibility to stressors and a decreased capability for homeostasis [
30].
4. Conclusions
Pneumothorax after cardiac-device implantation can occur in approximately 3% of patients. These pneumothoraxes can also be observed if patients are asymptomatic. There are some significant limitations to this retrospective study but this could pave the way for large, randomised, and controlled trials in iatrogenic pneumothoraxes.
Author Contributions
Conceptualization, A.A.; methodology, A.A.; software, A.A.; validation, A.A.; formal analysis, A.A.; investigation, G.G.; writing—original draft preparation, A.A.; writing—review and editing, A.A. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (Information Governance Department) of Northumbria Healthcare NHS Foundation Trust (protocol code RPI-C3737 and date of approval was 12 April 2021).
Informed Consent Statement
Patient consent was waived due to the study being an anonymized review.
Data Availability Statement
Anonymized data is available with reasonable requests.
Acknowledgments
We thank Honey Thomas for providing access to the cardiology database.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raatikainen, M.J.P.; Arnar, D.O.; Merkely, B.; Nielsen, J.C.; Hindricks, G.; Heidbuchel, H.; Camm, J. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 2017, 19 (Suppl. S2), ii1–ii90. [Google Scholar] [CrossRef]
- Danish Pacemaker and ICD Register Annual Report. 2019. Available online: https://www.sundhed.dk/content/cms/21/109821_dpir_annual_report_2019_final_211220.pdf (accessed on 27 April 2022).
- Kotsakou, M.; Kioumis, I.; Lazaridis, G.; Pitsiou, G.; Lampaki, S.; Papaiwannou, A.; Karavergou, A.; Tsakiridis, K.; Katsikogiannis, N.; Karapantzos, I.; et al. Pacemaker insertion. Ann. Transl. Med. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. ESC Scientific Document Group, 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Hung, S.-P.; Chen, P.-R.; Yang, C.-H.; Wo, H.-T.; Chang, P.-C.; Wang, C.-C.; Chou, C.-C.; Wen, M.-S.; Chung, C.-M.; et al. Risk factors influencing complications of cardiac implantable electronic device implantation: Infection, pneumothorax and heart perforation: A nationwide population-based cohort study. Medicine 2014, 93, e213. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.E.; Gleva, M.J.; Mela, T.; Chung, M.K.; Uslan, D.Z.; Borge, R.; Gottipaty, V.; Shinn, T.; Dan, D.; Feldman, L.A.; et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: Results from the REPLACE registry. Circulation 2010, 122, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Benz, A.P.; Vamos, M.; Erath, J.W.; Hohnloser, S.H. Cephalic vs. subclavian lead implantation in cardiac implantable electronic devices: A systematic review and meta-analysis. EP Eur. 2018, 21, 121–129. [Google Scholar] [CrossRef]
- MacDuff, A.; Arnold, A.; Harvey, J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. S2), ii18–ii31. [Google Scholar] [CrossRef]
- Domokos, D.; Szabo, A.; Banhegyi, G.; Polgar, B.; Bari, Z.; Bogyi, P.; Marczell, I.; Papp, L.; Kiss, R.G.; Duray, G.Z.; et al. Needle aspiration for treating iatrogenic pneumothorax after cardiac electronic device implantation: A pilot study. J. Interv. Card. Electrophysiol. 2020, 57, 295–301. [Google Scholar] [CrossRef]
- Papanikolaou, J.; Platogiannis, N.; Gialamas, I.; Platogiannis, D. Pneumothorax post-pacemaker implantation: The novelty of Heimlich valve. EP Eur. 2020, 23, 927. [Google Scholar] [CrossRef]
- Brown, S.G.; Ball, E.L.; Perrin, K.; Asha, S.E.; Braithwaite, I.; Egerton-Warburton, D.; Jones, P.G.; Keijzers, G.; Kinnear, F.B.; Kwan, B.C.; et al. Conservative versus Interventional Treatment for Spontaneous Pneumothorax. N. Engl. J. Med. 2020, 382, 405–415. [Google Scholar] [CrossRef]
- Gerhardy, B.C.; Liebenberg, P.; Simpson, G. Conservative management of traumatic pneumothoraces: A retrospective cohort study. Emerg. Med. Australas. 2022, 34, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.; Barratt, S.L.; Thompson, J.; Maskell, N.A. Conservative Management in Traumatic Pneumothoraces: An Observational Study. Chest 2018, 153, 946–953. [Google Scholar] [CrossRef]
- Conservative Management in Traumatic Pneumothoraces in the Emergency Department (CoMiT-ED): A Randomised Controlled Trial. 2021. Available online: https://fundingawards.nihr.ac.uk/award/NIHR132889 (accessed on 27 April 2022).
- Gerhardy, B.C.; Simpson, G. Conservative versus invasive management of secondary spontaneous pneumothorax: A retrospective cohort study. Acute Med. Surg. 2021, 8, e663. [Google Scholar] [CrossRef] [PubMed]
- Bates-Powell, J.; Basterfield, D.; Jackson, K.; Aujayeb, A. Physician-Led Thoracic Trauma Management in a Specialist Emergency Care Centre. J. Clin. Med. 2021, 10, 5806. [Google Scholar] [CrossRef] [PubMed]
- Aujayeb, A.; Parker, S.; Bourke, S.; Miller, J.; Cooper, D. A review of a pleural service. J. R. Coll. Physicians Edinb. 2016, 46, 26–31. [Google Scholar] [CrossRef]
- Aujayeb, A.; Jackson, K. A review of the outcomes of rigid medical thoracoscopy in a large UK district general hospital. Pleura Peritoneum 2020, 5, 20200131. [Google Scholar] [CrossRef]
- Moad, M.; Narkhede, P.; Jackson, K.; Aujayeb, A. A note on pneumothorax post-CT-guided biopsy. Br. J. Radiol. 2021, 94, 20201010. [Google Scholar] [CrossRef]
- Stradling, P.; Poole, G. Conservative management of spontaneous pneumothorax. Thorax 1966, 21, 145–149. [Google Scholar] [CrossRef]
- Kircher, L.T.; Swartzel, R.L., Jr. Spontaneous pneumothorax and its treatment. J. Am. Med Assoc. 1954, 155, 24–29. [Google Scholar] [CrossRef]
- Simpson, G. Spontaneous pneumothorax: Time for some fresh air. Intern. Med. J. 2010, 40, 231–234. [Google Scholar] [CrossRef]
- Walker, S.P.; Hallifax, R.; Rahman, N.M.; Maskell, N.A. Challenging the Paradigm of Persistent Air Leak: Are We Prolonging the Problem? Am. J. Respir. Crit. Care Med. 2022, 206, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Kafi, O.; Bhullar, D.; Scott, J.; Storey, C.; Hyatali, S.; Carlin, H.; Brown, A.; Grimshaw, E.; Miller, J.; et al. Complications after Thoracocentesis and Chest Drain Insertion: A Single Centre Study from the North East of England. J. Respir. 2021, 1, 135–140. [Google Scholar] [CrossRef]
- Sundaralingam, A.; Bedawi, E.O.; Harriss, E.K.; Munavvar, M.; Rahman, N.M. The Frequency, Risk Factors, and Management of Complications From Pleural Procedures. Chest 2021, 161, 1407–1425. [Google Scholar] [CrossRef] [PubMed]
- Vogler, J.; Geisler, A.; Gosau, N.; Hakmi, S.; Willems, S.; Rassaf, T.; Wakili, R.; Kaya, E. Triple lead cephalic versus subclavian vein approach in cardiac resynchronization therapy device implantation. Sci. Rep. 2018, 8, 17709. [Google Scholar] [CrossRef] [PubMed]
- Senes, J.; Mascia, G.; Bottoni, N.; Oddone, D.; Donateo, P.; Grimaldi, T.; Minneci, C.; Bertolozzi, I.; Brignole, M.; Puggioni, E.; et al. Is His-optimized superior to conventional cardiac resynchronization therapy in improving heart failure? Results from a propensity-matched study. Pacing Clin. Electrophysiol. 2021, 44, 1532–1539. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Q.; Sun, H.; Qin, X.; Zheng, Q. Left Bundle Branch Pacing: Current Knowledge and Future Prospects. Front. Cardiovasc. Med. 2021, 8, 630399. [Google Scholar] [CrossRef]
- Fumagalli, S.; Pieragnoli, P.; Haugaa, K.H.; Potpara, T.S.; Rasero, L.; Ramacciati, N.; Ricciardi, G.; Solimene, F.; Mascia, G.; Mascioli, G.; et al. The influence of age on the psychological profile of patients with cardiac implantable electronic devices: Results from the Italian population in a multicenter study conducted by the European Heart Rhythm Association. Aging Clin. Exp. Res. 2019, 31, 1219–1226. [Google Scholar] [CrossRef]
- Fumagalli, S.; Potpara, T.S.; Larsen, T.B.; Haugaa, K.H.; Dobreanu, D.; Proclemer, A.; Dagres, N. Frailty syndrome: An emerging clinical problem in the everyday management of clinical arrhythmias. The results of the European Heart Rhythm Association survey. Europace 2017, 19, 1896–1902. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).